Abstract
Light has profound effects on mood, as exemplified by seasonal affective disorder (SAD) and the beneficial effects of bright light therapy. However, the underlying neural pathways through which light regulates mood are not well understood. Our previous work has developed the diurnal grass rat, Arvicanthis niloticus, as an animal model of SAD (Leach et al., 2013a, Leach et al., 2013b). By utilizing a 12:12hr Dim Light:Dark (DLD) paradigm that simulates the lower light intensity of winter, we showed that the animals housed in DLD exhibited increased depression-like behaviors in the forced swim test (FST) and sweet solution preference (SSP) compared to animals housed in bright light during the day (BLD). The objective of the present study was to test the hypothesis that light affects mood by acting on the brain orexinergic system in the diurnal grass rat model of SAD. First, orexinA immunoreactivity (OXA-ir) was examined in DLD and BLD grass rats. The results revealed a reduction in the number of OXA-ir neurons in the hypothalamus and attenuated OXA-ir fiber density in the dorsal raphe nucleus of animals in the DLD compared to those in the BLD group. Then, the animals in BLD were treated systemically with SB-334867, a selective orexin 1 receptor (OX1R) antagonist, which led to a depressive phenotype characterized by increased immobility in the FST and a decrease in SSP compared to vehicle-treated controls. The results suggest that attenuated orexinergic signaling is associated with increased depression-like behaviors in grass rats, and support the hypothesis that the orexinergic system mediates the effects of light on mood.
Keywords: orexin, seasonal affective disorder, diurnal grass rats, SB-334867
Introduction
Environmental lighting conditions have a profound effect on mood, which is best exemplified in seasonal affective disorder (SAD). SAD is a major depressive disorder, in which affected individuals experience regularly recurring episodes of depression and anxiety each fall and winter, when there is less sunlight (Rosenthal et al., 1984). Symptoms associated with SAD remit in spring and summer when the ambient light gets brighter, and can be alleviated by bright-light exposure in winter (Rosenthal et al., 1984, Lewy et al., 1987). Although these phenomena have been characterized over decades, the mechanisms underlying the light-dependent changes in affective state have not been fully elucidated (Levitan, 2007).
To explore the neural substrates involved in SAD, we have utilized the Nile grass rat, Arvicanthis niloticus, a diurnal equatorial rodent species (McElhinny et al., 1997, Blanchong et al., 1999). Depression-like behaviors have been consistently observed by our group and others in diurnal grass rats housed in winter-like lighting conditions involving short day-length (Ashkenazy-Frolinger et al., 2009, Leach et al., 2013b) or low light intensity during the day (Leach et al., 2013a). For humans, due to the use of artificial lights, the duration of daily light exposure we experience across seasons does not fluctuate as much as the quality/intensity of the light (Hebert et al., 1998). Therefore, the changes in light intensity over the seasons may be a more salient determinant than changes in light duration for regulating mood in humans. By manipulating light intensity during the day, which is more etiologically relevant to humans, we have found increased depressive behaviors in grass rats housed in 12 hr dim-light/12 hr dark (DLD) compared to those housed in bright-light/dark (BLD) (Leach et al., 2013a). The reliable depression-like behavior under winter-like lighting conditions strongly supports the face validity of the diurnal grass rat as a model of SAD.
Using the grass rat model of SAD, the present study explored the hypothesis that light affects mood related behaviors by acting on the brain’s orexinergic (OXergic) system. The neuropeptide orexin (OX), also known as hypocretin, has been implicated in many important physiological functions including wakefulness, energy homeostasis, reward and mood regulation (Tsujino and Sakurai, 2009). In laboratory rats, OXergic neurons receive indirect retinal input (Deurveilher and Semba, 2005). Similar pathways are likely conserved in the diurnal grass rats. Although direct retinal innervation of OXergic neurons remains to be confirmed, in both laboratory rats and grass rats, there are direct retinal projections to the lateral hypothalamus where most OXergic cells are found (Leak and Moore, 1997, Johnson et al, 1988, Gaillard et al., 2013). Critically important for modulating mood and anxiety, OXergic cells project very heavily to the prefrontal cortex, limbic structures including the amygdala and bed nucleus of stria terminalis (BNST), and monoaminergic systems in both nocturnal laboratory rats and diurnal grass rats (Peyron et al., 1998, Nixon and Smale, 2007). Furthermore, the OX receptors have been found in these regions in laboratory rats (Gotter et al, 2012). Recently, we have found that in grass rats, a light pulse stimulates immediate-early gene activity in OXergic cells and cell in the dorsal raphe (DRN), and that blocking OXergic signaling with a selective OX receptor 1 (OX1R) antagonist SB-334867 inhibits light-induced activation of neurons in the DRN (Adidharma et al., 2012). Based on these results, we hypothesize that OXergic system mediates the effects of light on neural pathways that ultimately regulate mood and anxiety. To evaluate this hypothesis further, the present study used the grass rat SAD model to determine 1) whether the level of OX abundance, measured by immunoreactivity, is affected by lighting condition and associated with depression-like behaviors elicited by light deficiency (DLD), and 2) if there is a causal link between OX receptor antagonism and depression-like behaviors. The results provide insights into the role of OXergic signaling in light-dependent fluctuations in affective state relevant to SAD.
Experimental procedures
Animals and housing conditions
Adult male grass rats (Arvicanthis niloticus) were obtained from our breeding colony established with animals originating from sub-Saharan Africa. The colony was maintained/bred as previously described (McElhinny et al., 1997, Leach et al., 2013a, Leach et al., 2013b). These equatorial animals were housed in a 12hr light:12hr dark (LD) cycle with food (Prolab 2000 #5P06, PMI Nutrition LLC, MO, USA) and water available ad libitum. The time of lights-on was defined as Zeitgeber time (ZT) 0. All procedures were conducted in accordance with the Michigan State University IACUC.
Experiment 1: Effects of daytime light intensity on orexin A immunoreactivity (OXA ir)
Brains (n = 6/group) used in the experiment were obtained from animals in a previous study, in which male grass rats were singly housed in either bright light:dark (BLD, 1000 lux/1 lux) or dim light:dark (DLD, 50 lux/1 lux) condition for four weeks prior to the assessment of depression-like behaviors (Leach et al., 2013a). Following the behavioral tests, the animals were left undisturbed under the same illumination conditions for 5 days before being sacrificed at the middle of the light phase (ZT6) for brain analysis as previously described (Leach et al., 2013a). Brains were fixed with 4% paraformaldehyde, cryoprotected, and sectioned at 40 µm using a cryostat (Leica, IL).
Immunocytochemistry (ICC)
ICC for orexin A (OXA) was carried out using methodology described in previous studies (Yan et al., 2010, Adidharma et al., 2012). Every third section was incubated with an antiserum against OXA (1:20,000, s-19, Santa Cruz Biotechnology, Inc, CA) and processed with the avidin-biotin-immunoperoxidase technique using DAB as the chromogen. The orexin-containing cell bodies and fibers were stained brown. Following the ICC, sections were mounted on slides, dehydrated with alcohol, cleared with xylene, and coverslipped with Permount (Fisher Scientific, NJ, USA).
Quantitative analysis of ICC results
For quantification, images of the brain sections were captured using a CCD video camera (CX9000, MBF bioscience, VM, USA) attached to a light microscope (Nikon Instruments Inc., NY, USA). The camera and microscope settings were identical for every image. All the images were analyzed by investigators who were unaware of the experimental conditions of the animals. The number of OXA-ir cells was counted in serial sections of the hypothalamic region from its rostral to caudal extent (Fig. 1). The number of OXA-ir cells was further analyzed subregionally in the lateral hypothalamus (LH) and perifornical/dorsomedial hypothalamic region (PFA/DMH) using a vertical line across the fornix (~ 0.6 mm from the third ventricle) to separate the two subregions, as done previously in laboratory rats (Harris et al., 2005). The density of OXA-ir fibers/terminals was also analyzed in the dorsal raphe nucleus (DRN) from four levels across its rostro-caudal extent (Janusonis and Fite, 2001). The sections from levels 1 to 3 where the 5-HT neurons are clustered along the midline were grouped as rostral, while those from level 4 where the clusters of the 5-HT neurons are lateralized were defined as the middle DRN as done in our previous study (Leach et al., 2013a). The density of fibers/terminals was quantified using NIH Image J as previously described (Adidharma et al., 2012, Leach et al., 2013a). The size of each area of interest being measured was kept consistent across the sections/animals. A threshold that distinguished the immunoreactive staining from the background was also set consistently for each area. The percentage of pixels above the threshold in the area of interest was measured and averaged across the sections from the same region. The average percentage represented the density of staining per animal. The number of OXA-ir cells was analyzed using two-way ANOVA. In the DRN, a previous study revealed regional effects in 5-HT-ir when BLD and DLD animals were compared, such that a reduction of 5-HT-ir in the DLD group was only observed in the middle but not in the rostral DRN (Leach et al., 2013a). Therefore, in the present study, the density of OXA-ir was analyzed within each subregion separately using unpaired t-tests.
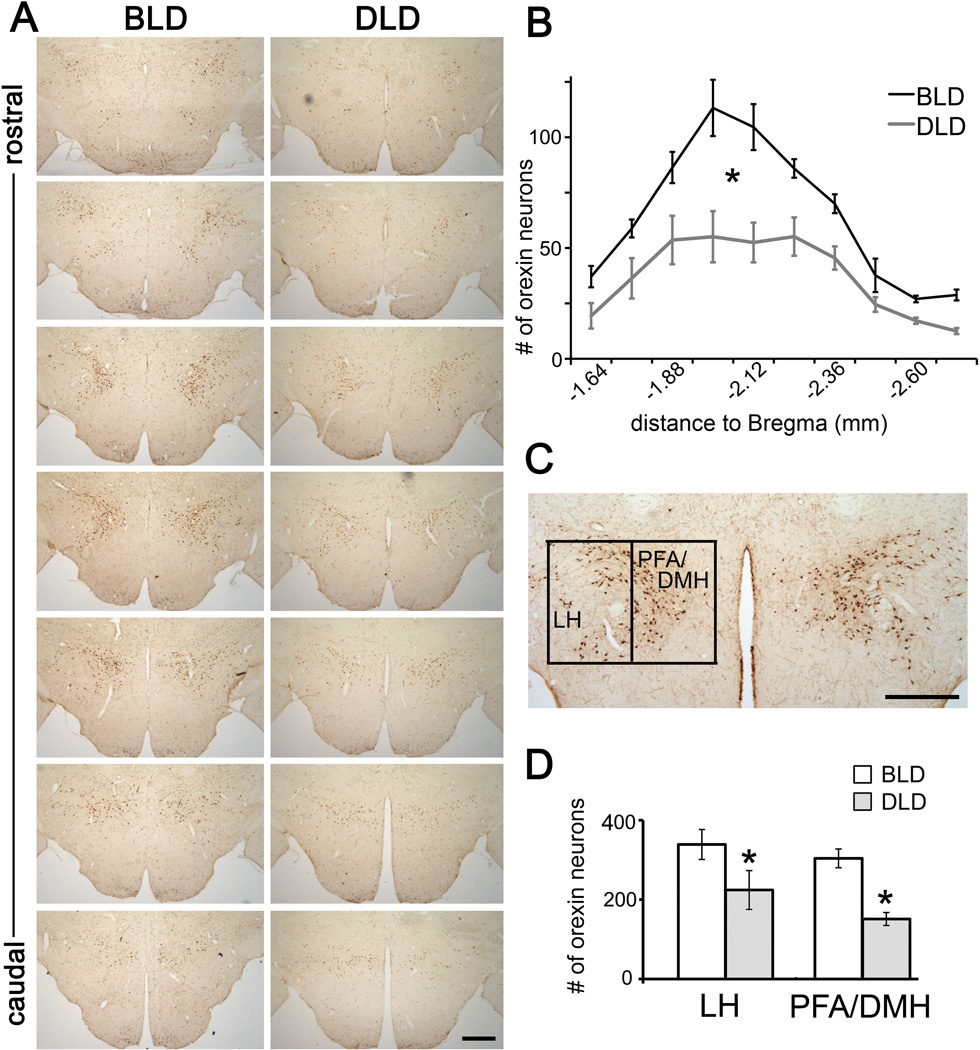
Figure 1.
Effects of light intensity on OXA-ir neurons in the grass rat hypothalamus. The representative photomicrographs (A) and the number (B) of OXA-ir neurons along the rostral-caudal axis of the posterior hypothalamus in animals housed in 12:12 hr bright light:dark (BLD) or dim light: dark (DLD) conditions. (C) The borders used to define the lateral (LH) and perifornical/dorsomedial hypothalamus (PFA/DMH) for quantification. (D) Number of OXA-ir neurons in LH and PFA/DMH subregions in the BLD and DLD group. Results are displayed as mean ± SEM (n = 6). * indicates p < 0.05 for the effect of lighting condition in ANOVA. Scale bar, 500 µm.
Experiment 2: Effects of OX1 receptor antagonism with SB-334867 on depression-like behavior
Animals were housed in the same manner as those in experiment 1. After four weeks of being housed under BLD conditions, animals were tested for depression-like behaviors following the treatment of a selective OX receptor 1 (OX1R) antagonist SB-334867 (10 mg/kg i.p., Tocris Biosciences, Bristol, UK) or vehicle (60/40 DMSO/saline, 0.4 ml). This dose was based on studies in mice (Ito et al., 2009, Scott et al., 2011) and our previous study in grass rats (Adidharma et al., 2012).
A control study was first performed to assess the effects of SB-334867 injection on general activity, as any acute effect of injection on the level of activity or arousal could affect the behaviors during the forced swim test (FST), and thus compromise the interpretation of the data. To control this potential confounding factor, general locomotor activity was recorded in a group of animals (n = 5) that were treated with SB-334867 or vehicle during the day (ZT5), to determine the optimal time course for FST following the treatment. Animals were singly housed in plexiglas cages (47 × 25 × 20 cm) under BLD conditions and monitored with IR motion sensors placed on the top of each cage. General locomotor activity was recorded in 5 min bins for three weeks by a laboratory computer and VitalView (Minimitter Inc., Bend, OR, USA). All animals were allowed to habituate to the apparatus for the first week. Afterwards, the animals received two injections at ZT5 of either SB-334867 (10 mg/kg i.p., Tocris Biosciences, Bristol, UK) or vehicle (60/40 DMSO/saline, 0.4 ml) with one week in between. A two-way repeated measure ANOVA was performed to analyze the general locomotor activity across time points. For each time point, a single-factor ANOVA with Geisser-Greenhouse corrections was performed to compare activity between the conditions, and the significant effect was followed by paired t-tests with a Bonferroni correction for multiple comparisons.
To assess depression-like behaviors, other groups of animals (n = 18) singly housed under BLD conditions were tested in the forced swim test (FST) and for their sweet solution preference (SSP) as described in previous studies from our lab (Leach et al., 2013a, Leach et al., 2013b). On the pre-test day of FST, animals received a 10 min training session in a cylindrical pool (35.5 cm tall × 30.5 cm diameter) filled with 25 cm of water maintained between 29–30 °C. The water was changed between each animal. Based on the immobility time during the last 5 min of the training session, paired littermates were placed into two groups to make sure there was no initial variability in the immobility time between the two groups. On the following test day, one group (n = 10) received an injection (i.p) of SB-334867 (10 mg/kg) and the other group (n = 8) was injected with vehicle between ZT3-7, followed by a 5 min testing session 4 hr later, as determined based on data of the control study on locomotor activity (see results section). Following the FST, the animals were supplied with a bottle of sweet solution containing 1.0% saccharin (Sigma, MI, USA) along with one containing tap water for two days. The animals were treated with SB-334867 or vehicle each day at ZT2 and the water bottles were weighed daily to measure intake.
Quantitative analysis for FST and SSP
The behaviors during the FST were videotaped and scored for three distinct behaviors: climbing, swimming, and immobility as described in previous studies (Leach et al., 2013a, Leach et al., 2013b). Analysis of group differences in these behaviors in the FST was conducted using student’s unpaired t-tests. The swim pattern of each animal during the five minute test-day session was also traced manually and distance traveled both in the entire pool (total movement) and in the inner half of the pool (center) was determined using Image J. Data were analyzed using unpaired t-tests. For the trace analysis, one animal from the SB-334867 treated group was removed prior to analysis because it was 2 SDs above the mean of its group.
SSP was calculated as the ratio of sweet solution to total liquid (tap water + sweet solution) intake. Daily SSP was compared between the two treatment groups using unpaired t-test. One animal from the SB-334867 treated group, which was a different subject from the outlier identified in the FST trace analysis, was removed for the SSP analysis because it was 2 SDs above the mean of its group.
Results
Effects of daytime light intensity on OXA-ir
As shown in Fig. 1A, many OXA-ir neurons were observed in the lateral hypothalamus (LH) and perifornical/dorsomedial hypothalamus (PFA/DMH), consistent with previous studies in grass rats (Novak and Albers, 2002, Nixon and Smale, 2007) and in other rodent species (Peyron et al., 1998, Chen et al., 1999, Cutler et al., 1999, Date et al., 1999, Nambu et al., 1999, Mintz et al., 2001). In the rostral end, there were more OXA-ir cells in the lateral region, while in the caudal end, most cells were found in the medial region near the third ventricle (Fig. 1A).
The number of OXA-ir neurons was significantly higher in the grass rats housed in BLD compared to DLD (Fig. 1B, two-way ANOVA, effect of light: F1,10 = 20.33, p = 0.001). A significant effect of rostral-caudal level (F9,90 = 38.61, p = 0.001) and interaction between light condition and rostral-caudal level (F9,90 = 4.31, p = 0.02) were also observed. The reduction in the number of OXA-ir cells was more concentrated in the middle portion than the rostral or caudal end. The number of OXA-ir cells was then analyzed separately in the LH and PFA/DMH (Fig. 1C, D). A two-way ANOVA revealed a significant main effect of lighting condition (F1,10 = 15.28, p = 0.003), but there was no significant effect of region (F1,10=2.761, p =.128) or an interaction between light and region (F1,10=.285, p =.605).
The density of OXA-ir fibers was analyzed in the dosal raphe nucleus (DRN, Fig. 2). In the rostral DRN, OXA-ir was found in both ventral and dorsal regions, while in the middle portion of the DRN, OXA-ir was most prominent in the lateral subregion of the nucleus (Fig. 2A). Quantitative analysis (Fig. 2B) on the density of OXA-ir fibers revealed a significant difference in the middle portion (t10 = 2.86, p = 0.01), but not the rostral portion of the DRN (t10 = 1.01, p = 0.34), with BLD animals having more OXA-ir than DLD animals.
Figure 2.
Effects of light intensity on OXA-ir in the dorsal raphe nucleus (DRN). (A), The representative photomicrographs showing OXA-ir fibers in the rostral and middle portions of the DRN in animals housed in 12:12 hr bright light:dark (BLD) or dim light: dark (DLD) conditions. (B), The histograms show the density of OXA-ir fibers in the rostral and middle DRN from animals in the BLD and DLD groups. Results are displayed as mean ± SEM (n=6). * indicates p < 0.05. Scale bar, 250 µm. aq, aqueduct; mlf, medial longitudinal fasciculus.
Effects of SB-334867 injections on depression-like behavior
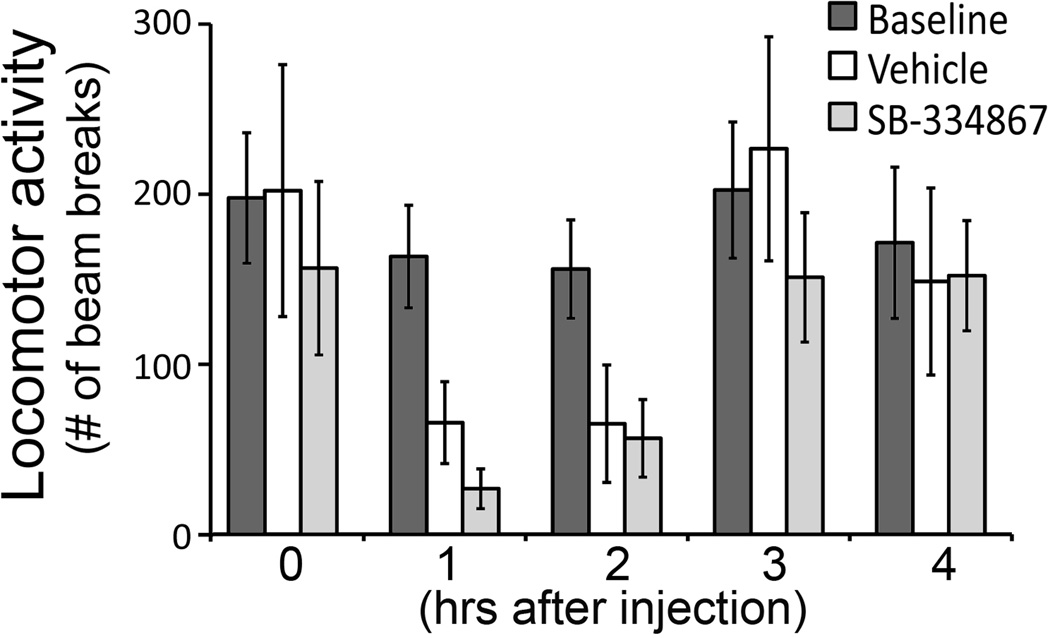
The effect of SB-334867 on locomotor activity was first assessed (Fig. 3) to control the potential confound of altered activity level on the performance during FST. There was no difference between the SB-334867 and vehicle treated group in locomotor activity at any time point (ANOVA, effect of treatment, F2, 8 = 2.76, p = 0.13, effect of time, F4, 16 = 8.56, p = 0.02, interaction between time and treatment, F8, 32 = 1.55, p = 0.27). However, injection of either SB-334867 or vehicle at ZT5 caused a decrease in locomotor activity in the first two hours following the injection compared to baseline activity during the same time window (ZT6-7, single factor ANOVA, F2, 8 = 39.73, p = 0.002). For both SB-334867 and vehicle treated groups, the locomotor activity recovered to baseline levels by 4 hours after the injection (single factor ANOVA, F2, 8 = 0.113, p = 0.79) (Fig. 3).
Figure 3.
Effects of SB-334867 i.p. injections on general locomotor activity. Exposure to both vehicle and SB-334867 significantly decreased locomotor activity during the first two hours following the injection (p < 0.05), which recovered by hour 4 after injection. Data are presented as mean ± SEM (n = 5).
Animals treated with SB-334867 showed more depression-like behaviors during the FST compared to the vehicle group (Fig. 4A), as revealed by significantly longer duration of immobility (t16 = 2.35, p = 0.03) and shorter duration of swimming (t16 = 2.13, p = 0.048). There was no significant difference in climbing behavior between the two groups (t16 = 0.52, p = 0.61). The swim pattern of the two groups was also different, with the SB-334867 treated group avoiding the center of the pool and showing more thigmotaxis (Fig. 4B). Quantitative analysis revealed that the overall distance traveled was comparable between the two groups (t15 = 1.75, p = 0.172), however, the distance traveled in the center of the pool was significantly less in SB-334867 treated group (t15 = 2.15, p = 0.048) (Fig 4C).
Figure 4.
Effects of SB-334867 treatment on depression-like behavior in the forced swim test (FST). (A), SB-334867 significantly increased immobility and decreased swimming. Data are presented as mean ± SEM (n = 8 in Vehicle, n = 10 in SB-334867 treated group). (B), Representative tracings of the swim pattern exhibited during the FST by an animal treated with either vehicle (left) or SB-334867 (right). (C), although the overall distance traveled was not different between the two groups, animals treated with the vehicle swam in the center of the pool significantly more than the SB-334867 treated group (n = 8 in Vehicle, n = 9 in SB-334867 treated group). * indicates p < 0.05.
The daily SSP was compared between animals that received daily injection of either SB-334867 or vehicle following the FST (Fig. 5). On the first day of assessment, there was no difference between the two groups. However, on the second day, the SB-334867 treated group showed a significant decline in their SSP compared to the vehicle treated group (t15 = 2.23, p = 0.04).
Figure 5.
Effects of SB-334867 treatment on depression-like behavior in the sweet solution preference test (SSP). A decreased preference for sweet solution by the drug treated group was observed on day 2. Data are presented as mean ± SEM (n = 8 in Vehicle, n = 9 in SB-334867 treated group). * indicates p < 0.05.
Discussion
The leading hypothesis about the etiology of SAD poses that the depression episodes are caused by misalignments between one’s circadian rhythms and their habitual sleep time (Lewy et al., 2007). The clinical practice of using light therapy is based on this theory (Terman and Terman, 2005, Lewy, 2009), which is derived from the fact that light is the most salient cue for resetting circadian rhythms (Pittendrigh, 1993). However, light can also affect mood through circadian-independent mechanisms (LeGates et al., 2012, Stephenson et al., 2012). Indeed, the light intensity required for effective light therapy in humans (>5000 lux, (Terman et al., 1990, Terman et al., 1996)) is much higher than that necessary for shifting our circadian rhythms (120 lux, (Zeitzer et al., 2000)), indicating that the therapeutic effects of bright light involve mechanisms beyond entraining daily rhythms.
In contrast to the circadian mechanisms, the circadian-independent mechanisms mediating the effects of light on mood are poorly understood (Stephenson et al., 2012). The objective of the present study was to explore the circadian-independent mechanisms by investigating the role that hypothalamic OXergic neurons played in mediating the effects of light on mood. OX, particularly OXA has been implicated in regulating mood and anxiety in both clinical and preclinical studies (Borgland and Labouebe, 2010, Gotter et al., 2012, Johnson et al., 2012a). A positive correlation has been found between OXA and positive emotions both in dogs and in humans (Wu et al., 2011, Blouin et al., 2013). In narcoleptic patients whose loss of OX neurons is associated with their condition (Peyron et al, 2000, Thannickal et al, 2000), depression and anxiety are prevalent (Mosko et al, 1989, Fortuyn et al, 2010, Ohayon, 2013). In patients suffering from major depressive disorder, the level of OXA peptide and mRNA is lower than that in healthy controls and is inversely correlated with symptom severity (Brundin et al., 2007a, Brundin et al., 2007b, Brundin et al., 2009, Rotter et al., 2011). Reduced central level of OXA has also been documented in comorbid depression and anxiety (Johnson et al., 2010). In animal depression models, decreased number/size of OXA neurons and diminished OXA content in the hypothalamic region have been reported (Allard et al., 2004, Nocjar et al., 2012).
To assess if the OXA system is also associated with the depressive responses observed in the SAD model of grass rat, we first examined the effects of light deficiency on OXA-ir in a cohort of grass rats whose depression-like behaviors had been assessed in a previous study (Leach et al., 2013a). The results show that the DLD animals with a verified increase in depression-like behaviors had fewer OXA-ir cells in the posterior hypothalamus. A functional dichotomy of OX neurons has been proposed, such that the neurons in LH regulate reward processing while those in PFA/DMH regulates arousal and response to stress (reviewed in (Harris and Aston-Jones, 2006)). In the present study, a reduction in the number of OXA-ir cells was observed in both the LH and PFA/DMH subregions in the DLD animals. There was a greater reduction in the number of OXA-ir cells in the PFA/DMH than LH region (50% vs. 35%) in DLD animals, suggesting that low light intensity may particularly affect arousal and stress responsiveness; however, the regional difference was not statistically significant so should be interpreted cautiously. It should be noted that in a recent study examining the response of OXA neurons to positive reinforcement also found the activation (measured by Fos-ir) of OXA neurons across the medial-lateral extent of the OX-containing region, without apparent subregional difference (McGregor et al., 2011), suggesting there may be overlapping functions between the two populations of cells.
In addition to the number of OXA-ir neurons, the density of OXA-ir fibers in the DRN was also lower in DLD animals compared to those in BLD. A significant reduction in OXA-ir fiber density was observed in the middle portion of the DRN, which is consistent with the attenuated 5-HT-ir in the same region in DLD animals (Leach et al., 2013a). It has been shown that the neurons in the rostral DRN mainly project to basal ganglia, while those in the middle send efferent projections to limbic and cortical regions that are involved in emotional behaviors (Hale and Lowry, 2011). A previous study found that OXergic signals mediate the light-induced activation of neurons in the DRN (Adidharma et al., 2012) and these results collectively suggest that light deficiency leads to attenuation in OXA-ir, which in turn down-regulates a monoaminergic system that ultimately affects mood and anxiety.
It should be noted that the OX system is influenced by circadian time and there are time-of-day effects on the level of the peptide and the number of OX-ir cells (Fujiki et al, 2001, Martinez et al, 2002, Nixon and Smale, 2004, Salomon et al, 2003, Blouin et al, 2013). Therefore, it is critically important to compare the peptide at the same circadian time between the different treatment groups. The BLD and DLD samples in the present study were collected at the same time of the day (ZT6). Furthermore, the daily rhythms and how they entrain or synchronize to the daily light/dark schedule have been compared between the BLD and DLD grass rats and found to be in the same manner (Leach et al., 2013a). The same sampling time and the same entraining pattern of the daily rhythm support the conclusion that the reduction in OXA-ir in the DLD group is not due to potential differences in their circadian timing, but rather caused by light deficiency.
OX binds to two receptors (type 1 and 2), with OXA binding with higher affinity over OXB to OX1R, while both OXA and OXB bind to OX2R with similar affinity (Sakurai et al., 1998). Null mutation of OX2R is associated with narcoleptic phenotype, while animals without OX1R are not narcoleptic (Sakurai, 2007), suggesting the OX1R is more relevant to other roles of OX system apart from promoting wakefulness, such as regulating reward seeking behaviors, stress, and anxiety and mood (Gotter et al., 2012). In a genetic study addressing the relationship between the OXergic system and mood disorders, a specific polymorphism of the OX1R gene was found to be significantly associated with unipolar depression (Rainero et al., 2011). The present study focused on the OXA-OX1R pathway by utilizing a selective OX1R antagonist SB-334867, to determine if there is a causal link between OXA-OX1R signaling and the depression-like behaviors in the grass rats. We found that systemic injection of SB-334867 induced depression-like behaviors in BLD animals, revealed by longer immobility during FST and decreased SSP (Figure 4A, 5). This suggests that an intact OXA-OX1R signaling pathway is required for the anti-depressive effects of bright light. It has been shown that intact OXergic signaling is also required for the anti-depressive effect of calorie restriction and of administration of Kososan, an herbal medicine that has anti-depressive effects (Lutter et al., 2008, Ito et al., 2009), and that SB-334867 blocks the anti-depressant effects of OXA in laboratory mice (Ito et al., 2008). To further explore the role of the OXergic pathway in light-dependent mood changes, a future study will augment OXergic activity through central infusion of OXA into DLD animals, which is expected to alleviate the depression-like behaviors. The results will lend further support to the hypothesis that attenuated OXergic signaling underlies the depression-like behaviors caused by daytime light deficiency.
In addition to the depression-like behaviors, thigmotaxis, an anxiety-like behavior (Treit and Fundytus, 1988) was also observed during the FST in the animals treated with SB-334867 (Fig. 4B, C). Intrigued by this observation, we analyzed the swim pattern in animals housed in BLD or DLD conditions from a previous study (Leach et al., 2013a). We found that although there was no difference in the total distance swam, the DLD group showed significantly more thigmotaxis and avoided the center area of the pool compared to the BLD group (t-test, p = 0.002), which is consistent with the behaviors of the SB-334867 treated group in the present study. Thigmotaxis in a water maze has been shown in laboratory rats to positively correlate with their trait anxiety and circulating corticosterone levels, which is an indicator of the HPA axis response to stress (Beiko et al., 2004, Herrero et al., 2006, Huang et al., 2012). Moreover, it has been reported that thigmostaxis in water is influenced by lighting conditions. In nocturnal mice, housing under bright light leads to more thigmotaxis in water compared to mice housed under dim light (Huang et al., 2012). This finding in nocturnal mice is opposite to what we observed in the diurnal grass rats, suggesting the anxiety-like responses in the FST that are associated with lighting condition are chronotype-dependent.
The brain regions that SB-334867 acted upon are of interest and are likely responsible, in part, for the behavioral effects of this antagonist observed in the present study. OXergic cells project to many brain regions that are involved in regulating mood and anxiety (Peyron et al., 1998), and where the expression of OX1R has been confirmed in nocturnal laboratory rats (Trivedi et al., 1998, Lu et al., 2000, Hervieu et al., 2001, Sunter et al., 2001). The involvement of the OXA-OX1R pathway in these sites has been assessed in various animal models of depression (Feng et al., 2007, Feng et al., 2008, Nocjar et al., 2012, Arendt et al., 2013). For example, in a social defeat model, reduced OXA levels were found in VTA and mPFC in defeated animals compared to undefeated controls (Nocjar et al., 2012). Using the inherent variability of immobility during FST, it has been shown that the depressive behaviors are associated with decreased OXA in the hippocampus and increased OX1R in the amygdala (Arendt et al., 2013). On the other hand, unilateral injection of SB-334867 into the BNST reduced anxiety-like behaviors in panic prone rats (Johnson et al., 2010). These results suggest that the role of OXA-OX1R signaling in emotion-related behaviors is unique for distinct brain regions. The distribution of OX receptors in the diurnal grass rats, how the expressions of receptors are affected by lighting condition in different brains, and their association with depression-like behaviors will be evaluated in future studies.
It should be noted that SB-334867 has also been reported to have anti-depressant effects in mice (Scott et al., 2011) and anxiolytic effects in mice following an acute stressor (Plaza-Zabala et al., 2010) and in a rat model of panic disorder (Johnson et al., 2010, Johnson et al., 2012a, Johnson et al., 2012b). The results from these studies seem to contradict our findings, but could be potentially due to factors such as time of day that the animals were tested (inactive vs. active phase), interval between injection and testing (30 min vs. hours to days), and the stress or anxiety paradigm used (acute stress, panic vs. chronic anxiety). In our grass rats, depression-like behaviors in the FST were observed 4 hr after SB-334867 treatment, while SSP was observed following 2 days of treatment (Fig. 4). This is consistent with the finding that SB-334867 blocks the antidepressant effects of Kossoan, a herbal medicine or OXA when given chronically or 3 to 4 days prior to FST (Ito et al., 2008, Ito et al., 2009). The involvement of OXergic pathways in chronic anxiety, especially anxiety comorbid with depression, has not been well studied in animals. It has been proposed that high OXA activity is associated with acute anxiety states (perhaps most analogous to panic), but that low OXA activity is associated with chronic anxiety (perhaps analogous to generalized anxiety) (Johnson et al., 2012a). In addition to the methodological differences, some of the apparent conflicts in the literature could also stem from chronotype-related differences in the day/night expression pattern of OX and in its response to light/dark. For instance, the highest OX level/activity is found at daytime for diurnal animals, but at nighttime for nocturnal animals (Estabrooke et al, 2001, Martinez et al, 2002, Nixon & Smale, 2004, Kodama et al, 2005). Furthermore, whereas OXergic neurons are activated by a dark pulse in nocturnal mice (Marston et al, 2008), they are activated by a light pulse in the diurnal grass rat (Adidharma et al, 2012). Future studies using the grass rat model will explore the interaction of the circadian phase, light and OXergic system in regulating depression- and/or anxiety-like behaviors.
In the diurnal grass rat model of SAD, depression-like and anxiety-like behaviors are elicited by decreased light intensity during the day, which is non-invasive and etiologically relevant for understanding SAD in diurnal humans. Given the distinctly different effects of light in diurnal and nocturnal species, i.e. arousal vs. sleep, this model offers a unique opportunity to answer questions about how light affects depression and anxiety in humans (Workman and Nelson, 2011). Elucidating the role that the OXergic system plays in mediating the effects of light on mood and anxiety will contribute to a better understanding of the neuropathology of SAD and lead to novel therapeutic strategies.
Highlights.
-
▪
In grass rats, a diurnal rodent model of SAD, the abundance of orexin A is affected by lighting condition.
-
▪
The abundence of orexin A is associated with depression-like behaviors elicited by light deficiency.
-
▪
Antagonism of orexin 1 receptor leads to depression- and anxiety-like behaviors in the diurnal grass rats.
-
▪
The results suggest orexinergic signaling plays a role in light-dependent fluctuations in affective state relevant to SAD.
Acknowledgements
We would like to thank Drs. Antonio A. Nunez and Cheryl Sisk for helpful comments on this study and the manuscript. This work is supported by NSF grant (IOS 1051919) and NIH grant (R03MH093760) to LY. The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies.
Abbreviations
- 5-HT
serotonin
- BLD
bright light:dark
- BNST
bed nucleus of stria terminalis
- DLD
dim light:dark
- DMH
dorsomedial hypothalamus
- DRN
dorsal raphe neucleus
- FST
forced swim test
- ir
immunoreactivity
- ICC
immunocytochemistry
- LH
lateral hypothalamus
- mPFC
medial prefrontal cortex
- OX
orexin
- OX1R
orexin 1 receptor
- PFA
perifornical area
- SAD
seasonal affective disorder
- SSP
sweet solution preference
- VTA
ventral tegmental area
References
- Adidharma W, Leach G, Yan L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience. 2012;220:201–207. doi: 10.1016/j.neuroscience.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38:311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH. Depressive behavior and activation of the orexin/hypocretin system. Behav Neurosci. 2013;127:86–94. doi: 10.1037/a0031442. [DOI] [PubMed] [Google Scholar]
- Ashkenazy-Frolinger T, Kronfeld-Schor N, Juetten J, Einat H. It is darkness and not light: Depression-like behaviors of diurnal unstriped Nile grass rats maintained under a short photoperiod schedule. J Neurosci Methods. 2009;186:165–170. doi: 10.1016/j.jneumeth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Blanchong JA, McElhinny TL, Mahoney MM, Smale L. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14:364–377. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
- Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson KAE, Lapierre JL, Siegel JM. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Labouebe G. Orexin/hypocretin in psychiatric disorders: present state of knowledge and future potential. Neuropsychopharmacology. 2010;35:353–354. doi: 10.1038/npp.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol. 2007a;17:573–579. doi: 10.1016/j.euroneuro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Traskman-Bendz L, Petersen A. Increased orexin levels in the cerebrospinal fluid the first year after a suicide attempt. J Affect Disord. 2009;113:179–182. doi: 10.1016/j.jad.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Brundin L, Petersen A, Bjorkqvist M, Traskman-Bendz L. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord. 2007b;100:259–263. doi: 10.1016/j.jad.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett. 1999;260:161–164. doi: 10.1016/s0304-3940(98)00977-x. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, Arch JR, Wilson S, Buckingham RE, Evans ML, Leslie RA, Williams G. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20:1455–1470. doi: 10.1016/s0196-9781(99)00157-6. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Hu Y, Strohl KP. Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychopharmacol. 2008;22:784–791. doi: 10.1177/0269881106082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res. 2007;1154:163–172. doi: 10.1016/j.brainres.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Fortuyn HA, Lappenschaar MA, Furer JW, Hodiamont PP, Rijnders CA, Renier WO, Buitelaar JK, Overeem S. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry. 2010;32:49–56. doi: 10.1016/j.genhosppsych.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Honda K, Mignot E, Nishino S. Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in response to food deprivation. Neuroreport. 2001;12:993–997. doi: 10.1097/00001756-200104170-00026. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Karten HJ, Sauve Y. Retinorecipient areas in the diurnal murine rodent Arvicanthis niloticus: a disproportionally large superior colliculus. J Comp Neurol. 2013;521:1699–1726. doi: 10.1002/cne.23303. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hebert M, Dumont M, Paquet J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 1998;15:59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- Herrero AI, Sandi C, Venero C. Individual differences in anxiety trait are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors. Neurobiol Learn Mem. 2006;86:150–159. doi: 10.1016/j.nlm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou W, Zhang Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav Brain Res. 2012;226:26–31. doi: 10.1016/j.bbr.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Gamo Y, Nagai T, Oikawa T, Yamada H, Hanawa T. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 2008;157:720–732. doi: 10.1016/j.neuroscience.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Nagai T, Oikawa T, Yamada H, Hanawa T. A possible mechanism underlying an antidepressive-like effect of Kososan, a Kampo medicine, via the hypothalamic orexinergic system in the stress-induced depression-like model mice. Biol Pharm Bull. 2009;32:1716–1722. doi: 10.1248/bpb.32.1716. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Fite KV. Diurnal variation of c-Fos expression in subdivisions of the dorsal raphe nucleus of the Mongolian gerbil (Meriones unguiculatus) J Comp Neurol. 2001;440:31–42. doi: 10.1002/cne.1368. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012a;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012b;107:733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 1988;462:301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- Kodama T, Usui S, Honda Y, Kimura M. High Fos expression during the active phase in orexin neurons of a diurnal rodent, Tamias sibiricus barberi. Peptides. 2005;26:631–638. doi: 10.1016/j.peptides.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Leach G, Adidharma W, Yan L. Depression-like responses induced by daytime light deficiency in the diurnal grass rat (Arvicanthis niloticus) PLoS One. 2013a;8:e57115. doi: 10.1371/journal.pone.0057115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach G, Ramanathan C, Langel J, Yan L. Responses of brain and behavior to changing day-length in the diurnal grass rat (Arvicanthis niloticus) Neuroscience. 2013b;234C:31–39. doi: 10.1016/j.neuroscience.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Identification of retinal ganglion cells projecting to the lateral hypothalamic area of the rat. Brain Res. 1997;770:105–114. doi: 10.1016/s0006-8993(97)00761-0. [DOI] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan RD. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues Clin Neurosci. 2007;9:315–324. doi: 10.31887/DCNS.2007.9.3/rlevitan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ. Circadian misalignment in mood disturbances. Curr Psychiatry Rep. 2009;11:459–465. doi: 10.1007/s11920-009-0070-5. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Rough JN, Songer JB, Mishra N, Yuhas K, Emens JS. The phase shift hypothesis for the circadian component of winter depression. Dialogues Clin Neurosci. 2007;9:291–300. doi: 10.31887/DCNS.2007.9.3/alewy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–354. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston OJ, Williams RH, Canal MM, Samuels RE, Upton N, Piggins HD. Circadian and dark-pulse activation of orexin/hypocretin neurons. Mol Brain. 2008;1:19. doi: 10.1186/1756-6606-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GS, Smale L, Nunez AA. Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res. 2002;955:1–7. doi: 10.1016/s0006-8993(02)03264-x. [DOI] [PubMed] [Google Scholar]
- McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62:91–96. doi: 10.1016/s0031-9384(97)00146-7. [DOI] [PubMed] [Google Scholar]
- McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31:15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, van den Pol AN, Casano AA, Albers HE. Distribution of hypocretin-(orexin) immunoreactivity in the central nervous system of Syrian hamsters (Mesocricetus auratus) J Chem Neuroanat. 2001;21:225–238. doi: 10.1016/s0891-0618(01)00111-9. [DOI] [PubMed] [Google Scholar]
- Mosko S, Zetin M, Glen S, Garber D, DeAntonio M, Sassin J, McAnich J, Warren S. Self-reported depressive symptomatology, mood ratings, and treatment outcome in sleep disorders patients. J Clin Psychol. 1989;45:51–60. doi: 10.1002/1097-4679(198901)45:1<51::aid-jclp2270450107>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Novak CM, Albers HE. Localization of hypocretin-like immunoreactivity in the brain of the diurnal rodent, Arvicanthis niloticus. J Chem Neuroanat. 2002;23:49–58. doi: 10.1016/s0891-0618(01)00144-2. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14:488–492. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainero I, Ostacoli L, Rubino E, Gallone S, Picci LR, Fenoglio P, Negro E, Rosso C, De Martino P, De Marchi M, Furlan PM, Pinessi L. Association between major mood disorders and the hypocretin receptor 1 gene. J Affect Disord. 2011;130:487–491. doi: 10.1016/j.jad.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Rotter A, Asemann R, Decker A, Kornhuber J, Biermann T. Orexin expression and promoter-methylation in peripheral blood of patients suffering from major depressive disorder. J Affect Disord. 2011;131:186–192. doi: 10.1016/j.jad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. page following 696. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, Mignot E. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 2011;222:289–294. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson KM, Schroder CM, Bertschy G, Bourgin P. Complex interaction of circadian and non-circadian effects of light on mood: Shedding new light on an old story. Sleep Med Rev. 2012;16:445–454. doi: 10.1016/j.smrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Sunter D, Morgan I, Edwards CM, Dakin CL, Murphy KG, Gardiner J, Taheri S, Rayes E, Bloom SR. Orexins: effects on behavior and localisation of orexin receptor 2 messenger ribonucleic acid in the rat brainstem. Brain Res. 2001;907:27–34. doi: 10.1016/s0006-8993(01)02344-7. [DOI] [PubMed] [Google Scholar]
- Terman JS, Terman M, Schlager D, Rafferty B, Rosofsky M, Link MJ, Gallin PF, Quitkin FM. Efficacy of brief, intense light exposure for treatment of winter depression. Psychopharmacol Bull. 1990;26:3–11. [PubMed] [Google Scholar]
- Terman M, Amira L, Terman JS, Ross DC. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153:1423–1429. doi: 10.1176/ajp.153.11.1423. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–663. doi: 10.1017/s1092852900019611. quiz 672. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Workman JL, Nelson RJ. Potential animal models of seasonal affective disorder. Neurosci Biobehav Rev. 2011;35:669–679. doi: 10.1016/j.neubiorev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Wu MF, Nienhuis R, Maidment N, Lam HA, Siegel JM. Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch Ital Biol. 2011;149:492–498. doi: 10.4449/aib.v149i4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R, Gorman M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J Biol Rhythms. 2010;25:19–27. doi: 10.1177/0748730409352054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]