Abstract
The accumulated evidence has shown that lipids and polymers each have distinct advantages as carriers for siRNA delivery. Composite materials comprising both lipids and polymers may present improved properties that combine the advantage of each. Cationic amphiphilic macromolecules (CAMs) containing a hydrophobic alkylated mucic acid segment and a hydrophilic poly(ethylene glycol) (PEG) tail were non-covalently complexed with two lipids.1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), to serve as a siRNA delivery vehicle. By varying the weight ratio of CAM to lipid, cationic complexes with varying compositions were obtained in aqueous media and their properties evaluated. CAM-lipid complex sizes were relatively independent of composition, ranging from 100 to 200 nm, and zeta potentials varied from 10 to 30 mV. Transmission electron microscopy confirmed the spherical morphology of the complexes. The optimal N/P ratio was 50 as determined by electrophoretic mobility shift assay. The ability to achieve gene silencing was evaluated by anti-luciferase siRNA delivery to a U87-luciferase cell line. Several weight ratios of CAM-lipid complexes were found to have similar delivery efficiency compared to the gold standard, Lipofectamine. Isothermal titration calorimetry revealed that siRNA binds more tightly at pH = 7.4 than pH = 5 to CAM-lipid (1:10 w/w). Further intracellular trafficking studies monitored the siRNA escape from the endosomes at 24 h following transfection of cells. The findings in the paper indicate that CAM-lipid complexes can serve as a novel and efficient siRNA delivery vehicle.
Keywords: Cationic Amphiphilic Macromolecule, Lipid, siRNA Delivery
1. Introduction
Small interfering ribonucleic acid (siRNA) has been viewed widely as a promising gene-based therapeutic for many diseases since the discovery of RNA interference (RNAi) by Fire et al[1]. Due to the anionic nature of siRNA and the presence of RNase in the bloodstream, delivery of naked siRNA is limited by inadequate cellular uptake and poor stability under physiological conditions. Therefore, modifications to currently developed siRNA delivery vehicles are necessary to improve efficiency[2–4]. While viral delivery systems have demonstrated high transfection efficiencies, typically only one copy of siRNA is encoded per virus. Furthermore, the use of virus is limited by safety concerns[5]. To circumvent these challenges, numerous non-viral delivery systems, including cationic polymer-based and lipid-based systems[6–8], cell-penetrating peptides[9, 10], and chemically modified siRNAs[11, 12], have been developed. To date, only a limited number of non-viral siRNA delivery systems have reached clinical trials due to low delivery efficiencies in vivo and high cytotoxicities[13]. Effective non-viral siRNA delivery vehicles with minimal cytotoxicity are thus still needed.
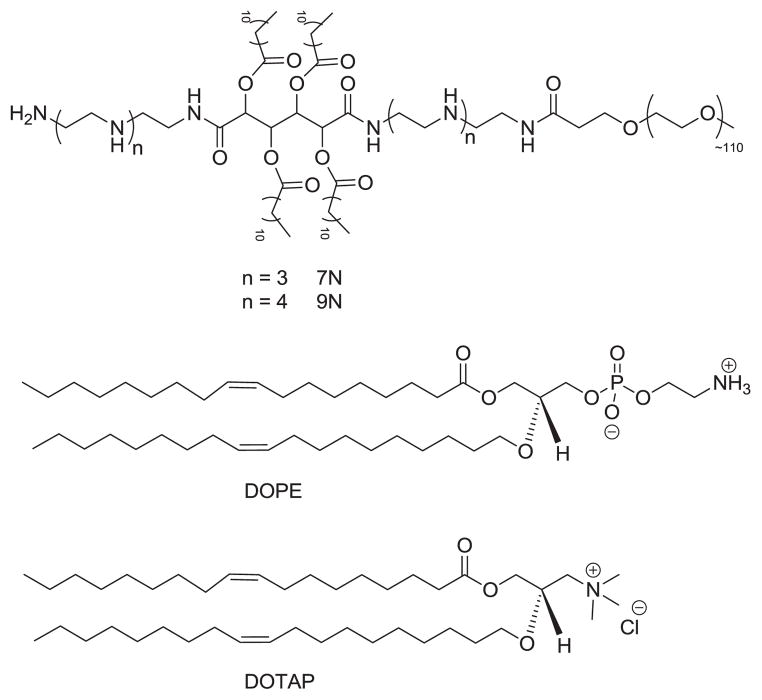
Cationic systems involving polymers and lipids have been developed for siRNA delivery as they prevent siRNA degradation, allow siRNA endosomal escape, and silence the target mRNA[14, 15]. Yet, these cationic systems have yet to overcome cytotoxicity, instability in the presence of serum, and low silencing efficiency. We previously developed cationic amphiphilic macromolecules (CAMs), which are comprised of hydrophobic acyl chains and hydrophilic poly(ethylene glycol) (PEG) chains. Within aqueous media, CAMs can self-assemble into micelles to present the PEG shell which increases the system’s circulation time in the bloodstream[16]. Our group has found the alkylated mucic acid backbone to be an effective and biocompatible hydrophobic segment in applications to chemotherapeutics [17]and therapeutics for atherosclerosis [18]. Two species of CAMs, differing by the number of amine groups in their backbone (Figure 1, 7N and 9N), were prepared previously and were shown to exhibit moderate gene-silencing efficiency with low cytotoxicity in vitro[16]. Thus, CAMs represent a promising delivery platform due to their self-assembly, biocompatibility, and tunable structure.
Figure 1.
Structure of CAMs (7N and 9N) (top), DOPE (middle), and DOTAP (bottom)
While CAMs alone are promising siRNA delivery systems[16], a greater gene silencing efficiency is desirable for practical applications. Cationic lipids are the most commonly used transfection agents for gene delivery as their cationic charge can complex the anionic gene fragment and their membrane fusion properties can enhance intracellular uptake[19]. However, lipid-based systems usually have poor stability in bloodstream and suffer relatively high cytotoxicity[19]. As CAM delivery systems are stable in physiological conditions and cytocompatible, we hypothesized that composite complexes containing CAMs and lipids would yield a more efficient and safe siRNA delivery system. To achieve this goal, a hybrid system containing CAMs and lipids was developed using 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) (Figure 1) at weight ratios of 1:1. DOPE was chosen for its ability to destabilize endosomal membranes and enhance siRNA release[20], while DOTAP was chosen for its high transfection efficiency due to its cationic features[21]. Complexes with varying CAM-lipid weight ratios were formulated to discern a CAM-lipid system with enhanced transfection efficiency as well as increased stability under physiological conditions. CAM-lipid complexes with various CAM to lipid weight ratios were prepared according to a previously reported method[22]. Physical as well as biological assays of CAM-lipid complexes were performed to elucidate their efficiency as a siRNA delivery vehicle. Physical properties were evaluated by dynamic light scattering (DLS) and transmission electron microscopy (TEM), which measured the sizes and morphologies of CAM-lipid complexes, respectively. Zeta potentials were also obtained to verify the cationic surface charge, which is critical for electrostatic interactions between siRNA and CAM-lipid complexes. Langmuir monolayers were used to show the mixing between CAM and lipid, and isothermal titration calorimetry (ITC) was used to illustrate the thermodynamic interaction between CAMs and lipid and CAM-lipid complexes and siRNA. To evaluate their function in cells, transfection efficiency and endosomal escape of CAM-lipid complexes were evaluated using an in vitro assay with a human primary glioblastoma U87 cell line and anti-Luciferase siRNA or Cy5-scrambled siRNA.
2. Materials and methods
2.1. Materials
DOPE and DOTAP were purchased from Avanti Polar Lipid (Alabaster, AL). The anti-luciferase siRNA (sense sequence: 5′-CUUACGCUGAGUACUUCGAdTdT-3′; antisense sequence: 5′-UCGAAGUACUCAGCGUAAGdTdT-3′) and Cy5-labeled negative control siRNA were purchased from Qiagen (Valencia, CA). All cell culture media and Lipofectamine were purchased from Invitrogen (Carlsbad, CA). The Luciferase assay kit and BCA protein assay kit were purchased from Promega (Madison, WI). U87-LUC, a human primary glioblastoma cell line with constitutive expression of firefly luciferase, was generously provided by Dr. Xu-Li Wang (Pharmaceutics and Pharmaceutical Chemistry, University of Utah). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) and used as received without further purification, except where noted.
2.2. Langmuir monolayer
Surface properties of CAM and mixed CAM-lipid monolayers were evaluated at the air-water interface using a Langmuir surface balance from KSV-Nima (Espoo, Finland) on a subphase of pure water (resistivity ≥ 18.2 MΩ · cm) at ambient temperature (~ 22 °C). CAM and lipid were dissolved in HPLC-grade chloroform to concentrations of ~1 mg/mL and mixtures prepared by adding appropriate volumes of each from stock solutions. Between each experiment, the Teflon trough (Biolin Scientific, MD) (total subphase volume = 109 mL) and barriers were cleaned with methanol and then rinsed repeatedly with ultra-pure water. Contaminants were removed from the platinum Wilhelmy plate (Biolin Scientific, MD) with an open fire from a Bunsen burner. All glassware was thoroughly cleaned with chloroform and methanol. The subphase surface was cleaned by aspirating during repeated sweeps of the computer-controlled barriers while monitoring the surface pressure, then continued until the change in pressure was negligible. CAM and CAM-lipid films were spread onto the subphase surface using a digital Hamilton syringe (Reno, NV). After a 10 min delay to allow for complete solvent evaporation, the films were compressed at a rate of 10 cm2/min. Data were collected by KSV-Nima’s LB Control software (v. 3.60) and analyzed using Origin (Northampton, MA).
2.3. Isothermal titration calorimetry (ITC)
Mixing of the CAMs with the lipids were further examined with the “uptake” ITC protocol as described by Heerklotz et. al[23] using a VP-ITC from Microcal (GE Healthcare, Northampton, MA). Briefly, CAM dispersions were titrated with lipid vesicle suspensions at 25 °C in 10 μL aliquots at 6 min intervals during stirring (280 rpm). The data were collected with Microcal’s dedicated Origin software program and the resulting heat signals were fitted using an Excel (Microsoft, CA) spreadsheet available for download[23].
2.4. CAM-lipid complex preparation
CAMs (7N and 9N) were synthesized and characterized based on previously published procedures [17]. The calculated molecular weights of 7N, 9N, DOPE, and DOTAP are 6167, 6252, 744, and 699, respectively. Gel permeation chromatography (GPC) data of 7N and 9N are as follows: 7N (6600, PDI: 1.09) and 9N (6800, PDI: 1.11). Complexes of various CAM-lipid ratios were prepared by a co-evaporation technique as previously described[22]. Briefly, the lipid component was comprised of a 1/1 (w/w) mixture of DOPE and DOTAP. CAM and lipid (DOPE/DOTAP) were co-dissolved in chloroform at various CAM-to-lipid weight ratios. The chloroform was removed by rotary evaporation. The resulting films were hydrated with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer at pH = 7.4 overnight at room temperature. The complex suspensions were then extruded 21 times with the 100 nm pore size polycarbonate filter (Avanti Lipid, AL) through a mini-extruder (Avanti Lipid, AL) to give nanoscale CAM-lipid complexes. Formation of CAM-lipid-siRNA complexes was performed by mixing CAM-lipid formulations with siRNA for 60 min at room temperature.
2.5. CAM-lipid size and zeta potential
CAM-lipid complexes (1 mg/mL in HEPES) with or without siRNA were analyzed using a NanoZS90 instrument (Malvern Instruments, UK) at room temperature. Each sample was run three separate times with 20 measurements per run to obtain the size and zeta potential.
2.6. Electrophoretic mobility shift assay
CAM-lipid/siRNA complexes were prepared as previously described for CAM/siRNA complexes[16]. Dispersions were briefly vortexed and incubated for 60 min at room temperature to allow for complex formation. Prior to electrophoresis, 2 μL of 10X BlueJuice gel loading buffer was added to each sample. Gel electrophoresis was performed using 0.8 % agarose E-gels containing ethidium bromide for DNA visualization and a PowerBase electrophoretic chamber (Invitrogen, CA). Gels were imaged using BioDoc-It Imaging System (UVP, CA).
2.7. Transmission electron microscopy (TEM)
A drop of CAM-lipid complex dispersion (0.05 mg/mL) with or without siRNA and a drop of uranyl acetate (0.5 mg/mL) were both dropped on a carbon film-coated copper grid. Excess solution was removed by tapping the edge of grid with filter paper. The grid was then dried for 30 min in a desiccator at room temperature. Images were taken on a TEM-Topcon 002B (TOPOCON, Japan).
2.8. siRNA binding affinity study
ITC was also used to compare CAM-lipid complex binding to siRNA. For these experiments, 10 μL aliquots of 3.8 μM (0.05 mg/mL) siRNA were injected into the calorimetry cell containing 64 μM (0.05 mg/mL total concentration) CAM-lipid complex at 25 °C at 2 min intervals. The experiments were repeated with both CAM species (7N and 9N) at pH 7.4 and pH 5.5. Data analysis was performed using Microcal’s “one-binding site” model on Origin. Prior to analysis, heat of dilution (obtained from titrating siRNA into respective buffers) was subtracted from each run, and the baselines manually adjusted to the average noise level between injections.[23] Heat changes from titration of CAM-lipid complexes with buffer were insignificant.
2.9. Cell culture
U87 and U87-LUC cells were maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were incubated at 37 °C in a 5% CO2 incubator (VWR, PA). For the U87-LUC cell line, which stably expresses luciferase, expression was maintained under selective pressure by G418 (500 μg/mL).
2.10. siRNA delivery assay
U87 cells were plated at a density of 5000 cells/well in 96-well plates approximately 20 h prior to transfection. Immediately prior to transfection, CAM-lipid/siRNA complexes were prepared in 20 μL of HEPES (N/P = 50). Lipofectamine was used as a positive control. A 100 nM siRNA solution was used, while CAM-lipid stock dispersions were prepared at 20 nM. An irrelevant siRNA sequence not targeted against firefly luciferase was delivered as a negative control. The CAM-lipid/siRNA complexes were brought to a total volume of 100 μL in OptiMEM medium. The serum-containing culture medium was aspirated from the cells and each well treated with 100 μL of CAM-lipid/siRNA complexes in OptiMEM medium. After a 4 h incubation period, cells were washed 3 times with HEPES and the transfection mixture was replaced with a serum-containing growth medium and maintained under normal growth conditions. After 48 h, the cells were assayed for firefly luciferase expression using a luminometer (Turner Biosystems, WI), and the values were normalized to total protein expression using a BCA assay kit (Promega, WI).
2.11. Intracellular trafficking
U87 cells were seeded in 24-well plates at 70% confluency and allowed to adhere overnight. Uptake and release of a fluorescently labeled siRNA (Dharmacon, CO) sequence into U87 cells was evaluated using fluorescence microscopy. After 4 h or 24 h of incubation with Cy5-scrambled-siRNA (Dharmacon, CO) and 1:10 CAM-lipids, 10:1 CAM-lipids, or Lipofectamine control, U87 cells were washed twice with HEPES and stained with LysoTracker Red (Molecular Probes, OR). After fixation in 4 % paraformaldehyde for 15 min and counterstaining with 4′,6-diamidino-2-phenylindole (DAPI), images were taken on an IX81 motorized inverted confocal microscope (Olympus, PA) to view siRNA localization within the cells. Colocalization of puncta was evaluated by merging images and quantifying their overlapping areas in ImageJ.
2.12. Statistical analysis
Statistical analyses were carried out using a one-way ANOVA test with a Fisher’s all-pairs post hoc comparison test. (Synergy Software, PA). The significance criteria assumed a 95% confidence level (P < 0.05). Standard error of the mean is reported in the form of error bars on the graphs of the final data.
3. Results and discussion
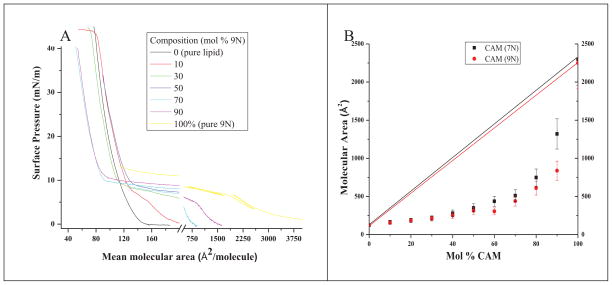
The two CAMs (7N and 9N) evaluated in this work were synthesized and their physicochemical characterizations were described in a previously published work[16]. They were then formulated into CAM-lipid complexes by addition of lipids (DOPE and DOTAP, in a 1:1 weight ratio). The interactions between CAM and lipid are dictated by electrostatic repulsion and hydrophobic attraction. The electrostatic repulsion is caused by the CAM and cationic DOTAP, whereas the hydrophobic attraction is due to the alkyl chains of the CAMs and lipids. Langmuir monolayers were employed to study this net interaction, the delicate balance between electrostatic repulsions and hydrophobic interactions, between CAMs and lipids. Compression of films comprised of surface active compounds at the air-aqueous interface is a well-established method for examining mixing of components. The condensation effect, in which the observed molecular area is decreased from that predicted molecular area (assuming ideality), is an indication of attractive interactions[24]. To determine miscibility, films of various CAM-lipid weight ratios were compressed and the resulting isotherms compared with each pure component. High water solubility of 9N (isotherms for 7N not shown, but similar to 9N) caused the films to collapse at ~10 mN/m (Figure 2A), so molecular areas were compared at 5 mN/m (Figure 2B). The large condensation effect observed for all compositions indicates strong attractive interactions of similar magnitude (except at 90 % CAM) and illustrates intimate CAM-lipid mixing. The stabilizing effect of even small amounts of lipid is demonstrated by the increase in collapse pressures when lipid is included in the films. Taken together, these results show that the combination of either of the CAM species (7N and 9N) investigated here with lipids form stable mixtures.
Figure 2.
(A) Compression isotherms of 9N-lipid on pure water. Compositions are expressed as mol % CAM (inset). (B) Area-composition plots for 7N (black) and 9N (red). Data were derived from compression isotherms at 5mN/m.
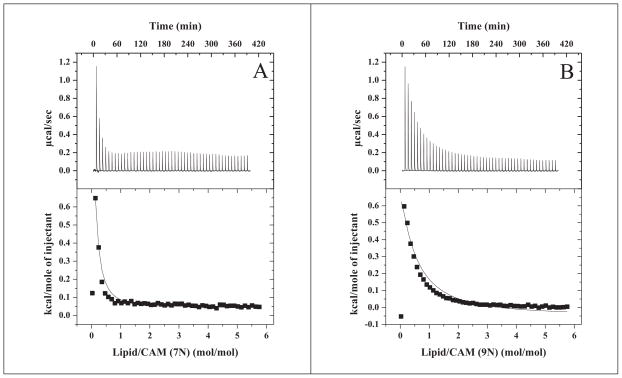
To quantify the affinities of each CAM species to lipid, we followed the ITC protocol of Tsamaloukas et al.[23]. Best global fits of the heats of binding derived from titration of 7N or 9N with lipids are shown respectively (Figure 3A and 3B). The curve-fitting routine allows the option of assuming CAM interactions with either the outer leaflet only or with both leaflets. Using the former assumption gave slightly improved global fits for these dataa. The results show that 7N binds lipids with ~7-fold higher affinity compared to 9N, based on partition coefficients (7.9 mM−1 for 7N and 1.2 mM−1 for 9N). However, the binding is endothermic, indicating hydrophobic interactions, and comparable for both species; this data suggests that the interactions involve membrane penetration rather than association of CAM with the liposome surface.
Figure 3.
Results from ITC “uptake” experiments for 0.5 mM 7N (A) and 9N (B) dispersions titrated with 20 mM DOTAP/DOPE liposomes at 25 °C, pH 7. Top panels: Raw data. Bottom panels: Integrated heats of binding (squares) and linear regression analysis of integrated heats (lines).
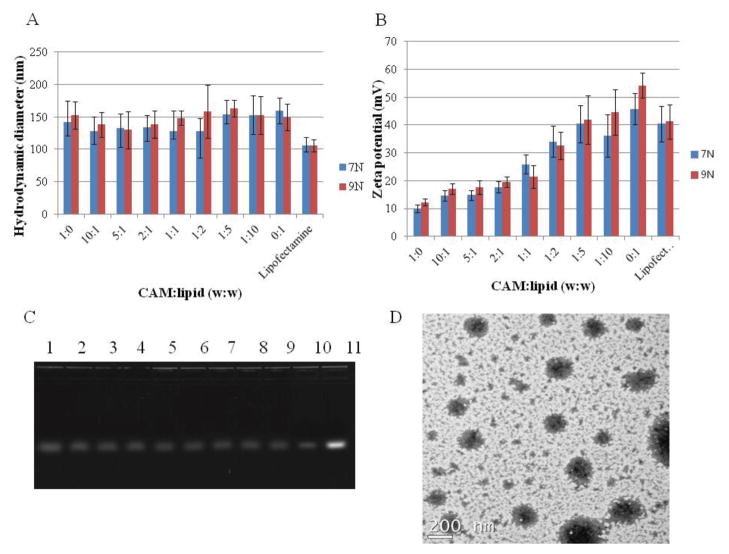
After CAM-lipid complexes were formulated using the previously reported method[20], their sizes were characterized using DLS. CAM-lipid complexes for all compositions were between 130 to 160 nm in diameter (Figure 4A), which is within the range generally considered ideal for both cellular uptake and systemic circulation [25–27]. DLS histograms showed a single peak with narrow distribution indicating that a distinct mono-dispersed complex was formed (PDI: 0.088 – 0.112). No aggregation was observed with any composition of the CAM-lipid complexes indicating that stable hybrid complexes were formed. The stable formation of the complexes was also supported by Langmuir monolayer and ITC studies (Figure 2 and 3). CAM-lipid complex zeta potentials at pH = 7.4 varied monotonically between that for CAM alone (~10 mV) and for lipid alone (~50 mV) (Figure 4B). The zeta potential difference between CAM and lipid was due to the nature of the cationic charges. CAM possesses primary (pKa = 10.7) and secondary amines (pKa = 9.7) of which protonation may not be complete due to electrostatic repulsion and insufficient access of the aqueous media to the hydrophobic core, while DOTAP possesses the quaternary ammonium cation which is pH-independent. When forming the hybrid complexes, the PEG tail of the CAM should shield the surface charge that lower the zeta potential as increasing the CAM composition. Gel electrophoresis was used to monitor siRNA complexation with the CAM-lipid complexes. As was the case for CAMs alone[16], it was found that an N/P ratio of 50 was necessary for efficient siRNA complexation with CAM-lipid mixtures. At an N/P ratio of 50, only a minor fraction of siRNA migrated on the gel, indicating complete complexation of siRNA to 9N-lipid for all compositions (Figure 4C). Similar results were observed when 7N-lipid was used (data not shown). TEM images of 9N-lipid complex with weight ratio of 1:1 is shown (Figure 4D). The complex size measured by TEM correlated with the DLS observations, the smaller particles observed with the hybrid complexes may be the assemblies from lipid or CAM monomer dissociated from the complexes. Furthermore, sizes of the CAM-lipid after siRNA complexation were also measured (Figure S1). These results indicated that the CAM-lipid/siRNA complex diameters remained at approximately 100–200 nm, suggesting that the size of the nanocomplex is not affected significantly by the presence of siRNA. Zeta potentials of CAM-lipid after complexation with siRNA (Figure S2) decreased as compared to CAM-lipid complexes alone due to the neutralization of cationic surface charge from anionic siRNA. Despite this decrease, all of the CAM-lipid/siRNA complexes maintained a net cationic charge rendering a favorable intracellular uptake of the complexes. To further investigate the stability of the CAM-lipid/siRNA complex under serum-containing condition, the complex sizes were monitored over a week in the presence of 10% fetal bovine serum (FBS) (Figure S3). Complexes with higher CAM weight ratios maintained a 100–200 nm size range, as PEG chains of the CAM can repulse the serum proteins. When decreasing CAM weight ratios, complex sizes increased as less PEG was available on the complex surfaces. For the complexes without CAMs, immediate visual aggregation was observed with FBS addition, indicating possible damage to the siRNA caused by RNase. This data suggests that CAM-lipid complexes can maintain the integrity and protect the siRNA from degradation under the serum-containing conditions.
Figure 4.
(A) Hydrodynamic diameter of CAM-lipid complexes in HEPES (10 mM, pH = 7.4) buffer with different weight ratios using DLS. (B) Zeta potentials of CAM-lipid complexes in HEPES (10 mM, pH = 7.4) with different weight ratios. Lipofectamine was used as control, data represent mean ± standard deviation (n=3). (C) Electrophoresis gel, lanes 1–9 correspond to 9N-lipid weight ratios of 1:0, 10:1, 5:1, 2:1, 1:1, 1:2, 1:5, 1:10, 0:1 at N/P ratio of 50, lane 10 is Lipofectamine, lane 11 is siRNA alone. (D) TEM image of CAM (9N)-lipid at 1:1 weight ratio.
ITC has been broadly applied in drug discovery processes. It can follow the heat change of binding between ligand and protein and gives not only precise measurement of binding affinity but also the thermodynamics of the binding. Notably, there is limited literature applying ITC to probe siRNA binding to delivery vehicle[28, 29]. In a related study, Keller et al. used ITC to illustrate the siRNA binding to chitosan[28], but, to our knowledge, our work is the first that uses ITC to characterize siRNA binding to polymer/lipid hybrid systems. Herein, ITC was used to monitor the thermodynamics of siRNA binding to 9N-lipid mixtures in both physiological and endosomal pH conditions (7.4 and 5, respectively). The siRNA binding to a 9N-lipid system at pH = 7.4 resulted in a large endothermic heat signal (Figure 5A). However, siRNA titration to the same 9N-lipid system at pH = 5 yielded a significantly reduced heat signal, similar to that observed from siRNA titration into the buffer alone (Figure 5C). The ITC study demonstrates that siRNA binds to 9N-lipid complexes with greater affinity at pH = 7.4 than pH = 5. However, monitoring the siRNA binding under a full serum condition is not feasible due to the large heat signal imposed by the serum protein. This finding may imply that once the siRNA complex enters the more acidic intracellular environment, siRNA is released from the CAM-lipid system because of weaker binding between siRNA and the CAM-lipid. Therefore, the binding affinity of siRNA to CAM-lipid is altered by pH changes, such that siRNA can be bound at neutral pH (e.g., in bloodstream) and then released under acidic conditions (i.e., within the cellular endosomes).
Figure 5.
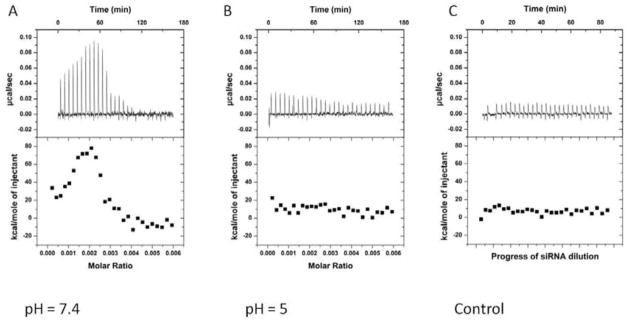
Comparison of data from isothermal titration of 0.128 mM 9N-lipid complex (1/10 (mass/mass)) with 3.8 μM siRNA at pH = 7.4 (A) and pH = 5 (B). Panel C is siRNA only addition. Top panels: raw heat signals. Lower panels: Integrated areas corresponding to each titration.
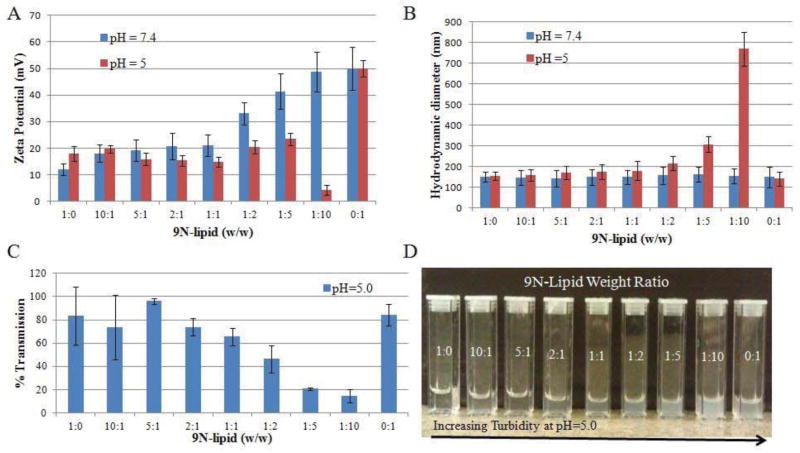
To understand these differences in siRNA binding affinity at pH 7.4 and 5, zeta potentials of 9N-lipid complexes were measured at both pH values. Zeta potentials of 9N-lipid with 1:10 weight ratio decreased drastically from 48 mV (pH = 7.4) to 5 mV (pH = 5) (Figure 6A). The descending trend of the zeta potential from pH = 7.4 to pH = 5 was also observed at 1:5, 1:2, and 1:1 weight ratios. The decreased binding affinity at pH = 5 can be anticipated by the decreased zeta potential of 9N-lipid complexes. To further study the decreased zeta potential of the complexes, sizes were measured to probe the colloidal stabilities of the complexes at pH = 5. It was shown that 9N-lipid with 1:10 weight ratio showed a steep increase from 150 nm to 770 nm (Figure 6B). Turbidities of the complexes were measured; the 9N-lipid with 1:10 ratio has less than 20% transmission (Figure 6C). Visual cloudiness in the dispersion (9N-lipid 1:1 weight ratio) suggests that precipitates form due to the complex instability at pH = 5 (Figure 6D). However, both the 9N and lipid alone were stable at pH = 5. Yet, 9N CAM has an increased zeta potential at pH = 5 compared to pH = 7.4, and when mixed with cationic lipid, the electrostatic repulsion can overcome the hydrophobic attraction leading to an instable complex that crashes out of solution. Hence, the pH-responsive effect between 9N and lipid with weight ratio 1:10 at pH = 5 was observed and further lead to the differential binding affinity between siRNA and the complexes.
Figure 6.
Stability studies of 9N-lipid complexes: (A) Zeta potentials of 9N-lipid complexes at pH = 7.4 and 5; (B) hydrodynamic volumes of 9N-lipid complexes at pH = 7.4 and 5; (C) turbidities of 9N-lipid complexes at pH = 5; and (D) visual appearances of 9N-lipid complexes at pH = 5.
To evaluate the gene silencing efficiency of CAM-lipid systems, the delivery of anti-luciferase siRNA to U87-Luc cells expressing luciferase was monitored. Nearly no silencing was observed with a scrambled siRNA control, indicating that the gene knockdown was specifically induced by anti-luciferase siRNA alone. Similar transfection efficiencies were found at 60% for both CAM alone and lipid alone (Figure 7). CAM-lipid complexes with weight ratios of 10:1, 5:1, and 2:1 showed decreased transfection efficiencies compared to the CAM or lipid alone. In contrast, the transfection efficiencies of CAM-lipid complexes with weight ratios of 1:1, 1:2, 1:5, and 1:10 were improved and comparable to Lipofectamine. The transfection efficiency trends reveal that increasing CAM ratio in the CAM-lipid complex yields decreasing transfection efficiency. Based on intensive literature precedence, increasing CAM ratios likely yield higher PEG coating percentages in the CAM-lipid complex which eventually impede the cellular uptake of the complex[17]. However, CAM alone showed higher efficiencies than the CAM-lipid complexes with weight ratios of 5:1, 2:1, and 10:1. This result suggests that the CAM-lipid complex is not simply a mixture of polymer and lipid systems; synergistic effects between the polymer and lipid appear to play an important role in the delivery process. As further support, CAM-lipid complexes with weight ratios of 1:1, 1:2, 1:5, and 1:10 gave enhanced transfection efficiencies compared to CAM or lipid alone. These formulations also had comparable efficiency to Lipofectamine. We postulate that CAM-lipid complexes possess a unique micelle-liposome mixed structure which requires additional detailed investigations. To examine the synergistic effect between CAM and lipid and to gain mechanistic insights, sequential studies were carried out using 9N-lipid with a weight ratio of 1:10, as the 9N-lipid with 1:10 ratio showed higher transfection efficiency than other formulations.
Figure 7.
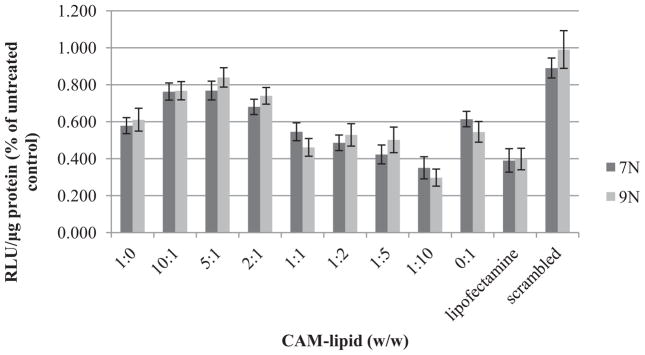
Luciferase reporter gene down-regulation assay performed over 48 h performed with the U87 luciferase cell line using complexes formulated from CAM-lipid complexes and anti-luciferase siRNA at N/P ratio of 50. Lipofectamine is used as a control. Data represent mean ± standard error (n=3).
To further investigate the endosomal escape of siRNA delivery, intracellular trafficking of 9N-lipid was examined using confocal microscopy. 9N-lipid/Cy5-siRNA (9N-lipid weight ratio 1:10) was co-localized with LysoTracker Red after 4 h of incubation (Figure 8). This observation suggests that the complexes were internalized but had not yet been released from endosomes or early lysosomes by 4 h. After 24 h, only minimal co-localization was observed and more extensive siRNA distribution was observed in the cytoplasm (data not shown), suggesting that Cy5-siRNA had undergone endosomal/lysosomal escape. Based on the previous pH-dependent data, the endosomal escape could be explained by the pH-responsive feature of the 9N-lipid at weight ratio of 1:10. When the complexes are internalized in endosomes, the collapse of the complexes at acidic pH caused the release of lipid, CAM, and siRNA. The lipid can serve as a destabilizing agent to disrupt the endosome membrane[30]. The CAM can induce endosome disruption via the well-studied proton sponge theory[31, 32]. The siRNA is then released into the cytoplasm to trigger the RNAi process after the endosomal disruption. The same trends were observed when using Lipofectamine as the carrier. For the less effective carrier (9N-lipid with weight ratio of 10:1), siRNA appeared to aggregate on the cell surface after 4 h. After 24 h, some CAM-lipid complexes were internalized, however, much more CAM-lipid complex remained on the cell surface as compared to the 1:10 formulation. These results suggest that siRNA efficiency is impaired at 10:1 weight ratio due to insufficient cell uptake and decreased intracellular release of siRNA. Therefore, to assure CAM-lipid complexes of optimized composition have great potential to be used as efficient non-viral carriers for siRNA delivery, cytotoxicity studies were conducted. Low cytotoxicity of the CAM-lipid complexes were observed (Figure S4).
Figure 8.
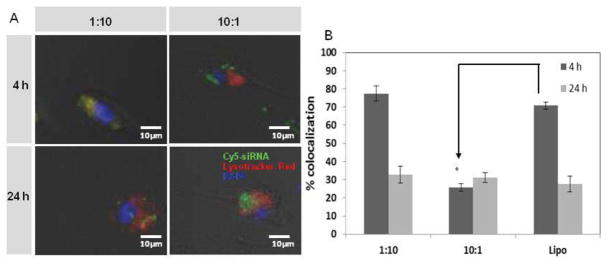
(A) Confocal microscope images of Cy5-siRNA (green) and endosomal (red) distribution in U87 cells when delivered by the indicated 9N-lipid complexes, at 4 h (top panel) and 24 h (bottom panel) post-transfection. (B) Colocalization of puncta was quantified using ImageJ. The percent colocalization of Lysotracker Red and Cy5-siRNA puncta was calculated as mean gray value from colocalized points divided by mean gray value from sum of points using Image J. Data represent mean ± standard error (n = 3).
4. Conclusion
We developed a novel complex that combines polymer and lipid to effectively delivery siRNA. Langmuir monolayer and isothermal titration calorimetry confirmed that formation of stable CAM-lipid complexes was based on the hydrophobic interactions. Size and zeta potential measurements further validate that CAM-lipid complexes are suitable for siRNA complexation and delivery. In vitro siRNA delivery experiments demonstrated that CAM-lipid complexes with specific CAM-lipid weight ratios have comparable gene silencing efficiencies compared to Lipofectamine control. Further, intracellular trafficking and ITC studies revealed that siRNA can escape from endosomes and are released from CAM-lipid complexes to down-regulate genes. Thus, these CAM-lipid complexes are a dramatic improvement upon prior developed systems. While most delivery systems that achieve delivery of nucleic acids do so at the cost of high cytoxicity, in contrast, here we have developed a system with good performance and low cytotoxicity. Notably, the ability to mediate gene silencing appears to be at least partially due to the pH-dependence of the interaction between the CAM-lipid complexes and the siRNA. Here, we showed the mechanism of differential binding affinities of siRNA the complexes at pH = 7.4 and 5, namely, that complexes were unstable under acidic conditions. The pH-responsive feature of the complexes appears to be a natural feature of the components that we designed and does not require additional complications such as attachments of endosomal disruption peptide or reducible disulfide bonds. These studies strongly suggest that CAM-lipid complexes can serve as efficient siRNA delivery vehicles and provide a novel method to probe delivery mechanisms.
Supplementary Material
Acknowledgments
Financial support from NIH (NHLBI HL107913), the Steven A Cox Foundation, Henry Kressel Scholarship and Jewish Federation for the Education of Women and Yeshiva University are gratefully acknowledged. The authors thank Dr. Bryan Langowski for assistance with reviewing and revising this manuscript.
Footnotes
The small difference in results makes positive determination of whether there is trans-bilayer equilibration within the time frame of the experiment impossible with this method.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Gooding M, Browne LP, Quinteiro FM, Selwood DL. siRNA Delivery: From Lipids to Cell-penetrating Peptides and Their Mimics. Chem Biol Drug Des. 2012;80:787–809. doi: 10.1111/cbdd.12052. [DOI] [PubMed] [Google Scholar]
- 3.Buyens K, De Smedt SC, Braeckmans K, Demeester J, Peeters L, van Grunsven LA, de Mollerat du Jeu X, Sawant R, Torchilin V, Farkasova K, Ogris M, Sanders NN. Liposome based systems for systemic siRNA delivery: Stability in blood sets the requirements for optimal carrier design. Journal of Controlled Release. 2012;158:362–370. doi: 10.1016/j.jconrel.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Dang J, Leong K. Natural polymers for gene delivery and tissue engineering☆. Advanced Drug Delivery Reviews. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Aagaard L, Rossi JJ. RNAi therapeutics: Principles, prospects and challenges. Advanced Drug Delivery Reviews. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong SY, Pelet JM, Putnam D. Polymer systems for gene delivery—Past, present, and future. Progress in Polymer Science. 2007;32:799–837. [Google Scholar]
- 7.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. Journal of Controlled Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Buyens K, De Smedt SC, Braeckmans K, Demeester J, Peeters L, van Grunsven LA, de Mollerat du Jeu X, Sawant R, Torchilin V, Farkasova K, Ogris M, Sanders NN. Liposome based systems for systemic siRNA delivery: stability in blood sets the requirements for optimal carrier design. J Control Release. 2012;158:362–370. doi: 10.1016/j.jconrel.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Endoh T, Ohtsuki T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Advanced Drug Delivery Reviews. 2009;61:704–709. doi: 10.1016/j.addr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Ezzat K, Zaghloul EM, El Andaloussi S, Lehto T, El-Sayed R, Magdy T, Smith CI, Langel U. Solid formulation of cell-penetrating peptide nanocomplexes with siRNA and their stability in simulated gastric conditions. J Control Release. 2012;162:1–8. doi: 10.1016/j.jconrel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung S, Lee SH, Mok H, Chung HJ, Park TG. Gene silencing efficiency of siRNA-PEG conjugates: Effect of PEGylation site and PEG molecular weight. Journal of Controlled Release. 2010;144:306–313. doi: 10.1016/j.jconrel.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Jeong JH, Mok H, Oh YK, Park TG. siRNA Conjugate Delivery Systems. Bioconjugate Chemistry. 2009;20:5–14. doi: 10.1021/bc800278e. [DOI] [PubMed] [Google Scholar]
- 13.Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J. 2011;6:1130–1146. doi: 10.1002/biot.201100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creusat G, Rinaldi AS, Weiss E, Elbaghdadi R, Remy JS, Mulherkar R, Zuber G. Proton sponge trick for pH-sensitive disassembly of polyethylenimine-based siRNA delivery systems. Bioconjug Chem. 2010;21:994–1002. doi: 10.1021/bc100010k. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Lv M, Gao Z. Polyethylenimine grafted with diblock copolymers of polyethylene glycol and polycaprolactone as siRNA delivery vector. J Control Release. 2011;152(Suppl 1):e143–145. doi: 10.1016/j.jconrel.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 16.Sparks SM, Waite CL, Harmon AM, Nusblat LM, Roth CM, Uhrich KE. Efficient intracellular siRNA delivery by ethyleneimine-modified amphiphilic macromolecules. Macromolecular bioscience. 2011;11:1192–1200. doi: 10.1002/mabi.201100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmon AM, Lash MH, Sparks SM, Uhrich KE. Preferential cellular uptake of amphiphilic macromolecule-lipid complexes with enhanced stability and biocompatibility. Journal of controlled release : official journal of the Controlled Release Society. 2011;153:233–239. doi: 10.1016/j.jconrel.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iverson NM, Sparks SM, Demirdirek B, Uhrich KE, Moghe PV. Controllable inhibition of cellular uptake of oxidized low-density lipoprotein: structure-function relationships for nanoscale amphiphilic polymers. Acta biomaterialia. 2010;6:3081–3091. doi: 10.1016/j.actbio.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monkkonen J, Urtti A. Lipid fusion in oligonucleotide and gene delivery with cationic lipids. Adv Drug Deliv Rev. 1998;34:37–49. doi: 10.1016/s0169-409x(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Li H, Sun J, Gao J, Liu W, Li B, Guo Y, Chen J. DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. Int J Pharm. 2010;390:198–207. doi: 10.1016/j.ijpharm.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Martino S, di Girolamo I, Tiribuzi R, D’Angelo F, Datti A, Orlacchio A. Efficient siRNA delivery by the cationic liposome DOTAP in human hematopoietic stem cells differentiating into dendritic cells. J Biomed Biotechnol. 2009;2009:410260. doi: 10.1155/2009/410260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmon AM, Lash MH, Sparks SM, Uhrich KE. Preferential cellular uptake of amphiphilic macromolecule–lipid complexes with enhanced stability and biocompatibility. Journal of Controlled Release. 2011;153:233–239. doi: 10.1016/j.jconrel.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsamaloukas AD, Keller S, Heerklotz H. Uptake and release protocol for assessing membrane binding and permeation by way of isothermal titration calorimetry. Nature protocols. 2007;2:695–704. doi: 10.1038/nprot.2007.98. [DOI] [PubMed] [Google Scholar]
- 24.Chapman D, Owens NF, Phillips MC, Walker DA. Mixed monolayers of phospholipids and cholesterol. Biochimica et biophysica acta. 1969;183:458–465. doi: 10.1016/0005-2736(69)90160-6. [DOI] [PubMed] [Google Scholar]
- 25.Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. pH-Responsive Polymeric Micelle Carriers for siRNA Drugs. Biomacromolecules. 2010 doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuhn L, Hirsch M, Krieg B, Koynov K, Fischer K, Schmidt M, Helm M, Zentel R. Cationic nanohydrogel particles as potential siRNA carriers for cellular delivery. ACS Nano. 2012;6:2198–2214. doi: 10.1021/nn204116u. [DOI] [PubMed] [Google Scholar]
- 27.Wang XL, Xu R, Lu ZR. A peptide-targeted delivery system with pH-sensitive amphiphilic cell membrane disruption for efficient receptor-mediated siRNA delivery. J Control Release. 2009;134:207–213. doi: 10.1016/j.jconrel.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Holzerny P, Ajdini B, Heusermann W, Bruno K, Schuleit M, Meinel L, Keller M. Biophysical properties of chitosan/siRNA polyplexes: profiling the polymer/siRNA interactions and bioactivity. J Control Release. 2012;157:297–304. doi: 10.1016/j.jconrel.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Jensen LB, Mortensen K, Pavan GM, Kasimova MR, Jensen DK, Gadzhyeva V, Nielsen HM, Foged C. Molecular characterization of the interaction between siRNA and PAMAM G7 dendrimers by SAXS, ITC, and molecular dynamics simulations. Biomacromolecules. 2010;11:3571–3577. doi: 10.1021/bm101033g. [DOI] [PubMed] [Google Scholar]
- 30.El Ouahabi A, Thiry M, Pector V, Fuks R, Ruysschaert JM, Vandenbranden M. The role of endosome destabilizing activity in the gene transfer process mediated by cationic lipids. FEBS letters. 1997;414:187–192. doi: 10.1016/s0014-5793(97)00973-3. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, May S. Release of cationic polymer-DNA complexes from the endosome: A theoretical investigation of the proton sponge hypothesis. The Journal of chemical physics. 2008;129:185105. doi: 10.1063/1.3009263. [DOI] [PubMed] [Google Scholar]
- 32.Hattori Y, Maitani Y. Low-molecular-weight polyethylenimine enhanced gene transfer by cationic cholesterol-based nanoparticle vector. Biological & pharmaceutical bulletin. 2007;30:1773–1778. doi: 10.1248/bpb.30.1773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.