Opinion Statement
The adult mammalian heart has limited capacity for generation, so a major injury such as a myocardial infarction results in the permanent loss of up to one billion cardiomyocytes. The field of cardiac cell therapy aims to replace these lost contractile units with de novo cardiomyocytes to restore lost systolic function and prevent progression to heart failure. Arguably the ideal cell for this application is the human cardiomyocyte itself, which can electromechanically couple with host myocardium and contribute active systolic force. Pluripotent stem cells from both human embryonic or induced pluripotent lineages are attractive sources for cardiomyocytes, and preclinical investigation of these cells is in progress. Recent work has focused on efficient generation and purification of cardiomyocytes, tissue engineering efforts, and examining the consequences of cell transplantation from mechanical, vascular, and electrical standpoints. Here we discuss historical and contemporary aspects of pluripotent stem cell-based cardiac cell therapy, with an emphasis on recent preclinical studies with translational goals.
Keywords: embryonic stem cells, pluripotent stem cells, cardiomyocyte, myocardial infarction, regenerative medicine, cardiac cell therapy
Introduction
Despite significant improvements in medical management, heart disease still claims one life every 40 seconds in the United States and is now the number one cause of mortality worldwide[1]. The adult heart lacks robust intrinsic regenerative capabilities, and any major injury to the myocardium results in the replacement of muscle with non-functional scar tissue, reduced contractile performance, and the initiation of a vicious cycle of adverse remodeling. Motivated by this challenge, a number of investigators began experimenting with exogenous cell transplantation in the early 1990s with the goal of remuscularizing the infarcted heart [2,3]. Early proof-of-concept experiments with neonatal and fetal cardiomyocytes showed that stable grafts of new myocardium could be formed in injured hearts [4,5], but these cell sources have obvious ethical and practical limitations that prevent clinical use. Skeletal myoblasts were next investigated as an alternative source of striated muscle tissue [6,7], but subsequent work raised concerns about arrhythmias [8–10] and efficacy [11] with this cell source. More recently, a variety of bone marrow-derived cell types have been explored in preclinical and clinical studies, but these cells do not generate significant numbers of de novo cardiomyocytes, and any beneficial contractile effects likely result from indirect mechanisms such as paracrine signaling [12,13].
If the ultimate goal of cardiac cell therapy is to regenerate human myocardium, then no cell type is better suited to the task than the human cardiomyocyte itself, as it is capable of electrically integrating with host muscle and generating systolic force. However, the initial practical challenge was to identify a suitable source for obtaining large quantities of phenotypically unambiguous cardiomyocytes. The isolation of human embryonic stem cells (hESCs) in 1998 [14] and human induced pluripotent stem cells (hiPSCs) a decade later [15] represented potential solutions to this problem. Here, we review the current status of cardiac repair using pluripotent stem cell-derived cardiomyocytes (PSC-CM), with an emphasis on recently published work that is aimed at moving this emerging technology towards clinical translation.
Sources of Pluripotent Stem Cells
Pluripotent hESCs are derived from preimplantation-stage human blastocysts donated after in vitro fertilization efforts [14]. In addition to political and ethical concerns, these cells also pose a practical challenge for therapeutic applications because their differentiated progeny will evoke an immune response from allogeneic recipients [16,17]. Some of these limitations are avoided at least in principle by the use of hiPSCs, which are derived by reprogramming somatic cells (e.g. dermal fibroblasts) to a pluripotent state [15]. While this reprogramming was initially accomplished by the forced over-expression of key stem cell-related transcription factors delivered via integrating viral vectors, a number of virus-free alternative approaches have since been developed including reprogramming via recombinant proteins [18], mRNA [19], non-integrating episomal vectors [20], and even small molecules [21].
Significant attention has been focused on comparing hESCs, hiPSCs, and their differentiated progeny. The Loring group has extensively investigated the genotype of hESC and hiPSC lines and found that both demonstrate similar degrees of genomic instability, underscoring the need for thorough characterization and validation before clinical application [22,23]. Toivonen and colleagues compared multiple hESC and hiPSC lines for transgene persistence and their ability to differentiate into cardiomyocytes and other lineages [24], and they reported differences in both differentiation potential and transgene silencing. Subsequent work by Sepac and colleagues found similar differences across several hESC and hiPSC lines in terms of cardiomyogenic potential [25]. While other studies have also highlighted potential epigenetic differences [26,27], it is nonetheless reassuring that differentiated hESC- and hiPSC-CMs seem to share a nearly identical functional phenotype [28,29].
Parthenogenetic stem cells are another pluripotent stem cell type that may avoid many of the ethical objections associated with hESCs, with the added potential benefit of reduced immune rejection since they display the HLA profile of only a single donor [30]. Although to our knowledge no one has characterized cardiomyocytes from human parthenogenetic stem cells, Didié and colleagues recently showed that cardiomyocytes from mouse parthenogenetic stem cells have a phenotype very similar to their counterparts from mouse ESCs and iPSCs [31]. These authors also showed that cardiomyocytes derived from mouse parthenogenetic stem cells integrated electrically with host myocardium and improved contractile function following transplantation in a mouse infarct model [31].
Generation and Enrichment of Cardiomyocytes from Pluripotent Stem Cells
hESC-CMs were first isolated from serum-stimulated embryoid bodies (EBs) [32,33] and later derived by co-culture with mouse endodermal cells [34]. Early differentiation protocols were inefficient with cardiomyocyte yields below 1%, but more recent directed cardiac differentiation protocols have resulted in dramatically improved cardiac purities (~30–70% cardiomyocytes) [35,36]. Additional refinements to these protocols include the addition of extracellular matrix proteins [37], modulation of Wnt signaling [38], and optimization of activin-nodal signaling [39]. These advancements now permit the reliable production of large quantities of relatively pure cardiomyocytes and give reason for optimism that the scaled production of clinical-grade cells for cardiac repair will be possible in the near future.
If an even higher degree of cardiomyocyte purity is required, subsequent enrichment steps are possible. Arguably the most successful strategy has been genetic selection using a cardiac-specific promoter that drives expression of a fluorescent protein and/or antibiotic resistance. Early proof of concept for genetic selection came from work by Field and colleagues using mouse ESC-CMs [40,41], and other groups have more recently extended this approach to human cells [42–44]. hESC-CMs have also been enriched based on sorting for surface markers (e.g. SIRPA [45], VCAM1 [46] and EMILIN2 [47]), via light-scattering properties via Raman micro-spectroscopy [48], and by differences in metabolic status[49]. While the latter approaches avoid the need for genetic modification, they are currently limited by cell yield and throughput. It remains to be determined what threshold of purity will be deemed acceptable for future clinical therapies.
The Phenotype of Stem Cell-Derived Cardiomyocytes
By light microscopy, early human PSC-CMs appear as small, nondescript, mononucleated cells of variable size and shape [29,32–34]. They express sarcomeric proteins including α-actinin, cardiac troponins I and T, α- and β-myosin heavy chains, atrial- and ventricular- myosin light chains, desmin, and tropomyosin [29,33,34,50,51]. Ultrastructurally, PSC-CMs show poorly organized sarcomeres and intercalated discs [28,50]. Interestingly, PSC-CMs show robust proliferative activity characteristic of early chamber myocardium, with cell cycle activity slowly tapering off over several weeks of in vitro maturation [52,53].
PSC-CMs have significant automaticity and exhibit action potentials (APs) that have been classified as either nodal- or ventricular-like [34,54]. In both subtypes, early hESC-CMs show immature AP properties (i.e. a more rapid spontaneous rate, a slow AP upstroke, and a depolarized maximum diastolic potential), but these parameters improve somewhat with prolonged duration in culture [54,55]. In voltage-clamp studies, hESC-CMs exhibit most of the major cardiac ion currents, including fast sodium, L- and T-type Ca2+, pacemaker, and transient outward and inward rectifier K+ currents [34,55–57]. Depolarization in these cells is dominated by Na+ influx via the NaV1.5 channel, and this current is at least partially responsible for their spontaneous electrical activity [57]. As in adult cardiomyocytes, depolarization activates L-type Ca2+ channels in hESC-CMs, which results in a Ca2+ influx that is amplified by release from sarcoplasmic reticulum stores [56,58,59]. Recent data from our group indicates that this calcium-induced-calcium release process operates via a tight “local control” mechanism, similar to that of adult myocardium [56].
The first measurements of hESC-CM contractility were performed by Binah and colleagues using videomicroscopic contraction analysis of EBs [60,61]. While the use of EBs has some limitations (including low cardiomyocyte purity and heterogeneity in size and shape), these investigators showed that hESC-CMs had relatively immature contractile properties, including a negative force-frequency relationship. Our own group later used videomicroscopy to quantify both the magnitude and kinetics of spontaneous contractions of individual hESC- and hiPSC-CMs, and we found that these cardiomyocytes had contraction amplitudes of ~5% after ~25 days of in vitro maturation [28]. Hazeltine and colleagues used traction force microscopy to show that force production by hESC-CMs increased with culture on stiffer substrates [62]. Other groups have used atomic force microscopy to directly measure contractile force generation by PSC-CMs and reported values of ~0.2nN per cell [63,64]. By any measure, however, the forces generated by immature PSC-CMs appear miniscule: approximately10- to 100-fold lower than those of mature adult cardiomyocytes. This remains a major challenge for the field, and much additional work will be required to identify scalable methods for enhancing the mechanical properties of PSC-CMs.
Cardiac Tissue Engineering
Motivated in part by this increasing recognition of the immature functional properties of PSC-CMs under two-dimensional monoculture conditions, a number of groups have explored cardiac tissue engineering as a means of imposing both maturation and multicellular organization [65–67]. In pioneering experiments, Zimmermann and Eschenhagen first seeded primary rat cardiomyocytes in hydrogels [68], and several groups have subsequently applied this general approach to PSC-CMs and fibrin-based hydrogels [69–72]. This technique allows constructs to be prepared in almost any pattern, including as an injectable delivery vehicle [73]. Working independently, both the Murry and Martin groups showed that static stretch induces an increase in cardiomyocyte alignment and maturation within collagen-based hydrogel constructs [70,74]. In an interesting variation on this approach, the Radisic group exposed their constructs, cylindrical-shaped “biowires” of hPSC-CMs and non-cardiac supportive cells, to electrical field stimulation [75], and they found this promoted a more mature structural and electrophysiological phenotype. Recently, Bursac and colleagues seeded hESC-CMs into fibrin-based hydrogels over a PDMS template to form relatively large three-dimensional patches, and the resultant constructs exhibited force generation and conduction velocity measures that far exceed those previously reported [72].
Engineered tissue constructs can also be created by seeding PSC-CMs into three-dimensional scaffolds specifically designed for the inclusion of supporting non-cardiac cell types. The Gepstein group was among the first to compare outcomes with hESC-CMs alone versus a “tri-cell” mixture of hESC-CMs, endothelial cells and fibroblasts, by seeding both cell preparations into a poly-L-lactic acid (PLLA) scaffold [76]. Both expressed cardiac markers, but scaffolds seeded with the tri-cell combination showed far greater vascular organization in vitro. Qualitatively similar results were observed in vivo following engraftment in healthy rat hearts [77].
Two alternatives to the preceding artificial scaffold-based strategies are the use of decellularized native heart tissue [78] and scaffold-free tissue constructs [79–81]. Lu and colleagues seeded multipotent cardiovascular progenitors from hiPSCs into decellularized mouse hearts and found that the resultant constructs exhibited spontaneous contractile activity and responded appropriately to chemical agonists [78]. In pursuing the scaffold-free approach, the Murry group showed that hESC-CMs and supportive non-myocyte cell types will spontaneously aggregate to form viable cardiac patches [79–81]. It remains to be seen if either of these approaches are scalable for human applications.
Taken collectively, these studies suggest a potential role for tissue engineering in future therapeutic applications, but the identity of the most appropriate construct for achieving cardiac repair remains uncertain.
In Vivo Studies
Small Animal Models
Early in vivo proof-of-concept for the use of PSCs in cardiac repair came from the Field group, who showed that genetically-selected mESC-CMs formed stable myocardial grafts following transplantation in the hearts of dystrophic mice [82]. Xiao and colleagues later extended this work by microdissecting mESC-CMs from spontaneously beating EBs and transplanting them into a rat infarct model [83]. Somewhat surprisingly, in the absence of immunosuppression, this xenogeneic cell transplantation resulted in the formation of graft myocardium with beneficial effects on LV dimensions, fractional shortening and hemodynamics that were sustained for up to 32 weeks [83].
Transplantation studies with hESC-CMs followed a similar trajectory, with work first commencing in uninjured rodent hearts [84,85] and then gradually transitioning to rodent infarct models [35,86–90]. Our group transplanted enriched populations of hESC-CMs (~80% cardiac) into infarcted athymic rat hearts and saw partial remuscularization of the infarct zone [35]. Compared to infarcted controls receiving either non-cardiac hESC derivatives or vehicle, infarcted recipients of hESC-CMs showed better preserved LV dimensions, fractional shortening and regional wall motion. Interestingly, Mummery and colleagues found that while hESC-CMs formed nascent myocardium and improved contractile function in the infarcted hearts of immunodeficient mice, these beneficial effects appeared to be transient in nature [87,89]. While there were major experimental differences between the latter studies and our own (including the formation of qualitatively smaller grafts and the use of a mouse model in which hESC-CMs were unlikely to couple electrically), the Mummery group’s findings nonetheless underscore the need for future studies with a greater duration of follow-up.
All of the preceding studies examined outcomes following hESC-CM transplantation in acute or subacute infarct models. More recently, Fernandes and colleagues transplanted hESC-CMs into chronically infarcted rat hearts, which perhaps represent a more relevant model for the end-stage patient in which such novel cell therapies would be first applied [88]. Interestingly, although hESC-CMs formed large myocardial grafts in chronically injured hearts, their engraftment was not accompanied by beneficial effects on contractile function. This study raises concerns that hESC-CMs cannot efficiently integrate and provide new force-generating units in chronically injured hearts or that they may be unable to reverse deleterious remodeling in hearts with already established failure.
Recent studies have also begun to focus on the application of iPSCs in rodent models of cardiac injury. Proof-of-concept experiments with murine iPSC-CMs have shown the ability of these cells to survive and form nascent myocardium [91,92]. Recently, the Gepstein group transplanted hiPSC-CMs from spontaneously beating EBs into uninjured rat hearts [93], and, while their study had a relatively short duration of follow-up (7–10 days), they did find histological evidence of engraftment and structural features of host-graft coupling (i.e. immunostaining for gap junctions between graft and host myocytes). Another recent study of interest by Carpenter and colleagues involved the transplantation of multipotent cardiovascular progenitors from hiPSCs in a rat infarct model [94]. Although the effect did not reach statistical significance, the cell-treated hearts showed a trend toward less deterioration in ejection fraction at 10 weeks post-infarction than untreated controls. The grafts in this study seemed modest in size, inviting speculation as to whether better graft survival post-transplantation might yield greater improvements in cardiac function.
Large Animal Models
The preceding experience in rodent infarct models set the stage for transplantation studies with hPSC-CMs in more relevant large animal preclinical models. Surprisingly, the first large animal study was performed nearly a decade ago when the Gepstein group transplanted hESC-CMs microdissected from beating EBs into the left ventricle of swine with complete atrioventricular block [50]. They detected ectopic pacemaking activity in the hearts of hESC-CM recipients and localized this signal to the site of cell transplantation by electroanatomic mapping. Although their study involved a relatively crude cell preparation and a very short duration of follow-up, it nonetheless remains a landmark study in the field.
The next relevant large animal study was reported in 2010 by the Menasché and Pucéat groups, who transplanted multipotent cardiovascular progenitors derived from rhesus ESCs in a primate infarct model [95]. These investigators found that while the recipients of undifferentiated ESCs grew teratomas, those receiving cardiovascular progenitors showed remuscularization of up to 20% of the infarct area. Unfortunately, their study did not include any functional endpoints, leaving open the question as to whether the partial remuscularization of the infarct results in a meaningful restoration of lost cardiac function in primates.
More recently, the Sawa group incorporated hiPSC-CMs into bioengineered sheets that were then applied to the epicardial surface of infarcted pig hearts [96]. At 8 weeks post-transplantation (12 weeks post-infarction), the recipient hearts had very few surviving graft cells, but they nonetheless showed better preserved ejection and left ventricular dimensions than untreated controls. More recently, this same group has shown that graft cell survival can be somewhat improved by delivering hiPSC-CM sheets with a pedicle omentum flap [97].
Remaining Hurdles to Translation
While this progress in animal models gives reason for hope, a number of major hurdles must still be overcome if PSC-based cardiovascular therapies are to reach clinical application. Some of these challenges and their potential solutions have been discussed above. For example, to avoid teratomas, it is likely that we will need highly purified preparations of cardiomyocytes (or cardiomyocytes with the appropriate supportive cell types). With improved directed differentiation protocols and enrichment strategies, such cell production now seems feasible. Another major issue is the immature phenotype of the PSC-CMs generated by existing protocols. While this issue will clearly require much additional work, a number of potential solutions have been previously mentioned, including prolonged duration in culture [28], electromechanical conditioning [75], and/or tissue engineering approaches [72]. In the following sections, we highlight four other remaining barriers to PSC-CM-based therapies: electromechanical integration and the risk of arrhythmias, graft cell death, graft vascularization, and immune rejection.
Electromechanical Integration and Arrhythmia Risk
Early work by Gepstein and others showed that hESC-CM grafts were capable of electromechanical integration in uninjured hearts [50,98], but their ability to couple with host myocardium following transplantation in injured hearts remained uncertain until quite recently. To address this question directly, our group generated transgenic hESC-CMs that stably express the fluorescent calcium indicator GCaMP3 [99]. GCaMP3+ hESC-CMs exhibit fluorescent transients with each contractile cycle, providing a convenient readout of graft activation that can then be correlated with the host electrocardiogram. Using this approach, we found that hESC-CM grafts in uninjured guinea pig hearts always activated synchronously with host myocardium. Outcomes following transplantation in injured hearts, however, were mixed, with only ~60% of injured hearts containing coupled hESC-CM graft regions. Equally concerning was the finding that even well-coupled graft regions in injured hearts typically showed relatively slow conduction velocities, a situation likely to favor pro-arrhythmic reentrant phenomena. In summary, while we found direct evidence that hESC-CMs could form functionally integrated myocardium in injured hearts, more work will be required to improve their integration and maximize the functional benefits.
A closely related issue is the risk of graft-related arrhythmias, the incidence of which could be conceivably either increased or decreased by improvements in graft electromechanical integration. Indeed, PSC-CM grafts could plausibly contribute to all three fundamental arrhythmia mechanisms: automaticity, triggered activity, and reentry. First, as immature cardiomyocytes, PSC-CMs exhibit some degree of automaticity, although this diminishes somewhat with duration in culture [28]. Second, some reports suggest that these myocytes are particularly prone to exhibiting early- and after-depolarizations and triggered activity that is thought to underlie many episodes of ventricular tachycardia [100]. Finally, PSC-CM transplantation may promote reentrant phenomenon by slow propagation through irregularly-shaped islands of graft myocardium isolated by scar tissue. While work in small animals suggested that hESC-CM transplantation might actually exert an arrhythmia-suppressive effect in injured hearts [99], more recent preliminary studies in larger animal models with slower heart rates suggest that these cells may instead promote arrhythmias [101]. In our opinion, if such pro-arrhythmic effects are significant, this may prove the most challenging hurdle to the successful development of PSC-based cardiovascular therapies.
Cell death
It is known that the vast majority of implanted cardiomyocytes die shortly after intra-cardiac transplantation as a consequence of anoikis, ischemia and inflammation [102,103]. While this initial wave of cell death is somewhat compensated for by the subsequent proliferative activity of PSC-CMs in vivo [84], it is nonetheless an inviting target for improving graft outcomes. Our group has identified a number of interventions that each help attenuate graft cell death to a degree, including transient heat shock of the cells pre-transplantation [84], delivery in the presence of a cocktail of pro-survival factors [35] and treatment with carbamylated erythropoietin [103]. Additionally, the Wu lab has described elegant, longitudinal and non-invasive imaging techniques that will likely prove useful in testing other methods of enhancing graft cell survival [104,105]. It is likely that the ideal strategy for improving cell survival and integration will involve a multifaceted approach.
Immune rejection
Another obvious cause of graft cell death is immune rejection. While undifferentiated PSCs have some degree of immune privilege [106], their differentiated progeny including cardiomyocytes are immunogenic and clearly evoke an immune response in allogeneic recipients [16,107]. A number of exotic solutions to this problem have been proposed including the creation of isogeneic PSCs by somatic cell nuclear transfer [108], the creation of “universal donor” PSC lines via HLA engineering [109,110], and the induction of tolerance via bone marrow microchimerism [111,112]. Because iPSCs can be genetically matched to their recipient, they represent another theoretical solution to this problem, although the creation of “customized” autologous iPSC therapies would likely present a new set of practical hurdles in terms of scalability, economics, and regulatory burden. In our opinion, initial PSC-based therapies will require pharmacological immunosuppression, although we speculate that stem cell banking and the exclusion of antigen-presenting cell types (e.g. endothelial cells) from grafts may allow the use of a less aggressive immunosuppression regimen than is required for conventional solid organ transplants. The Wu group is using the aforementioned longitudinal imaging techniques to help define optimal immunosuppressive strategies for PSC-CMs [113,114].
Graft vascularization
A robust vascular supply is critical for PSC-CM grafts to thrive in the heart, but surprisingly the vascular consequences of PSC-CM transplantation have not been extensively investigated. In our own hESC-CM transplantation studies in intact and injured rodent hearts, we demonstrated the formation of new microvessels within the graft myocardium [35,84,99]. These neovessels were largely of host origin, but we did find chimeric vessels when less pure populations of cardiomyocytes were injected. Importantly, while the density of new capillaries within the graft tissue approached that in the surviving distant host myocardium, vessel densities in the border zone and scar tissue outside of the graft were unchanged relative to those in untreated controls [35,84]. Working independently, both the Murry and Gepstein groups have compared outcomes following the implantation of bioengineered constructs seeded with hESC-CMs alone versus those seeded with a mixture of hESC-CMs, endothelial cells, and fibroblasts [77,79]. Not surprisingly, the latter preparation produced more graft-derived vessels, but both were perfused by vessels that were connected to the host vasculature, as evidenced by the presence of either intraluminal erythrocytes or fluorescent microspheres. It remains to be demonstrated how the function of these vessels will compare to those obtained following the injection of cell suspensions alone.
In recent work, our group imaged engrafted hearts by micro-CT angiography to look for effects on larger vessels that cannot be readily examined by histology [115]. Interestingly, skeletal myoblast grafts evoked significant remodeling of larger conducting vessels in myocardium distant to the graft, an effect that might enhance perfusion of both host and graft tissue. It remains to be seen whether PSC-CM grafts can mediate a similar response.
Closing Perspectives
While the development of cardiovascular therapies based on PSCs certainly lags that of certain adult stem cell types (some of which have reached human trials), substantial progress has been made in recent years. PSC-CMs can now be made in large quantities at high purities using reagents that comply with “Good Manufacturing Practice” standards [116]. The phenotype of PSC-CMs has been extensively characterized in vitro, and promising new strategies for promoting their maturation are currently under development. Preclinical studies in small animal models have shown convincingly that these cells survive in both healthy and infarcted myocardium; and, in most studies, they also exert modest beneficial effects on cardiac function. This work has provided compelling rationale for large animal studies, which are now well underway. Despite these advances, there are still critical gaps in understanding that should be addressed before clinical trials can commence. For example, while recent studies have shown that hESC-CM grafts are indeed capable of coupling with host myocardium and contributing new force-generating units, it is still uncertain whether this mechanism accounts for the observed beneficial effects on contractile function. Finally, valid concerns regarding the risk of arrhythmias must be addressed. In summary, while the long-term prospects for PSC-based cardiac repair are favorable, much work remains to be done.
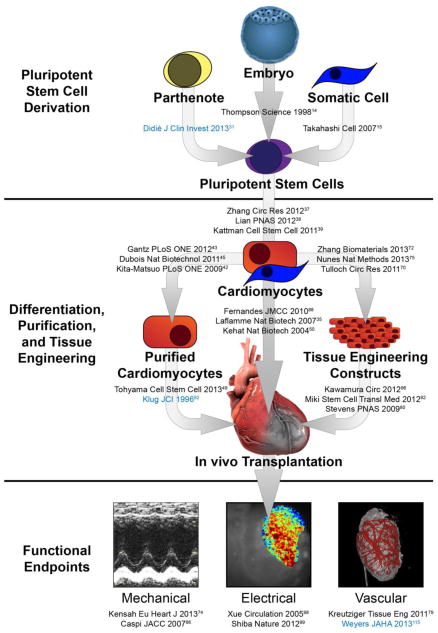
Figure 1. Schematic representing the generation, transplantation, and functional endpoints associated with stem cell-derived cardiomyocyte therapy.
Critical manuscripts in each area are listed, with rodent cell work denoted in blue and human cell work denoted in black.
Footnotes
Conflict of Interest
Dr. Scott D. Lundy, Dr. Jay A. Gantz, Dr. Chelsea M. Pagan, Dr. Dominic Filice, and Dr. Michael A. Laflamme each declare no potential conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
• Of importance
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013 Jan 1;127(1):143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996 Dec 1;98(11):2512–23. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh GY, Klug MG, Soonpaa MH, Field LJ. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993 Sep;92(3):1548–54. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation. 1996 Nov 1;94(9 Suppl):II332–6. [PubMed] [Google Scholar]
- 5.Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994 Apr 1;264(5155):98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 6.Siminiak T, Fiszer D, Jerzykowska O, Grygielska B, Rozwadowska N, Kałmucki P, et al. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J. 2005 Jun;26(12):1188–95. doi: 10.1093/eurheartj/ehi159. [DOI] [PubMed] [Google Scholar]
- 7.Dib N, McCarthy P, Campbell A, Yeager M, Pagani FD, Wright S, et al. Feasibility and safety of autologous myoblast transplantation in patients with ischemic cardiomyopathy. Cell Transplant. 2005;14(1):11–9. doi: 10.3727/000000005783983296. [DOI] [PubMed] [Google Scholar]
- 8.Hagège AA, Marolleau J-P, Vilquin J-T, Alhéritière A, Peyrard S, Duboc D, et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006 Jul 4;114(1 Suppl):I108–13. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 9.Menasché P, Hagège AA, Vilquin J-T, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003 Apr 2;41(7):1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 10.Povsic TJ, O’Connor CM, Henry T, Taussig A, Kereiakes DJ, Fortuin FD, et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J. 2011 Oct;162(4):654–662. e1. doi: 10.1016/j.ahj.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008 Mar 4;117(9):1189–200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 12.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004 Apr 8;428(6983):664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 13.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Research. 2008 Nov 21;103(11):1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Nussbaum J, Minami E, Laflamme MA, Virag JAI, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007 May;21(7):1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 17.Kofidis T, deBruin JL, Tanaka M, Zwierzchoniewska M, Weissman I, Fedoseyeva E, et al. They are not stealthy in the heart: embryonic stem cells trigger cell infiltration, humoral and T-lymphocyte-based host immune response. Eur J Cardiothorac Surg. 2005 Sep;28(3):461–6. doi: 10.1016/j.ejcts.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Wu S, Joo J, Zhu S, Han D, Lin T, et al. Generation of Induced Pluripotent Stem Cells Using Recombinant Proteins. Cell Stem Cell. 2009 Apr 22; doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren L, Manos PD, Ahfeldt T, Loh Y-H, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowey SN, Huang X, Chou B-K, Ye Z, Cheng L. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat Protoc. 2012 Nov;7(11):2013–21. doi: 10.1038/nprot.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013 Aug 9;341(6146):651–4. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 22.Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011 Jan 7;8(1):106–18. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller F-J, Schuldt BM, Williams R, Mason D, Altun G, Papapetrou EP, et al. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011 Apr;8(4):315–7. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toivonen S, Ojala M, Hyysalo A, Ilmarinen T, Rajala K, Pekkanen-Mattila M, et al. Comparative analysis of targeted differentiation of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells reveals variability associated with incomplete transgene silencing in retrovirally derived hiPSC lines. Stem Cells Transl Med. 2013 Feb;2(2):83–93. doi: 10.5966/sctm.2012-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepac A, Si-Tayeb K, Sedlic F, Barrett S, Canfield S, Duncan SA, et al. Comparison of Cardiomyogenic Potential among Human ESC and iPSC Lines. Cell Transplant. 2012 Aug 2; doi: 10.3727/096368912X653165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011 Feb 4;144(3):439–52. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol. 2011 May;13(5):541–9. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Lundy SD, Zhu W-Z, Regnier M, Laflamme M. Structural and Functional Maturation of Cardiomyocytes Derived From Human Pluripotent Stem Cells. Stem Cells Dev. 2013 Mar 6; doi: 10.1089/scd.2012.0490. Report by our group demonstrating the functional maturation of PSC-CMs in vitro toward a more adult-like phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research. 2009 Feb 27;104(4):e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brevini TAL, Gandolfi F. Parthenotes as a source of embryonic stem cells. Cell Prolif. 2008 Feb;41( Suppl 1):20–30. doi: 10.1111/j.1365-2184.2008.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Didié M, Christalla P, Rubart M, Muppala V, Döker S, Unsöld B, et al. Parthenogenetic stem cells for tissue-engineered heart repair. J Clin Invest. 2013 Feb 22; doi: 10.1172/JCI66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001 Aug;108(3):407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circulation Research. 2002 Sep 20;91(6):501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 34.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003 Jun 3;107(21):2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 35.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007 Sep;25(9):1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008 May 22;453(7194):524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circulation Research. 2012 Oct 12;111(9):1125–36. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012 Jul 3;109(27):E1848–57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011 Feb 4;8(2):228–40. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Pasumarthi KB, Field LJ. Cardiomyocyte enrichment in differentiating ES cell cultures: strategies and applications. Methods in molecular biology (Clifton, NJ) 2002;185:157–68. doi: 10.1385/1-59259-241-4:157. [DOI] [PubMed] [Google Scholar]
- 41.Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, et al. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9(4):767–78. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 42.Kita-Matsuo H, Barcova M, Prigozhina N, Salomonis N, Wei K, Jacot JG, et al. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS ONE. 2009;4(4):e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gantz JA, Palpant NJ, Welikson RE, Hauschka SD. Targeted Genomic Integration of a Selectable Floxed Dual Fluorescence Reporter in Human Embryonic Stem Cells. PLoS ONE. 2012 doi: 10.1371/journal.pone.0046971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther. 2007 Nov;15(11):2027–36. doi: 10.1038/sj.mt.6300303. [DOI] [PubMed] [Google Scholar]
- 45.Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29(11):1011–8. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, et al. Efficient and Scalable Purification of Cardiomyocytes from Human Embryonic and Induced Pluripotent Stem Cells by VCAM1 Surface Expression. In: Prosper F, editor. PLoS ONE. 8. Vol. 6. 2011. p. e23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Hoof D, Dormeyer W, Braam SR, Passier R, Monshouwer-Kloots J, Ward-van Oostwaard D, et al. Identification of cell surface proteins for antibody-based selection of human embryonic stem cell-derived cardiomyocytes. J Proteome Res. 2010 Mar 5;9(3):1610–8. doi: 10.1021/pr901138a. [DOI] [PubMed] [Google Scholar]
- 48.Pascut FC, Goh HT, George V, Denning C, Notingher I. Toward label-free Raman-activated cell sorting of cardiomyocytes derived from human embryonic stem cells. J Biomed Opt. 2011:045002. doi: 10.1117/1.3570302. [DOI] [PubMed] [Google Scholar]
- 49.Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013 Jan 3;12(1):127–37. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004 Oct;22(10):1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Tohyama S, Murata M, Nomura F, Kaneko T, Chen H, et al. In vitro pharmacologic testing using human induced pluripotent stem cell-derived cardiomyocytes. Biochem Biophys Res Commun. 2009 Aug 7;385(4):497–502. doi: 10.1016/j.bbrc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 52.Norström A, Akesson K, Hardarson T, Hamberger L, Björquist P, Sartipy P. Molecular and pharmacological properties of human embryonic stem cell-derived cardiomyocytes. Exp Biol Med (Maywood) 2006 Dec;231(11):1753–62. doi: 10.1177/153537020623101113. [DOI] [PubMed] [Google Scholar]
- 53.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005 Dec;39(6):865–73. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He J-Q, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circulation Research. 2003 Jul 11;93(1):32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 55.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007 May;25(5):1136–44. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 56.Zhu W-Z, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2009;4(4):e5407. doi: 10.1371/journal.pone.0005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol (Lond) 2004 Sep 1;559(Pt 2):479–96. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Fu J-D, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007 Dec;25(12):3038–44. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 59.Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, et al. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008 Aug;26(8):1961–72. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 60.Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, et al. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006 Feb;24(2):236–45. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 61.Binah O, Dolnikov K, Sadan O, Shilkrut M, Zeevi-Levin N, Amit M, et al. Functional and developmental properties of human embryonic stem cells-derived cardiomyocytes. Journal of electrocardiology. 2007 Oct;40(6 Suppl):S192–6. doi: 10.1016/j.jelectrocard.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 62.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, Sun N, Bruce MA, Wu JC, Butte MJ. Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS ONE. 2012;7(5):e37559. doi: 10.1371/journal.pone.0037559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang I-NE, Wang X, Ge X, Anderson J, Ho M, Ashley E, et al. Apelin enhances directed cardiac differentiation of mouse and human embryonic stem cells. PLoS ONE. 2012;7(6):e38328. doi: 10.1371/journal.pone.0038328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kreutziger KL, Murry CE. Engineered human cardiac tissue. Pediatr Cardiol. 2011 Mar;32(3):334–41. doi: 10.1007/s00246-011-9888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radisic M, Christman KL. Materials science and tissue engineering: repairing the heart. Mayo Clinic proceedings Mayo Clinic Elsevier Inc. 2013;88(8):884–98. doi: 10.1016/j.mayocp.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiburcy M, Zimmermann W-H. Modeling myocardial growth and hypertrophy in engineered heart muscle. Trends Cardiovasc Med Elsevier. 2013:1–7. doi: 10.1016/j.tcm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Zimmermann W-H, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000 Apr 5;68(1):106–14. [PubMed] [Google Scholar]
- 69.Soong PL, Tiburcy M, Zimmermann W-H. Cardiac differentiation of human embryonic stem cells and their assembly into engineered heart muscle. Curr Protoc Cell Biol. 2012 Jun;Chapter 23(Unit 23.8) doi: 10.1002/0471143030.cb2308s55. [DOI] [PubMed] [Google Scholar]
- 70.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation Research. 2011 Jun 24;109(1):47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kensah G, Gruh I, Viering J, Schumann H, Dahlmann J, Meyer H, et al. A novel miniaturized multimodal bioreactor for continuous in situ assessment of bioartificial cardiac tissue during stimulation and maturation. Tissue Eng Part C Methods. 2011 Apr;17(4):463–73. doi: 10.1089/ten.tec.2010.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Zhang D, Shadrin IY, Lam J, Xian H-Q, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013 May 2; doi: 10.1016/j.biomaterials.2013.04.026. Recent publication in which tissue engineering approaches were used to organize hESC-CMs into three-dimensional constructs with impressive structural and functional properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Habib M, Shapira-Schweitzer K, Caspi O, Gepstein A, Arbel G, Aronson D, et al. A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials. 2011 Jul 21; doi: 10.1016/j.biomaterials.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 74.Kensah G, Roa Lara A, Dahlmann J, Zweigerdt R, Schwanke K, Hegermann J, et al. Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur Heart J. 2013;34(15):1134–46. doi: 10.1093/eurheartj/ehs349. [DOI] [PubMed] [Google Scholar]
- 75•.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013 Jun 23; doi: 10.1038/nmeth.2524. The authors created “biowires” of hiPSC-CMs and showed that electrical stimulation helped promote their structural and functional maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IHM, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circulation Research. 2007 Feb 2;100(2):263–72. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 77.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue engineering Part A. 2010 Jan;16(1):115–25. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 78.Lu T-Y, Lin B, Kim J, Sullivan M, Tobita K, Salama G, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nature communications. 2013;4 doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 79.Kreutziger KL, Muskheli V, Johnson P, Braun K, Wight TN, Murry CE. Developing vasculature and stroma in engineered human myocardium. Tissue engineering Part A. 2011;17(9–10):1219–28. doi: 10.1089/ten.tea.2010.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stevens KR. Physiological function and transplantation of scaffold-free and vascularized human muscle tissue. Proceedings of the …. 2009;106(39) doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue engineering Part A. 2009 Jun;15(6):1211–22. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996 Jul 1;98(1):216–24. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Min J-Y, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, et al. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003 Feb;125(2):361–9. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 84.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005 Sep;167(3):663–71. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, et al. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol. 2007 Oct;43(4):504–16. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007 Nov 6;50(19):1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 87.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007 Oct;1(1):9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010 Dec;49(6):941–9. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Laake LW, Passier R, Ouden den K, Schreurs C, Monshouwer-Kloots J, Ward-van Oostwaard D, et al. Improvement of mouse cardiac function by hESC-derived cardiomyocytes correlates with vascularity but not graft size. Stem Cell Res. 2009 Aug;3(2–3):106–12. doi: 10.1016/j.scr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 90.van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circulation Research. 2008 May 9;102(9):1008–10. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 91.Halbach M, Peinkofer G, Baumgartner S, Maass M, Wiedey M, Neef K, et al. electrophysiological integration and action potential properties of transplanted cardiomyocytes derived from induced pluripotent stem cells. Cardiovasc Res. 2013:1–32. doi: 10.1093/cvr/cvt213. [DOI] [PubMed] [Google Scholar]
- 92.Miki K, Uenaka H, Saito A, Miyagawa S, Sakaguchi T, Higuchi T, et al. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl Med. 2012 May;1(5):430–7. doi: 10.5966/sctm.2011-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zwi-Dantsis L, Huber I, Habib M, Winterstern A, Gepstein A, Arbel G, et al. Derivation and cardiomyocyte differentiation of induced pluripotent stem cells from heart failure patients. Eur Heart J. 2013 Jun;34(21):1575–86. doi: 10.1093/eurheartj/ehs096. [DOI] [PubMed] [Google Scholar]
- 94.Carpenter L, Carr C, Yang CT, Stuckey DJ, Clarke K, Watt SM. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012 Apr 10;21(6):977–86. doi: 10.1089/scd.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010 Apr;120(4):1125–39. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96•.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012 Sep 11;126(11 Suppl 1):S29–37. doi: 10.1161/CIRCULATIONAHA.111.084343. Large animal study showing partial restoration of systolic function following the transplantation of hiPSC-CMs organized into two-dimensional tissue sheets. [DOI] [PubMed] [Google Scholar]
- 97.Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, et al. Enhanced Survival of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by the Combination of Cell Sheets With the Pedicled Omental Flap Technique in a Porcine Heart. Circulation. 2013;128(26_suppl_1):S87–S94. doi: 10.1161/CIRCULATIONAHA.112.000366. [DOI] [PubMed] [Google Scholar]
- 98.Xue T, Cho HC, Akar FG, Tsang S-Y, Jones SP, Marbán E, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005 Jan 4;111(1):11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 99•.Shiba Y, Fernandes S, Zhu W-Z, Filice D, Muskheli V, Kim J, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012 Aug 5; doi: 10.1038/nature11317. Recent publication from our group showing that hESC-CM grafts can couple synchronously with host myocardium and that their transplantation suppresses arrhythmias in injured guinea pig hearts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jonsson MKB, Duker G, Tropp C, Andersson B, Sartipy P, Vos MA, et al. Quantified proarrhythmic potential of selected human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010 May;4(3):189–200. doi: 10.1016/j.scr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 101.Christoffels VM, Pu WT. Developing insights into cardiac regeneration. Development. 2013 Oct;140(19):3933–7. doi: 10.1242/dev.096867. [DOI] [PubMed] [Google Scholar]
- 102.Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999 Jul 13;100(2):193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- 103.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol Elsevier Inc. 2008;45(4):567–81. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu E, Chen W-Y, Gu J, Burridge P, Wu JC. Molecular imaging of stem cells: tracking survival, biodistribution, tumorigenicity, and immunogenicity. Theranostics. 2012;2(4):335–45. doi: 10.7150/thno.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee AS, Wu JC. Imaging of embryonic stem cell migration in vivo. Methods Mol Biol. 2011;750:101–14. doi: 10.1007/978-1-61779-145-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22(4):448–56. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 107.Swijnenburg R-J, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005 Aug 30;112(9 Suppl):I166–72. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 108.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013 Jun 6;153(6):1228–38. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riolobos L, Hirata RK, Turtle CJ, Wang P-R, Gornalusse GG, Zavajlevski M, et al. HLA engineering of human pluripotent stem cells. Mol Ther. 2013 Jun;21(6):1232–41. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deuse T, Seifert M, Phillips N, Fire A, Tyan D, Kay M, et al. Human leukocyte antigen I knockdown human embryonic stem cells induce host ignorance and achieve prolonged xenogeneic survival. Circulation. 2011 Sep 13;124(11 Suppl):S3–9. doi: 10.1161/CIRCULATIONAHA.111.020727. [DOI] [PubMed] [Google Scholar]
- 111.Baertschiger RM, Gonelle-Gispert C, Morel P, Sgroi A, Serre-Beinier V, Stouffs M, et al. Transplantation of mouse embryonic stem cells induces hematopoietic and tissue chimerism in rats. Xenotransplantation. 2010 Sep;17(5):362–9. doi: 10.1111/j.1399-3089.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- 112.Yuan X, Zhang H, Wei Y-J, Hu S-S. Embryonic stem cell transplantation for the treatment of myocardial infarction: immune privilege or rejection. Transpl Immunol. 2007 Nov;18(2):88–93. doi: 10.1016/j.trim.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 113.Huber BC, Ransohoff JD, Ransohoff KJ, Riegler J, Ebert A, Kodo K, et al. Costimulation-Adhesion Blockade is Superior to Cyclosporine A and Prednisone Immunosuppressive Therapy for Preventing Rejection of Differentiated Human Embryonic Stem Cells Following Transplantation. Stem Cells. 2013 Aug 16; doi: 10.1002/stem.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pearl JI, Lee AS, Leveson-Gower DB, Sun N, Ghosh Z, Lan F, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011 Mar 4;8(3):309–17. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weyers JJ, Schwartz SM, Minami E, Carlson DD, Dupras SK, Weitz K, et al. Effects of cell grafting on coronary remodeling after myocardial infarction. J Am Heart Assoc. 2013 Jun;2(3):e000202. doi: 10.1161/JAHA.113.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lecina M, Ting S, Choo A, Reuveny S, Oh S. Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng Part C Methods. 2010 Dec;16(6):1609–19. doi: 10.1089/ten.TEC.2010.0104. [DOI] [PubMed] [Google Scholar]