Summary
The anaphase-promoting complex/cyclosome (APC/C) is a protein-ubiquitin ligase (E3) that initiates the final events of mitosis by catalyzing the ubiquitination and proteasomal destruction of securin, cyclins, and other substrates [1, 2]. Like other members of the RING family of E3s [3, 4], the APC/C catalyzes direct ubiquitin transfer from an E2-ubiquitin conjugate (E2-Ub) to lysine residues on the protein substrate. The APC/C is activated at specific cell-cycle stages by association with an activator subunit, Cdc20 or Cdh1, which provides binding sites for specific substrate sequence motifs, or degrons. Activator might also stimulate catalytic activity [5, 6], but the underlying mechanisms are not known. Here, we dissected activator function using an artificial fusion substrate in which the N-terminal region of securin was linked to an APC/C core subunit. This fusion substrate bound tightly to the APC/C and was ubiquitinated at a low rate in the absence of activator. Ubiquitination of this substrate was stimulated by activator, due primarily to a dramatic stimulation of E2 sensitivity (Km) and catalytic rate (kcat), which together resulted in a 670-fold stimulation of kcat/Km. Thus, activator is not simply a substrate adaptor but also enhances catalysis by promoting a more efficient interaction with the E2-Ub. Interestingly, full E2 stimulation required activator interaction with degron motifs on the substrate. We conclude that formation of a complete APC/C-activator-substrate complex leads to a major enhancement of E2 efficiency, providing an unusual substrate-assisted catalytic mechanism that limits efficient ubiquitin transfer to specific substrates.
Results
Activator Stimulates Ubiquitination of a Securin-Apc10 Fusion Substrate
The APC/C is a large (1.2-1.5 MDa) complex composed of 13-14 subunits, and recent structural studies have unveiled the positioning of many of its key subunits (Figure S1) [1, 2]. Conserved cullin and RING subunits (Apc2 and Apc11) provide the presumed binding site for the E2-Ub. Substrate binding is mediated primarily by the activator subunit, which contains a long, disordered N-terminal region followed by a C-terminal WD40 domain that interacts with substrate degron motifs, including the D box and KEN box [7-11]. The D box is thought to be sandwiched between the activator WD40 domain and the core APC/C subunit Apc10/Doc1 [12-14] (Figure S1).
The goal of this work was to determine whether activator is simply a substrate adaptor or also promotes APC/C activity through other mechanisms. We constructed protein substrates that are recruited to the APC/C in the absence of activator, predicting that these substrates would be modified efficiently if activator serves solely as a substrate adaptor. We took advantage of our previous findings that purified Apc10 binds with high affinity to its normal site when added to purified yeast APC/C lacking this subunit [14, 15]. We engineered two fusion substrates in which yeast Apc10 was fused to the 110-residue N-terminal region of yeast securin (Pds1) or the 124-residue N-terminal region of the mitotic cyclin Clb2 (Figure 1A; Figure S1B). These unstructured substrate regions contain the D and KEN boxes that mediate APC/C recognition, as well as multiple lysines that are modified processively in APC/C reactions.
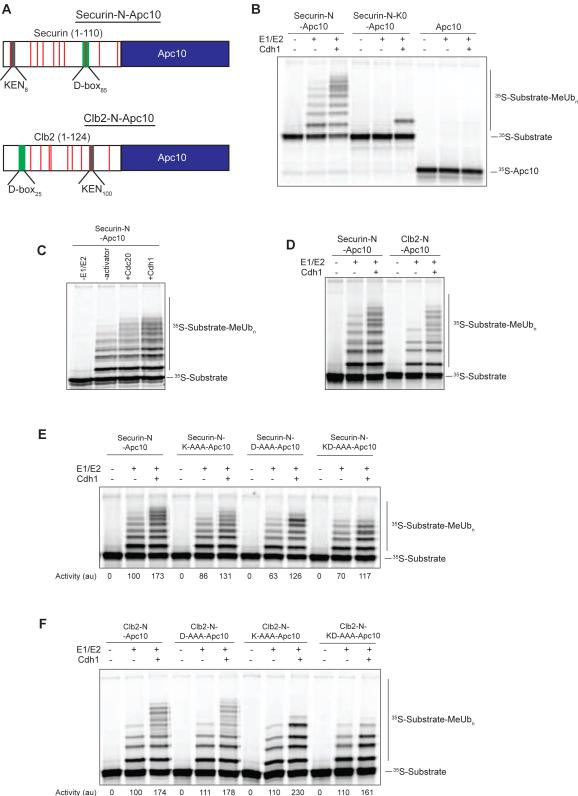
Figure 1. Fusion Substrate Ubiquitination by the APC/C.
(A) Schematic diagram of the securin-N-Apc10 and Clb2-N-Apc10 fusion substrates used in this study. Lysine residues in substrate regions are indicated by red bars. See also Figure S1B.
(B) APC/C (~5 nM) was immunoprecipitated from strains lacking Apc10 and Cdh1 and incubated with the indicated 35S-labeled substrates, translated in rabbit reticulocyte lysates. After washing, the APC/C-substrate complex was incubated with Cdh1 as indicated, and reactions were started by the addition of E1 (300 nM), E2 (50 μM Ubc4), and methyl-ubiquitin. Reaction products were analyzed by SDS-PAGE and autoradiography. Results are representative of two independent experiments.
(C) APC/C reactions were performed as in (B) with the securin-N-Apc10 fusion substrate, using either Cdc20 or Cdh1 as the added activator. Results are representative of three independent experiments. The greater stimulation by Cdh1 was likely due to the fact that this activator is generally more stable in vitro than Cdc20.
(D) APC/C reactions were performed with either the securin-N-Apc10 or Clb2-N-Apc10 fusion substrate, as in (B). Results are representative of three independent experiments.
(E, F) APC/C reactions were performed as in (B) with the indicated 35S-labeled fusion substrates, including mutant substrates in which KEN and/or D box residues were replaced with alanines (K-AAA and D-AAA mutants, respectively). Activity (bottom) reflects the total amount of ubiquitinated substrate, quantified with ImageQuant software and normalized relative to activity with wild-type fusion protein in the absence of activator (lanes 2). Results are representative of two independent experiments.
To analyze ubiquitin ligation to these fusion substrates, we immunopurified TAP-tagged APC/C on magnetic beads from fresh lysates of a yeast strain lacking Apc10 and the activator Cdh1. The APC/C-bound beads were incubated with an excess of radiolabeled fusion substrate produced by translation in vitro, and unbound substrate was washed away. Fusion substrate bound with normal high affinity to the Apc10-binding site of the APC/C (Figure S2). The APC/C-fusion substrate complex was incubated with a saturating amount (50 μM) of the E2, Ubc4, charged with methylated ubiquitin (MeUb), which blocks polyubiquitin chain assembly and greatly facilitates clear detection and quantification of reaction products [16]. The number of distinct products generated in these reactions reflects the number of lysines that have been modified on the substrate.
The securin fusion substrate was ubiquitinated at 5-6 lysines by the APC/C in the absence of added activator (Figure 1B, lane 2). This activity was not due to activator protein from the rabbit reticulocyte lysate used to produce the radiolabeled fusion substrate (Figure S3). In addition, this activity was not likely to be due to Cdc20 in the purified APC/C preparation: in all our previous work, APC/C isolated from asynchronous cdh1Δ cells displays no detectable activity toward any substrate. Finally, our later studies of E2 responsiveness (below) clearly indicate that this activator-independent activity is distinct from that seen in the presence of activator. Thus, we conclude that recruitment of substrate alone is sufficient to allow some ubiquitination.
Addition of the purified activator Cdh1 increased the rate of fusion-substrate ubiquitination, resulting in increased substrate turnover (i.e., total modified substrate) and increased formation of larger products (Figure 1B, lane 3). Ubiquitination was greatly reduced in reactions with a securin fusion substrate lacking all 10 lysine residues in the N-terminal fragment (Figure 1B). The single modification of the lysine-free substrate in the presence of activator likely occurred at the N-terminus of the substrate [17], as Apc10 alone was not ubiquitinated in the absence or presence of activator (Figure 1B). Thus, the securin fusion substrate was ubiquitinated at multiple lysines in the N-terminal securin region.
Both Cdc20 and Cdh1 stimulated securin fusion substrate ubiquitination (Figure 1C). We also observed activator-stimulated ubiquitination of a fusion protein containing the N-terminal region of Clb2 (Figure 1A, D).
We next analyzed securin fusion mutants in which key residues of the KEN box, D box, or both were mutated to alanine (KEN was changed to AAA, and the D box, RxxLxxxN, was changed to AxxAxxxA). For each mutant, we quantified APC/C activity by measuring substrate turnover: i.e., the total amount of ubiquitinated protein substrate in all protein bands above the unmodified protein on the autoradiographs. This method simply provides the rate at which the unmodified substrate in the lower band is ligated to the first methyl-ubiquitin only, and this rate is not affected by processivity or the rates at which additional ubiquitins are added to the substrate or to ubiquitin itself.
Mutations in the KEN and/or D box caused small but reproducible decreases in rates of fusion-substrate ubiquitination in the absence of activator (Figure 1E). Addition of activator caused a 1.5- to 2-fold increase in activity toward the wild-type substrate and all degron mutants, suggesting that intact degrons are not required for activator to stimulate initial ubiquitin attachment in the presence of saturating E2 concentrations. However, degron mutations, particularly in the D box, did reduce the processivity of substrate ubiquitination, as indicated by a lower number of modified lysines.
Similar KEN and D-box alanine mutations were made in the Clb2-Apc10 fusion substrate (Figure 1F). These mutations did not decrease the rate of ubiquitination of the Clb2-fusion substrate in the absence of activator and did not greatly affect the 1.5- to 2-fold stimulation of activity in the presence of activator. Mutation of the KEN box did cause a significant decrease in processivity. Thus, despite the fact that the fusion substrates are already linked tightly to the APC/C, their patterns of ubiquitination appear to be influenced by engagement of the KEN or D box, and the importance of each motif varies in different substrates.
Stimulation of E2 Efficiency by Activator
We next addressed the mechanism by which activator promotes fusion substrate ubiquitination. One intriguing possibility was suggested by previous studies of the ubiquitin ligase SCF, in which neddylation activates the enzyme by enhancing E2 affinity and catalytic rate [18, 19]. We hypothesized that activator binding to the APC/C might serve a similar function. Because our fusion substrate, unlike normal APC/C substrates, is ubiquitinated in the absence of activator, it provided a unique reagent for comparing E2 responsiveness in the absence and presence of activator.
We measured the initial velocity of APC/C activity toward fusion substrates over a range of E2 concentrations. As before, we quantified APC/C activity by measuring the turnover of unmodified fusion substrate into the first ubiquitinated form. Reactions were performed under conditions of excess E1, methylated ubiquitin, and ATP, resulting in a multiple-turnover reaction in which E2 is replenished with ubiquitin after each ubiquitin discharge. The APC/C, like other RING E3s, can be viewed as a two-substrate enzyme in which the protein substrate and E2-Ub are the two reactants, whose binding affinities both influence the reaction rate. However, because we measured activity with a tightly-bound (i.e. saturated) fusion-substrate that does not dissociate appreciably during the reaction, our reactions were performed under pseudo-first-order conditions in which the half-maximal E2 concentration should be equivalent to an apparent Km value for the E2-Ub substrate, which reflects E2-Ub affinity for the APC/C-substrate complex, rather than affinity for the APC/C alone.
It should also be noted that in these reactions it is the E2-Ub, and not the securin fusion protein, that is the ‘substrate’ whose concentration is being titrated and whose concentration should remain constant for accurate determination of apparent Km. Because these reactions are performed under conditions of considerable E2 excess over the low APC/C concentration (~1 nM), and because E2-Ub is regenerated by E1, there is unlikely to be significant depletion of E2-Ub during these reactions.
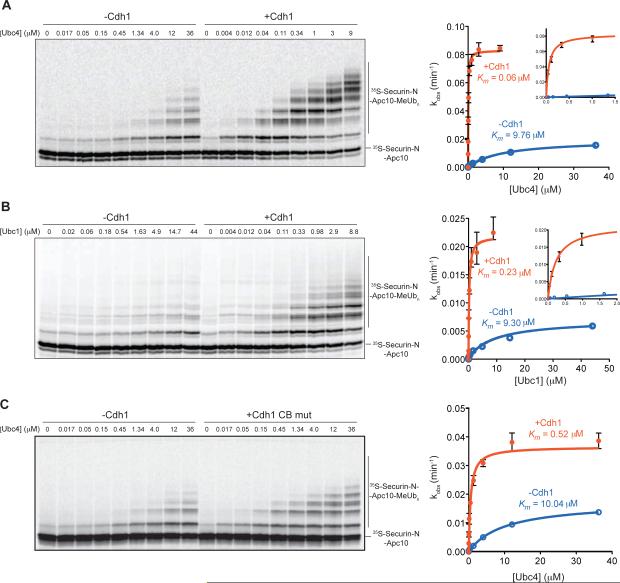
Addition of activator resulted in a striking increase in E2 sensitivity: the apparent Km for Ubc4 decreased about 160-fold (Figure 2A; Table S1). As seen in our earlier experiments with saturating E2 concentrations (Figure 1), activator also enhanced the maximal rate of substrate ubiquitination (4-fold stimulation of kcat; Table S1). The combined effect was a remarkable 670-fold increase in kcat/Km.
Figure 2. Activator Stimulateskcat/Km of Ubc4 and Ubc1.
(A, B) APC/C (~1 nM) reactions were performed as in Figure 1B with 35S-labeled securin-NApc10 fusion substrate, in the absence (left) or presence (right) of wild-type Cdh1. Purified E2 was titrated into the reactions at the indicated concentrations. Reactions with Ubc4 (A) were incubated for 20 min in the absence of Cdh1 and 10 min in the presence of Cdh1, and reactions with Ubc1 (B) were incubated for 30 min. The rate of substrate turnover (kobs) was determined by dividing the moles of total substrate modified per minute by the moles of APC/C. Values represent the means (+/− SEM) from three independent experiments. Data were analyzed using Prism and fit using Michaelis-Menten parameters to determine the apparent Km and maximal kobs (kcat), which are summarized in Table S1. Insets show close-ups of activity at lower E2 concentrations.
(C) APC/C reactions were performed at various Ubc4 concentrations as in (A), in the absence or presence of a mutant form of Cdh1 in which critical residues of the C-box were mutated (I57A, P58A). Data from three independent experiments (means +/− SEM) are presented, and the apparent Km and maximal kobs (kcat) are summarized in Table S1.
We also analyzed Ubc1, an APC/C E2 that is specialized for lysine 48-linked polyubiquitin chain assembly and has relatively low activity toward unmodified substrates [16, 20]. The Km for Ubc1 was also reduced by activator, in this case about 40-fold, and catalytic rate increased 3-fold, resulting in a 137-fold increase in kcat/Km (Figure 2B; Table S1).
Previous studies suggest that the N-terminal region of the activator promotes APC/C activity, and stimulation in those studies depended on a short linear sequence motif in this region called the C-box [5, 6] (Figure S1). Consistent with these results, we found that mutation of two key residues in the Cdh1 C-box (I57A, P58A) [21] greatly reduced the stimulatory effect of Cdh1 on the efficiency of Ubc4 (only a 43-fold increase in kcat/Km; Figure 2C; Table S1). Consistent with previous studies, we also found that the C-box mutation caused a 4-fold reduction in activator binding to the APC/C under these conditions (Figure S4) [22]. Thus, reduced stimulation by this activator mutant is due in part to a reduction in activator binding.
Substrate Binding is Required for Full Stimulation of E2 Sensitivity by Activator
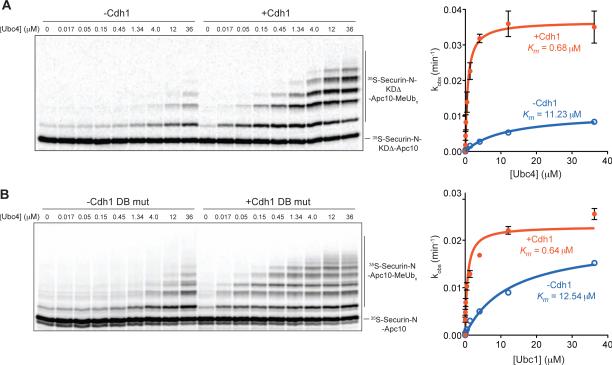
Our initial studies of the securin and Clb2 fusion substrates (Figure 1E, F) hinted that the stimulation of APC/C activity by activator is influenced by substrate degron motifs. We addressed this possibility in more detail by measuring E2 responsiveness with a securin fusion substrate from which both the 3-residue KEN box and nine-residue D box were deleted. Similar results were obtained with a fusion substrate carrying alanine mutations in both degrons (data not shown). Consistent with the results we obtained under saturating E2 conditions (Figure 1E), we found that the degron mutations caused a 2-fold decrease in maximal activity (kcat) in the absence of activator (Figure 3A; Table S1). Also consistent with the studies in Figure 1E, degron mutations had only a mild effect on the stimulation of kcat by activator (3-fold stimulation, compared to a 4-fold stimulation with wild-type substrate). Most importantly, we observed that degron mutations greatly reduced the stimulatory effect of activator on the Ubc4 Km (16-fold decrease in Km, compared to the 160-fold decrease with wild-type substrate; Figure 3A; Table S1). Thus, full activator stimulation of E2 Km depends on substrate degron motifs.
Figure 3. Stimulation of E2 Depends on Substrate Binding.
(A, B) APC/C (~1 nM) reactions were performed at various Ubc4 concentrations, as in Figure 2A, using 35S-labeled securin-N-Apc10 fusion substrate in the absence (left) or presence (right) of Cdh1. The experiments in (A) were performed with a mutant fusion substrate from which both the 3-residue KEN box and nine-residue D box were deleted. The experiments in (B) were performed with a mutant form of Cdh1 in which a key residue involved in D-box binding was mutated (V279M). For both panels, data from three independent experiments (means +/− SEM) are presented, and the apparent Km and maximal kobs (kcat) are summarized in Table S1.
Recent structural work has pinpointed the residues on the Cdh1 WD40 domain that interact with the RxxL sequence of the D box, and mutation of these residues reduces substrate binding [8, 9]. We found that mutation of a key residue (V279M) in the leucine-binding pocket of Cdh1 reduced the effect of activator on E2 Km, resulting in only a 20-fold decrease (Figure 3B; Table 1). These results are consistent with a role for D-box binding in the stimulatory effects of activator, although we cannot rule out the possibility that the defect in this mutant is due in part to decreased activator binding affinity for the APC/C.
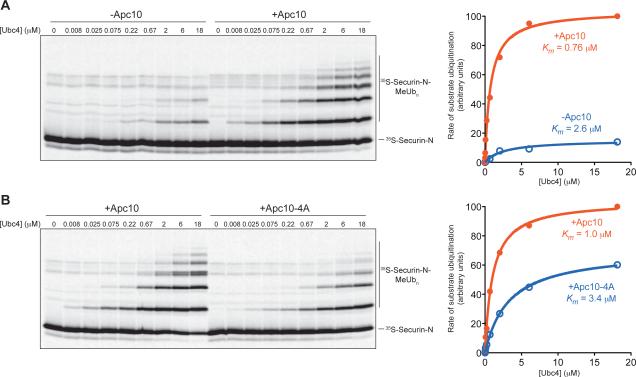
We addressed the role of the D box further by measuring E2 responsiveness in the absence of Apc10, which is known to contribute to D-box binding [12-14]. To test the effect of Apc10 deletion, we could not use the fusion substrate and instead measured activity toward a conventional soluble substrate: the N-terminal 110 residues of securin [23]. We compared the activity of APC/C-Apc10Δ in the absence and presence of excess purified Apc10. Activator was present in all reactions, because activity toward a soluble substrate is not detectable in the absence of activator. As seen previously [15], APC/C activity in the absence of Apc10 was very low and non-processive (Figure 4A). Addition of Apc10 stimulated substrate ubiquitination and reduced the apparent Km for Ubc4 about 3-fold. We also tested APC/C activity toward the soluble securin fragment using the Apc10-4A mutant protein, which carries mutations in a cluster of four residues that are required for Apc10 binding to the D box [14]. In reactions with the Apc10-4A protein, we found that the apparent Km for Ubc4 was 3-fold higher than that with wild-type Apc10 (Figure 4B). We conclude that Apc10 is required for full E2 responsiveness with the securin substrate, likely through its role in binding the substrate D box.
Figure 4. Activator Stimulation of Ubc4 Depends on Apc10.
(A) APC/C-Apc10Δ (~1 nM) reactions were performed as in Figure 2A with 35S-labeled securin N-terminal fragment (residues 1-110) translated in reticulocyte lysate and purified. Purified Cdh1, also produced by translation in vitro, was added to all reactions. Recombinant Apc10 was added to a final concentration of 20 μM to half the reactions as indicated, and purified Ubc4 was titrated into the reactions. Data were analyzed using Prism and fit using Michaelis-Menten parameters to determine the half-maximal E2 concentration, or apparent Km. Results are representative of two independent experiments.
(B) APC/C reactions were performed with soluble securin fragment as in (A), using immunopurified wild-type APC/C (left panel) or APC/C carrying the Apc10-4A mutant subunit (right panel). Results are representative of two independent experiments. APC/C-Apc10-4A activity is more robust than the activity of APC/C lacking Apc10 (A) because deletion of Apc10 causes a nonspecific loss of activity [15].
The apparent Km for Ubc4 in reactions with soluble securin (~1 μM in the presence of activator and Apc10; Figure 4A, B) is similar to that seen in our previous studies with soluble substrates [16] but is considerably higher than the Km for Ubc4 in our reactions with fusion substrate plus activator (~0.06 μM; Figure 2A; Table 1). This difference presumably results from the fact that substrate is far below half-maximal concentrations in reactions with soluble substrate, in which case the apparent E2 Km reflects E2-Ub affinity for the APC/C-activator complex rather than the APC/C-activator-substrate complex. These data argue that E2-Ub has a higher affinity in the presence of substrate, consistent with our other evidence that substrate degrons are required for maximal E2 stimulation by activator.
Discussion
We conclude that the binding of activator to the APC/C enhances E2 efficiency by multiple mechanisms. These mechanisms are reminiscent of the effects of neddylation on SCF activity [18, 19] and might therefore reveal conserved general mechanisms in ubiquitin ligase regulation. The dramatic decrease in the Km for E2 upon activator binding suggests that the major effect of activator is to increase the affinity of the interaction between APC/C and E2-Ub. The relatively small increase in APC/C turnover rate at saturating E2 concentrations is less easily explained, but could result from changes in E2-Ub orientation or positioning near the substrate [3, 18, 19], or from some change in the ability of the RING domain to stimulate E2 activity [24-26]. How might the activator elicit these effects? Previous studies in Xenopus extracts suggest that the N-terminal region of the activator is responsible for promoting ubiquitination of a substrate, Nek2A, that associates with the APC/C in the absence of activator [6]. Consistent with these studies, we found that the C box of the N-terminal region is required for the stimulatory effect of activator. Interestingly, high-resolution EM studies of human APC/C structures have recently revealed that the activator N-terminal region promotes a major shift in the positioning of the E2-binding site formed from the cullin and RING subunits [27]. These conformational changes are consistent with the shifts in E2 biochemical behavior that we observe in the presence of activator.
We observed that mutation of substrate degrons greatly reduces the impact of activator on the Km for E2, suggesting that degron binding promotes an increase in E2 affinity. How might this occur? Perhaps substrate binding to the activator C-terminal WD40 domain influences the ability of the activator N-terminal region to exert its effects on the E2-binding site. Given that substrate enhances activator binding to the APC/C [28], one possibility is that the fusion substrate locks down the WD40 domain on the APC/C, leading to higher affinity binding by the N-terminal region.
It seems likely that substrate degron binding also influences activity through E2-independent mechanisms. Interaction of the fusion substrate with activator should restrain the mobility of the large substrate N-terminal region, promoting more productive substrate positioning and thereby enhancing catalytic rate. In addition, substrate-activator interactions might affect the accessibility of certain lysines, as suggested by the changes in patterns of lysine modification in reactions with some degron mutants (Figure 1E, F). Interestingly, in the case of securin at least, the D box stimulated fusion substrate ubiquitination even in the absence of activator (Figure 1E; Table S1), perhaps as a result of the low affinity interaction between the D box and Apc10 alone [12].
Our results are most readily explained by a model in which the activator, together with Apc10, helps position the substrate for maximal activity while also triggering changes in the E2-binding site that promote ubiquitin transfer. Optimal E2 function and substrate ubiquitination depend on a cooperative network of protein contacts between all components: APC/C, activator, substrate, and E2-Ub. By ensuring that full E2 activity is unleashed only in the presence of the APC/C-activator-substrate complex, this system could provide an important mechanism for ensuring that only the appropriate substrate is modified––an important feature for an enzyme whose substrates are irreversibly destroyed.
Supplementary Material
Highlights.
APC/C ubiquitinates a bound substrate in the absence of activator subunit.
Activator increases the rate of substrate ubiquitination.
Activator stimulates E2 sensitivity and catalytic rate.
Stimulation of E2 efficiency by activator depends on substrate degrons.
Acknowledgements
We thank Juliet Girard, Scott Foster, Mary Matyskiela, Geeta Narlikar, and members of the Morgan lab for valuable discussions and reagents, and David Barford for sharing results prior to publication. This work was supported by a Graduate Research Fellowship from the National Science Foundation and by funding from the National Institute of General Medical Sciences (R37-GM053270).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental Information includes four figures, a table, supplemental experimental procedures, and supplemental references.
References
- 1.Barford D. Structure, function and mechanism of the anaphase promoting complex (APC/C). Q. Rev. Biophys. 2011;44:153–190. doi: 10.1017/S0033583510000259. [DOI] [PubMed] [Google Scholar]
- 2.Primorac I, Musacchio A. Panta rhei: the APC/C at steady state. J. Cell Biol. 2013;201:177–189. doi: 10.1083/jcb.201301130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 4.Metzger MB, Pruneda JN, Klevit RE, Weissman AM. RING-type E3 ligases: master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim. Biophys. Acta. 2014;1843:47–60. doi: 10.1016/j.bbamcr.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- 6.Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol. Cell. 2008;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Chao WC, Kulkarni K, Zhang Z, Kong EH, Barford D. Structure of the mitotic checkpoint complex. Nature. 2012;484:208–213. doi: 10.1038/nature10896. [DOI] [PubMed] [Google Scholar]
- 9.He J, Chao WC, Zhang Z, Yang J, Cronin N, Barford D. Insights into degron recognition by APC/C coactivators from the structure of an Acm1-Cdh1 complex. Mol. Cell. 2013;50:649–660. doi: 10.1016/j.molcel.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 11.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 12.da Fonseca PC, Kong EH, Zhang Z, Schreiber A, Williams MA, Morris EP, Barford D. Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature. 2011;470:274–278. doi: 10.1038/nature09625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buschhorn BA, Petzold G, Galova M, Dube P, Kraft C, Herzog F, Stark H, Peters JM. Substrate binding on the APC/C occurs between the coactivator Cdh1 and the processivity factor Doc1. Nat. Struct. Mol. Biol. 2011;18:6–13. doi: 10.1038/nsmb.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll CW, Enquist-Newman M, Morgan DO. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 2005;15:11–18. doi: 10.1016/j.cub.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 15.Carroll CW, Morgan DO. The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nat. Cell Biol. 2002;4:880–887. doi: 10.1038/ncb871. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Ciechanover A, Ben-Saadon R. N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol. 2004;14:103–106. doi: 10.1016/j.tcb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha A, Deshaies RJ. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol. Cell. 2008;32:21–31. doi: 10.1016/j.molcel.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigo-Brenni MC, Foster SA, Morgan DO. Catalysis of lysine 48-specific ubiquitin chain assembly by residues in E2 and ubiquitin. Mol. Cell. 2010;39:548–559. doi: 10.1016/j.molcel.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton BR, Ng TM, Matyskiela ME, Carroll CW, Morgan DO, Toczyski DP. An architectural map of the anaphase-promoting complex. Genes Dev. 2006;20:449–460. doi: 10.1101/gad.1396906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab M, Neutzner M, Mocker D, Seufert W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 2001;20:5165–5175. doi: 10.1093/emboj/20.18.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaefer JB, Morgan DO. Protein-linked ubiquitin chain structure restricts activity of deubiquitinating enzymes. J. Biol. Chem. 2011;286:45186–45196. doi: 10.1074/jbc.M111.310094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dou H, Buetow L, Sibbet GJ, Cameron K, Huang DT. BIRC7-E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat. Struct. Mol. Biol. 2012;19:876–883. doi: 10.1038/nsmb.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plechanovova A, Jaffray EG, Tatham MH, Naismith JH, Hay RT. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature. 2012;489:115–120. doi: 10.1038/nature11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruneda JN, Littlefield PJ, Soss SE, Nordquist KA, Chazin WJ, Brzovic PS, Klevit RE. Structure of an E3:E2~Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol. Cell. 2012;47:933–942. doi: 10.1016/j.molcel.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang L, Zhang Z, Yang J, McLaughlin SH, Barford D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature. 2014 doi: 10.1038/nature13543. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matyskiela ME, Morgan DO. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol. Cell. 2009;34:68–80. doi: 10.1016/j.molcel.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.