Abstract
Evidence contributing to the understanding of neurobiological mechanisms underlying appetite dysregulation in anorexia nervosa draws heavily on separate lines of research into neuroendocrine and neural circuitry functioning. In particular, studies consistently cite elevated ghrelin and abnormal activation patterns in homeostatic (hypothalamus) and hedonic (striatum, amygdala, insula) regions governing appetite. The current preliminary study examined the interaction of these systems, based on research demonstrating associations between circulating ghrelin levels and activity in these regions in healthy individuals. In a cross-sectional design, we studied 13 women with active anorexia nervosa (AN), 9 women weight-recovered from AN (AN-WR), and 12 healthy-weight control women using a food cue functional magnetic resonance imaging paradigm, with assessment of fasting levels of acylated ghrelin. Healthy-weight control women exhibited significant positive associations between fasting acylated ghrelin and activity in the right amygdala, hippocampus, insula, and orbitofrontal cortex in response to high-calorie foods, associations which were absent in the AN and AN-WR groups. Women with AN-WR demonstrated a negative relationship between ghrelin and activity in the left hippocampus in response to high-calorie foods, while women with AN showed a positive association between ghrelin and activity in the right orbitofrontal cortex in response to low-calorie foods. Findings suggest a breakdown in the interaction between ghrelin signaling and neural activity in relation to reward responsivity in AN, a phenomenon that may be further characterized using pharmacogenetic studies.
Keywords: Appetite, Reward, fMRI, Hormones, Eating disorders
1. Introduction
Mounting evidence supports disruption of food motivation and reward circuitry functioning in women with anorexia nervosa (AN) (Ellison et al., 1998; Uher et al., 2003, 2004; Santel et al., 2006; Wagner et al., 2007, 2008; Kaye et al., 2009; Fladung et al., 2010; Gizewski et al., 2010; Cowdrey et al., 2011; Joos et al., 2011; Frank et al., 2012; Holsen et al., 2012). Functional neuroimaging studies that directly compare active and recovered patients with healthy-weight controls offer insight into state vs. potential “trait” features of the neural signature of AN (Uher et al., 2003; Holsen et al., 2012). Importantly, the studies indicate that dysfunction in these circuits extends beyond the acute effects of severe self-starvation (Uher et al., 2003; Wagner et al., 2008; Holsen et al., 2012), and may serve as a marker for vulnerability to relapse. Similar state and trait characteristics typify the neuroendocrine profile associated with AN. For example, levels of acylated ghrelin (an orexigenic neuropeptide mainly produced in the stomach) are abnormally high in active AN, and secretion may remain elevated in women after weight gain (Nakahara et al., 2007). However, despite data from human and animal studies demonstrating the effects of ghrelin on activity in the brain (Olszewski et al., 2003a; Malik et al., 2008; Kroemer et al., 2012; Li et al., 2012), little is known regarding the interplay between circulating peptides and neural functioning in active and recovered phases of AN.
A growing body of studies demonstrate evidence of neural dysfunction in AN, with reports citing abnormal patterns of activation in regions implicated in reward, appetitive motivation, and integration of the visceral state with emotion processing. Although the directionality of the effect (i.e., hyper- or hypo-activation vs. healthy-weight controls) is often mixed, the nucleus accumbens (Wagner et al., 2007; Fladung et al., 2010; Frank et al., 2012; Holsen et al., 2012), amygdala (Ellison et al., 1998; Gizewski et al., 2010; Joos et al., 2011; Holsen et al., 2012), and insula (Ellison et al., 1998; Wagner et al., 2008; Gizewski et al., 2010; Frank et al., 2012; Holsen et al., 2012) have emerged as candidate regions in AN, both with regard to state abnormalities in women with active AN (Uher et al., 2003; Santel et al., 2006; Fladung et al., 2010; Gizewski et al., 2010; Joos et al., 2011; Frank et al., 2012; Holsen et al., 2012) and persistent dysfunction following weight gain in women weight-restored from AN (Uher et al., 2003, 2004; Holsen et al., 2005; Wagner et al., 2007, 2008). Although some studies focused solely on reward functioning have included trials with negatively valenced hedonic value (Wagner et al., 2007; Fladung et al., 2010), most investigations on neural response to food stimuli have probed functioning in these regions using stimuli of a high hedonic value (high-calorie foods). Thus, while reward circuitry dysfunction is now well established in AN, the degree to which the neural response to food stimuli of varying reward value may be abnormal in this population is unknown. Foods of low caloric value, for example, may still signal homeostatic relevance (and in AN, potentially hedonic relevance), especially in a state of starvation. Using a neuroanatomical approach, we previously demonstrated significant hypoactivation in both hedonic [amygdala, hippocampus, orbitofrontal cortex (OFC), and insula] and homeo-static (hypothalamus) food-motivation regions in response to high-calorie foods (vs. objects) in a fasting state in women with active AN and those with AN-WR, compared with healthy-weight controls (Holsen et al., 2012). We additionally found that cortisol levels accounted for substantial variability between AN, AN-WR and healthy-weight control groups in blood oxygenation level dependent (BOLD) activation in response to high-calorie foods in the insula (Lawson et al., 2012). In particular, our results of state and trait hypoactivation in the hypothalamus (Holsen et al., 2012) provided the first evidence of dysfunction in this region in AN, and further suggested a link between abnormal appetitive drive and well-established neuroendocrine disruption in AN.
Among the neuroendocrine systems controlling human appetite, studies of active AN have consistently implicated elevated ghrelin in the pathophysiology of abnormal appetite in this disorder (e.g., see Prince et al., 2009; Ogiso et al., 2011; Yi et al., 2011; Mequinion et al., 2013). Ghrelin is a potent orexigenic peptide, with levels rising sharply just before meals and falling to nadir within an hour after eating (Cummings et al., 2001; Shiiya et al., 2002). Produced largely in the stomach and secreted in a pulsatile pattern (Tolle et al., 2002), ghrelin circulates as highly unstable acylated (“active”) forms of ghrelin, which bind to post-synaptic ghrelin receptors (GHS-R1a), and stable des-acylated ghrelin (Hosoda et al., 2006; Soares and Leite-Moreira, 2008). Des-acylated ghrelin is less well characterized and does not have activity at the GHS-R1a receptor, but also appears to be biologically active. While orexigenic activity of acylated ghrelin is well established, the role of des-acylated ghrelin in food intake is less clear. Females with active AN demonstrated elevated total (acylated+des-acylated) ghrelin in a fasted state (Misra et al., 2004; Janas-Kozik et al., 2007; Monteleone et al., 2008; Karczewska-Kupczewska et al., 2010; Sedlackova et al., 2011), in response to a mixed meal (Nakahara et al., 2007; Sedlackova et al., 2011, 2012) and in the 24-h circadian profile (Germain et al., 2007, 2010), which has been shown to be due to increased secretory burst mass and amplitude (Misra et al., 2005). Excessive secretion of acylated ghrelin has also been reported in women with AN (Germain et al., 2010; Koyama et al., 2010). Short-term weight gain in AN is generally associated with decreases in overnight total ghrelin secretion (Misra et al., 2005), and in fasting (Janas-Kozik et al., 2007) and postprandial (Otto et al., 2005) levels of total ghrelin, with most studies focused on changes within individuals in the symptomatic AN group, limiting the ability to examine normalization following treatment. One study, however, reported decreases but not normalization of postprandial total ghrelin in women with AN compared with healthy-weight controls (Nakahara et al., 2007), suggesting that abnormalities may persist after short-term recovery. Whether there are changes in ghrelin secretion, in particular the active forms, after long-term weight recovery from AN is unknown and would be particularly important in thinking about disease pathophysiology.
Ghrelin acts primarily on neuropeptide Y (NPY) and proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus, and ghrelin receptors have been identified in other hypothalamic nuclei [paraventricular nucleus (PVN), ventromedial nucleus (VMN)] (Howard et al., 1996; Guan et al., 1997; Nakazato et al., 2001; Hosoda et al., 2006). Beyond its role in the coordination of homeostatic hunger signaling, ghrelin accesses the mesolimbic reward circuitry through receptors on dopaminergic neurons in the ventral tegmental area (VTA), hippocampus, and amygdala (Nakazato et al., 2001; Lawrence et al., 2002; Olszewski et al., 2003a, 2003b; Alvarez-Crespo et al., 2012), providing a possible pathway that mediates the relationship between appetite dysregulation and reward circuitry dysfunction in AN. For example, in animal models, ghrelin administration in the nucleus accumbens increased food intake by a factor of six, and in the VTA by a factor of 11 (Naleid et al., 2005), with injection into the shell of the nucleus accumbens resulting in increased dopamine levels (Quarta et al., 2009). Further, in humans, intravenous ghrelin administration heightened neural activation in the VTA, amygdala, insula, and OFC in response to food images (Malik et al., 2008). Relationships between endogenous ghrelin and brain activity in healthy-weight individuals provide critical evidence that peripheral levels are associated with central nervous system target action. In particular, fasting total ghrelin was positively related to activity in the hypothalamus, amygdala, and prefrontal cortex in response to palatable food stimuli during a state of hunger (Kroemer et al., 2012), and in response to varying macronutrient preloads in the amygdala, insula, and OFC (Li et al., 2012). Thus, in healthy-weight individuals, peripheral ghrelin may be involved in the brain response to homeostatic (through action in the hypothalamus) and hedonic (through action on the amygdala, insula, and OFC) aspects of food intake. The nature of these associations in women with AN, however, remains unknown.
With an overall goal of delineating the complex pathophysiology of appetite disruption in AN, the current study was aimed at determining the relationship between abnormal peripheral ghrelin and hypoactivation in homeostatic and hedonic food motivation circuitry in women with active and weight-restored AN compared with healthy-weight controls. Given the heightened discrepancy between self-reported lack of hunger and objective state of starvation in AN (Robinson et al., 1983; Huse and Lucas, 1984; Robinson, 1989; Hetherington and Rolls, 1991), these combined approaches provide the advantage of representing unbiased measures of hunger and response to food cues, allowing for greater validity in our results and interpretation. We hypothesized that healthy-weight control women would demonstrate signifi-cant relationships between acylated ghrelin and BOLD activity in homeostatic (hypothalamus) and hedonic (nucleus accumbens, amygdala, hippocampus, insula, and OFC) circuitry in response to food stimuli in a fasting state, and further that the direction of these relationships would vary across stimuli of different hedonic value: positive associations for high-calorie foods and negative relationships in response to low-calorie foods. Based on our previous findings of significant hypoactivation in response to high-calorie foods in women with active AN and women who were weight-recovered from AN (AN-WR) (Holsen et al., 2012), and evidence in prior AN samples of abnormal ghrelin levels (Misra et al., 2004, 2005), we additionally hypothesized that this pattern of associations would be disrupted in women with active AN and ANWR.
2. Methods
2.1. Subjects
Female participants were a community sample of women with anorexia nervosa—restricting type (n=13; AN group) or with a history of anorexia, weight-recovered (n=10; AN-WR group) between the ages of 19–28, recruited from surrounding treatment centers and the community through advertisements. [Subject characteristics and fMRI results related to the contrast of high-calorie food > objects have been previously reported (Holsen et al., 2012; Lawson et al., 2012, 2013). However, ghrelin levels, functional magnetic resonance imaging results related to the contrast of low-calorie foods > objects, and relationships between ghrelin and neural activation have not been described previously.] All subjects met diagnostic criteria for anorexia nervosa, either present or past, according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV). Diagnoses were made using the Structured Clinical Interview for the DSM-IV, administered by a trained psychiatric nurse practitioner or doctoral level clinical psychologist. All AN-WR women had regular menses. Weight recovery was defined as maintenance of 90–110% ideal body weight (which takes into account height, weight, and frame size) for at least 6 months (Metropolitan Life Foundation, 1983); AN-WR women had been in recovery for an average of 3.7 ± 3.0 (mean±SD) years. Exclusion criteria included use of hormones, history of psychosis, objective binging/ purging behaviors > once/month within the last 3 months, history of diabetes mellitus, active substance abuse, contraindication to magnetic resonance imaging, and past gastrointestinal tract surgery. A control group of healthy-weight women (n=13; HC group) with regular menses, no pubertal delay, and 90–110% ideal body weight were recruited from the community. Exclusion criteria were the same as for the AN groups, with the following exceptions: history of any objective binging/ purging behaviors, amenorrhea, excessive exercise within the last 3 months, and any psychiatric disorder. After complete description of the study to the participants, written informed consent was obtained. The study was approved by the Partners HealthCare Institutional Review Board.
2.2. Procedures
Subjects arrived at the Massachusetts General Hospital Clinical Research Center having fasted for 12 h. HC and AN-WR women presented during the follicular phase of the menstrual cycle (days 1–10). Subjects underwent a fasting functional magnetic resonance imaging (fMRI) session at 08:00 h. Subjects rated their appetite using visual analog scales before and after the fMRI scanning session. Fasting blood samples were drawn on ice and immediately spun at around 09:00 h by trained nursing staff. Plasma samples were stored at –80 °C in plastic tubes containing a 10-mg/ml solution of PMSF (phenylmethanesulfonyl fluoride) in methanol. Acylated ghrelin levels were determined using a radioimmunoassay (LINCO Research, St. Charles, MO, USA; intra-assay coefficient of variation (CV) 6.5–9.5%; inter-assay CV 9.6–16.2%). The distribution of acylated ghrelin levels was significantly skewed, and raw values were therefore log-transformed. Given the sensitivity of planned brain-ghrelin association analyses to outliers, within-group distributions of log-transformed acylated ghrelin were examined for extreme values, and participants with log-transformed values greater than 2 SD above the group mean were excluded, yielding a final sample of n=13 AN, n=12 HC, and n=9 AN-WR.
2.3. Behavioral and hormone data analysis
Behavioral and hormone data were analyzed using SPSS software (version 19; SPSS, Inc., Chicago, IL, USA). Between-group differences were assessed using independent samples t-tests, χ2 tests, or overall analysis of variance (ANOVA) with significantly different values compared by Fisher's Least Significant Difference Test. Pearson correlations were used to quantify relationships between hormones and appetite ratings in each group, and statistical differences between correlations quantified using r to Z Fisher transformation. A P value of <0.05 was designated for statistical significance.
2.4. fMRI paradigm
A visual food stimuli task [validated in previous publications (Holsen et al., 2012; Lawson et al., 2012)], during which subjects viewed high- and low-calorie food stimuli, non-food stimuli (household objects), and fixation stimuli in a block design was presented while subjects underwent standard gradient echo planar functional imaging on a Siemens 3T Trio. For additional experimental setup details, see our earlier report (Holsen et al., 2012).
2.5. fMRI data analysis
The fMRI data were preprocessed using Statistical Parametric Mapping (SPM8) (Wellcome Department of Cognitive Neurology, 2008) and custom routines in MATLAB (Mathworks, Inc., 2000) (see Holsen et al., 2012). For single subject analyses, a generalized linear model (GLM) was applied to the fMRI data of each subject, with anticipated hemodynamic responses to visual stimuli (high-calorie foods, low-calorie foods, objects) convolved with a canonical hemodynamic response function and subsequently used as regressors. Specific comparisons of interest (high-calorie foods vs. objects; low-calorie foods vs. objects) were tested using linear contrasts, and SPM maps were created based on these contrasts. Results from the single subject level for these contrasts of interest were submitted to a second level random effects regression analysis to examine the relationship between fasting acylated ghrelin and brain activity in response to foods of varying reward value in the fasting state. For each group (HC, AN, and AN-WR), acylated ghrelin levels entered as the covariate of interest, specific relationships of interest (positive association, negative association) were tested using linear contrasts, and SPM maps were created based on these contrasts. These effects of interest were examined in each group using the small-volume correction approach in SPM8, which limits voxel-wise analyses to voxels within a priori regions of interest [ROIs: hypothalamus, nucleus accumbens, amygdala, hippocampus, orbitofrontal cortex (OFC), and anterior insula]. Anatomical borders of hypothesized regions were defined using a manually segmented Montreal Neurological Institute-152 brain and implemented as overlays on the SPM8 canonical brain using the Wake Forest University PickAtlas (Maldjian et al., 2003) toolbox for SPM. False positives were controlled using family-wise error (FWE) correction. Within an anatomical ROI, significant results identified using small volume correction (initial voxel-wise height threshold: P < 0.05 uncorrected) were reported as significant if they additionally met the peak level threshold of P < 0.05, FWE-corrected. The a priori ROI approach was guided by our published fMRI results in these women (Holsen et al., 2012), previous findings in the literature on ghrelin–brain activity relationships (Malik et al., 2008; Kroemer et al., 2012; Li et al., 2012) and by studies providing evidence of GHS-R1 receptor localization (Nakazato et al., 2001; Lawrence et al., 2002; Olszewski et al., 2003a, 2003b; Alvarez-Crespo et al., 2012). Although these regions were the main focus of this study, we also report results at the whole brain level at a more conservative threshold of P < 0.001, FWE-corrected across whole brain.
Finally, percent signal change (psc) values were obtained and used to fully quantify brain-ghrelin relationships. First, using the MarsBaR toolbox in SPM, 4-mm spheres drawn around the peak voxel identified areas significant within each anatomical ROI for the within-group contrasts for positive and negative associations between ghrelin and brain activity. Next, using the REX toolbox (Whitfield-Gabrieli, 2009), psc values within these 4-mm spheres were extracted for each subject from their individual (first level) statistical parameter maps. The psc values for individual subjects values were then exported to SPSS for calculation of Pearson correlation coefficients within each group and tests of statistical differences between correlations quantified using r to Z Fisher transformation (see Section 2.3).
3. Results
3.1. Subject characteristics
Groups were comparable in age and handedness (see Table 1). AN-WR and HC groups had similar body-mass index (BMI) and percent ideal body weight (%IBW), while women with AN had significantly lower BMI and %IBW compared with both the HC and AN-WR groups. The AN and AN-WR groups did not significantly differ in age of symptom onset, duration of illness, rate of current medication treatment, or rate of current/past comorbid diagnoses. More women with AN-WR had a history of purging behavior than women with AN. Groups differed significantly on the Eating Disorders Examination-Questionnaire (EDE-Q) ratings, with HC women endorsing the fewest symptoms, women with AN-WR exhibiting intermediate ratings, and women with AN scoring the highest. HC women had significantly lower scores on the Beck Depression Inventory (BDI) than the AN and AN-WR groups, who did not differ significantly from each other. Women with AN-WR had been weight-recovered and menses-recovered for an average of over 3 years, indicating they were in long-term recovery. There were no significant differences between groups on smoking status.
Table 1.
Demographic and clinical characteristics.
| Characteristic | Group | Between-group comparisonsa | |||||
|---|---|---|---|---|---|---|---|
| HC (n=12) | AN (n=13) | AN-WR (n=9) | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age | 21.9 | 1.6 | 21.9 | 2.6 | 23.2 | 2.3 | HC vs. AN: t(23) = 0.08, P=0.936 |
| HC vs. AN-WR: t(19) =.54, P=0.141 | |||||||
| AN vs. AN-WR: t(20) =.27, P=.219 | |||||||
| % IBW | 97.6 | 6.9 | 81.7 | 4.0 | 99.1 | 10.8 | HC vs. AN: t(23) =7.17, P<0.001 |
| HC vs. AN-WR: t(19) =0.39, P=.700 | |||||||
| AN vs. AN-WR: t(20) =5.38, P=.001 | |||||||
| BMI | 22.5 | 1.4 | 18.1 | 0.8 | 22.2 | 2.3 | HC vs. AN: t(23) =10.08, P<0.001 |
| HC vs. AN-WR: t(19) =0.41, P=.689 | |||||||
| AN vs. AN-WR: t(20) = 6.08, P < 0.001 | |||||||
| Age at symptom onset | 16.8 | 3.0 | 15.4 | 2.4 | AN vs. AN-WR: t(20) =1.09, P=0.288 | ||
| Duration of illness | 4.9 | 2.6 | 4.1 | 2.4 | AN vs. AN-WR: t(20) = 0.75, P=0.461 | ||
| Duration of weight recovery | 3.3 | 3.1 | |||||
| Duration of menses recovery | 3.4 | 3.7 | |||||
| EDEQ | 0.3 | 0.2 | 3.2 | 1.4 | 1.8 | 0.8 | HC vs. AN: t(23) = 7.20, P < 0.001 |
| HC vs. AN-WR: t(19) = 6.12, P<0.001 | |||||||
| AN vs. AN-WR: t(20) = 3.45, P=0.003 | |||||||
| BDI | 0.7 | 1.4 | 14.5 | 11.0 | 8.5 | 6.7 | HC vs. AN: t(23)=4.29, P=0.001 |
| HC vs. AN-WR: t(19) = 3.96, P=0.001 | |||||||
| AN vs. AN-WR: t(20) =1.44, P=0.165 | |||||||
| n | % | n | % | n | % | ||
| Handedness (right) | 10 | 83.3 | 12 | 92.3 | 9 | 100 | HC vs. AN: X2(1) = 0.48, FET=0.593 |
| HC vs. AN-WR: X2(1) =1.66, FET=0.486 | |||||||
| AN vs. AN-WR: X2(1) = 0.73, FET =1.000 | |||||||
| History of purging | 2 | 15.4 | 6 | 67.7 | AN vs. AN-WR: X2(1) = 6.04, FET=0.026 | ||
| Current psychotropic medicationb | 5 | 38.5 | 2 | 22.2 | AN vs. AN-WR: X2(1) = 0.65, FET= 0.648 | ||
| Comorbid diagnosisc | |||||||
| Current | 6 | 46.2 | 1 | 11.1 | AN vs. AN-WR: X2(1) = 3.01, FET=0.165 | ||
| Past | 4 | 30.8 | 1 | 11.1 | AN vs. AN-WR: X2(1) = 1.17, FET=0.360 | ||
| Smoking statusd | |||||||
| Current | 0 | 0.0 | 0 | 0.0 | 2 | 22.2 | HC vs. AN: X2(1) = 0.03, FET =1.000 |
| HC vs. AN-WR: X2(1) = 2.95, AS=0.289 | |||||||
| AN vs. AN-WR: X2(t) = 3.18, AS = 0.204 | |||||||
| Past | 2 | 16.7 | 1 | 7.7 | 2 | 22.2 | HC vs. AN: X2(1) = 0.48, FET=0.593 |
| HC vs. AN-WR: X2(1) = 0.10, FET=1.000 | |||||||
| AN vs. AN-WR: X2(1) = 0.95, FET= 0.544 | |||||||
Significant results bolded; FET= Fisher's Exact Test; AS=Asymptotic Significance.
Five active women were taking psychotropic medications: 1 was taking venlafaxine, 1 was taking fluoxetine, 1 was taking a low dose of amphetamine/dextroamphetamine (5 mg 24 h prior to the scan), 1 was taking escitalopram and aripiprazole, 1 was taking desvenlafaxine. Two weight-restored women were taking psychotropic medications: 1 was taking bupropion and lorazepam, 1 was taking fluoxetine.
Comorbid Axis 1 diagnoses in the active group included: 3 subjects with current Generalized Anxiety Disorder, 1 subject with current ADHD-NOS, 1 subject with current GAD, history of Bipolar 1, history of ADHD-NOS, and history of PTSD, 1 subject with current Anxiety Disorder-NOS and current Depressive Disorder-NOS, 2 subjects with a past history of Major Depressive Disorder, and 1 subject with a history of Depressive Disorder-NOS. Comorbid Axis 1 diagnoses in the weight-restored group included 1 subject with current Major Depressive Disorder and Generalized Anxiety Disorder; 1 subject with a past history of Major Depressive Disorder and Social Phobia.
Past smokers include any that are also current smokers. Where FET could not be calculated due to minimal expected counts, the AS (asymptotic significance) is presented. Of the n = 2 subjects (both AN-WR) who endorsed current smoking status, 1 had abstained from cigarettes on the day of the study visit, and the other had smoked half of a cigarette on the morning of the study visit (a few hours before the start research protocol). This subject's hormone and brain data were carefully examined to determine whether this event influenced her data, but her data fell near the mean of the AN-WR group, indicating that having smoked half a cigarette had no effect on our primary variables.
3.2. Appetite ratings and ghrelin levels
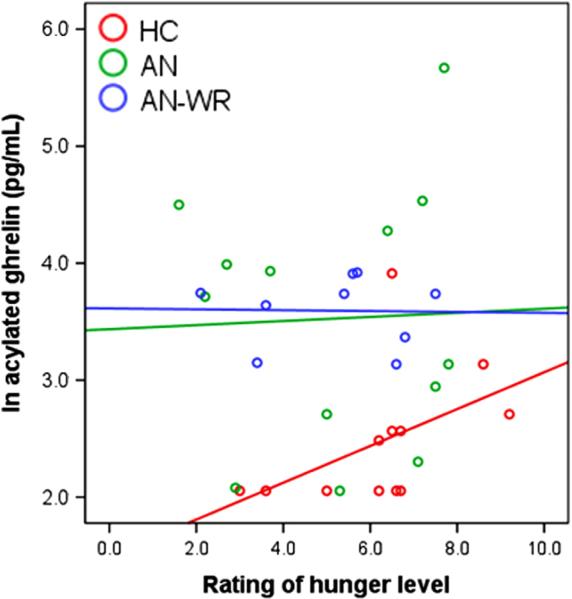
Groups did not differ significantly in their ratings of overall fasting hunger level (see Table 2). However, HC women rated their desire to eat a favorite food significantly higher than both the AN and AN-WR groups. The AN and AN-WR women had significantly higher fasting (log-transformed) acylated ghrelin compared with HC women (see Table 2; raw acylated ghrelin levels, which were significantly higher in AN than HC women, are listed for reference). Acylated ghrelin levels did not differ between the AN and the AN-WR women. In the HC women, but not the AN or AN-WR women, acylated ghrelin was positively associated with self-reported hunger level at a trend level (P=0.113) but not with desire to eat a favorite food (P=0.212); the magnitude of relationships between acylated ghrelin and hunger/desire to eat a favorite food did not significantly differ between groups (see Table 2 and Fig. 1). Ratings of hunger and desire to eat a favorite food were significantly related to each other in the AN group (r=0.61, P=0.028), but not within the HC and AN-WR groups (|r|=0.24 to 0.35; P=0.446 to 0.359, respectively). In the AN and AN-WR groups, acylated ghrelin levels were not significantly different between those currently on medication compared with unmedicated participants (AN: t(11)=1.53, P=0.134; AN-WR: t(7)=1.27, P=0.246).
Table 2.
Group differences in and relationships between fasting plasma acylated ghrelin levels and appetite ratings.
| Group | Between-group comparisonsa | ||||||
|---|---|---|---|---|---|---|---|
| HC | AN | AN-WR | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Acylated ghrelin (pg/ml), raw | 14.4 | 12.1 | 59.5 | 75.6 | 37.8 | 10.4 | F(2,31) = 2.77, P=0.078 |
| HC vs. AN: Mean Diff =45.12, P=0.053 | |||||||
| HC vs. AN-WR: Mean Diff= 23.38, P=0.001 | |||||||
| AN vs. AN-WR: Mean Diff=21.74, P=0.405 | |||||||
| Acylated ghrelin (pg/ml), log-transformed | 2.5 | 0.6 | 3.5 | 1.1 | 3.6 | 0.3 | F(2,31) = 7.47, P=0.002 |
| HC vs. AN: Mean Diff=1.05, P=0.007 | |||||||
| HC vs. AN-WR: Mean Diff= 1.12, P= 0.001 | |||||||
| AN vs. AN-WR: Mean Diff =0.07, P= 0.860 | |||||||
| Hunger | 6.2 | 1.8 | 5.2 | 2.3 | 5.2 | 1.8 | F(2,31) = 1.10, P= 0.345 |
| HC vs. AN: Mean Diff=1.07, P=0.206 | |||||||
| HC vs. AN-WR: Mean Diff= 1.04, P= 0.197 | |||||||
| AN vs. AN-WR: Mean Diff=0.03, P=0.976 | |||||||
| Desire to eat favorite food | 5.7 | 2.4 | 2.5 | 2.5 | 3.3 | 2.2 | F(2,31) = 5.40, P=0.010 |
| HC vs. AN: Mean Diff =3.01, P=0.005 | |||||||
| HC vs. AN-WR: Mean Diff= 2.37, P=0.029 | |||||||
| AN vs. AN-WR: Mean Diff=0.63, P=0.551 | |||||||
| r (P) | r ( P) | r ( P) | |||||
| Correlation between acylated ghrelin and: | |||||||
| Hunger | 0.48 (0.113) | 0.04 (0.906) | – 0.02 (0.955) | HC vs. AN: Z = 1.06, P= 0.145 | |||
| HC vs. AN-WR: Z =1.04, P=0.149 | |||||||
| AN vs. AN-WR: Z=0.11, P=0.456 | |||||||
| Desire to eat favorite food | 0.39 (0.212) | – 0.11 (0.712) | – 0.20 (0.604) | HC vs. AN: Z = 1.14, P= 0.127 | |||
| HC vs. AN-WR: Z =1.17, P=0.121 | |||||||
| AN vs. AN-WR: Z=0.17, P=0.433 | |||||||
Significant results bolded.
Fig. 1.
Scatterplot demonstrating the relationship between serum fasting acylated ghrelin (log-transformed values) and hunger rating, with data markers and fit lines stratified by group according to the legend.
3.3. Brain-hormone associations
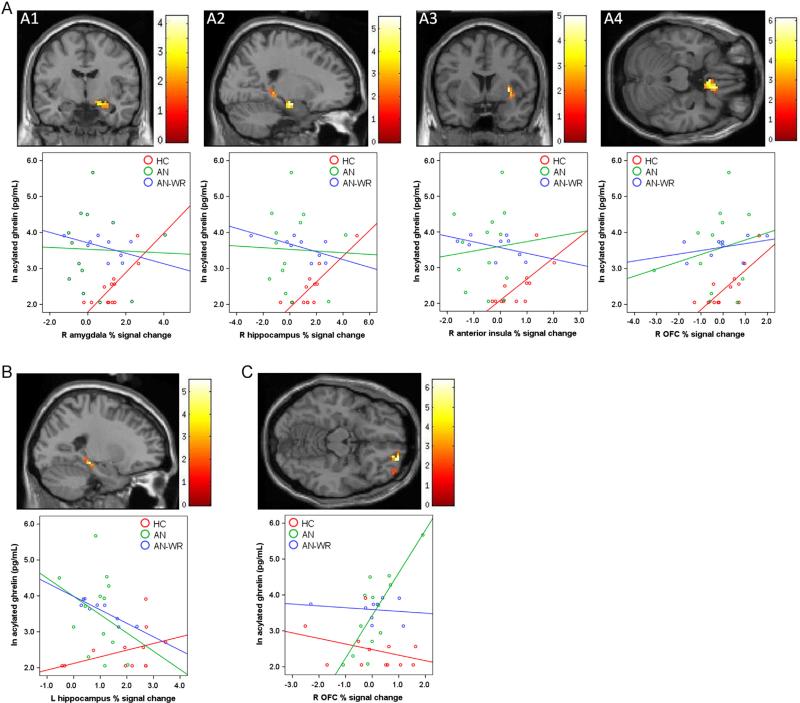
In our previous report of brain-activity deficits in women with AN, we found pre-meal hypoactivation in response to high-calorie foods vs. objects in AN (vs. HC) in the hypothalamus, amygdala, hippocampus, OFC, and anterior insula, and in the AN-WR group (vs. HC) in the hypothalamus, amygdala, and anterior insula (Holsen et al., 2012). Here we report effects of orexigenic (acylated ghrelin) hormone levels on these group differences. In particular, fasting acylated ghrelin was significantly associated with the response to high-calorie foods (vs. objects) in the fasting state in the HC group (see Table 3, Fig. 2A). As shown in Table 3, acylated ghrelin was positively associated with BOLD activation to high-calorie foods > objects in the right amygdala, hippocampus, anterior insula, and OFC. However, in the AN and AN-WR groups, there were no regions that showed positive associations between activation in response to high-calorie foods > objects and acylated ghrelin levels (see Table 3). In contrast to these positive relationships in the HC group, women with AN-WR exhibited a significant negative relationship between acylated ghrelin levels and BOLD activation to high-calorie foods > objects in the left hippocampus, associations that were not observed in the AN and HC groups (see Fig. 2B).
Table 3.
Multiple regression model in SPM: relationship between ROI activity and fasting plasma acylated ghrelin.
| k | x | y | z a | Positive relationshipsb, tmax (voxel-level FWE-corrected P) | Negative relationshipsb, tmax (voxel-level FWE-corrected P) | ||
|---|---|---|---|---|---|---|---|
| HC | AN | AN-WR | |||||
| High-calorie foods vs. objects | |||||||
| R Amygdala | 19 | 21 | –10 | –14 | 4.25 (0.030) | NS | NS |
| R Hippocampus | 27 | 21 | –13 | –17 | 5.51 (0.018) | NS | NS |
| L Hippocampus | 24 | –21 | –28 | –11 | NS | NS | 6.72 (0.029) |
| R Anterior insula | 27 | 33 | 5 | 1 | 4.96 (0.051) | NS | NS |
| R Orbitofrontal cortex | 45 | 12 | 23 | –23 | 6.11 (0.037) | NS | NS |
| Low-calorie foods vs. objects | |||||||
| R Orbitofrontal cortex | 26 | 21 | 56 | –17 | NS | 6.40 (0.019) | NS |
Coordinates are presented in MNI space.
Clusters identified using small volume correction within an anatomical ROI (initial voxel-wise height threshold: P< 0.05 uncorrected) are reported (in bold)as significant if they additionally met the peak-level threshold of P< 0.05, FWE-corrected. NS = no significant voxels met threshold levels in SPM8 regression model.
Fig. 2.
Regions demonstrating significant relationships between serum fasting acylated ghrelin and BOLD response to food (vs. objects): (A) Positive relationships between ghrelin and high-calorie vs. object BOLD activity in healthy-weight control subjects in the right amygdala (A1), right hippocampus (A2) right anterior insula (A3), and right OFC (A4); (B) Negative relationship between ghrelin and high-calorie vs. object BOLD activity in AN-WR women in the left hippocampus; (C) Positive relationship between ghrelin and low-calorie vs. object BOLD activity in AN women in the right OFC. Voxels surviving the statistical threshold [identified using small volume correction (initial voxel-wise height threshold: P < 0.05 uncorrected) and reported as significant if they additionally met the peak-level threshold of P < 0.05, FWE-corrected] are projected on a single-subject T1, centered at tmax. Within-group associations between acylated ghrelin and extracted % signal change values in each ROI are visualized in corresponding scatterplots below each ROI, with data markers and fit lines stratified by group according to the legend.
To test whether the associations identified in the HC and ANWR groups differed from relationships in the other groups, percent signal change (psc) values were extracted for each subject from within the significant ROI clusters, and Pearson correlation coefficients between these psc values and ghrelin levels computed for each group. As demonstrated in Table 4, results of the subsequent r to Z Fisher transformations for each ROI confirmed that HC women had significantly different correlations in the amygdala, hippo-campus, anterior insula, and OFC than AN or AN-WR women. Additionally, the negative relationship between acylated ghrelin and hippocampus percent signal change noted in AN-WR women was significantly different from the correlation in the HC group and the AN group.
Table 4.
Between-group differences in correlations between ROI activity and fasting plasma acylated ghrelin.
| Pearson’s correlation coefficient, r (P)a | Between-group comparisonsa | |||
|---|---|---|---|---|
| HC | AN | AN-WR | ||
| High-calorie foods vs. objects | ||||
| R Amygdala | 0.78 (0.003) | –0.03 (0.912) | –0.53 (0.146) | HC vs. AN: Z=2.34, P=0.010 |
| HC vs. AN-WR: Z=3.10, P= 0.001 | ||||
| R Hippocampus | 0.85 (0.001) | –0.04 (0.907) | –0.64 (0.064) | HC vs. AN: Z=2.82, P=0.005 |
| HC vs. AN-WR: Z=3.82, P=0.0001 | ||||
| L Hippocampus | 0.41 (0.191) | –0.31 (0.306) | –0.90 (0.001) | AN-WR vs. HC: Z=3.62, P=0.0001 |
| AN-WR vs. AN: Z=2.23, P= 0.013 | ||||
| R Anterior insula | 0.72 (0.008) | 0.09 (0.779) | –0.48 (0.194) | HC vs. AN: Z=1.78, P= 0.038 |
| HC vs. AN-WR: Z= 2.71, P=0.003 | ||||
| R Orbitofrontal cortex | 0.78 (0.003) | 0.20 (0.514) | 0.39 (0.295) | HC vs. AN: Z=1.83, P=0.034 |
| HC vs. AN-WR: Z = 1.20, P= 0.115 | ||||
| Low-calorie foods vs. objects | ||||
| R Orbitofrontal cortex | –0.33 (0.289) | 0.80 (0.001) | –0.17 (0.667) | AN vs. HC: Z=3.14, P=0.0008 |
| AN vs. AN-WR: Z=2.46, P=0.007 | ||||
Significant results bolded.
In addition to the association between hormone levels and group differences in brain activity during processing of stimuli with a high reward value (i.e., high-calorie foods vs. objects), we were interested in whether these findings extended to evaluation of stimuli of differing reward value. Regression of acylated ghrelin on activation in response to low-calorie foods vs. objects yielded a positive association in the AN group in the right OFC (see Table 3, Fig. 2C). The correlation between acylated ghrelin and OFC psc values in AN women was significantly different from those in the HC and AN-WR groups (see Table 4). In the HC and AN-WR groups, there were no regions that showed positive associations between activation in response to low-calorie foods and acylated ghrelin levels (see Table 3). At the whole brain level, there were no additional regions that were identified as demonstrating positive or negative relationships between acylated ghrelin and either high- or low-calorie foods in any of the groups (HC, AN, and AN-WR) at the P < 0.001 FWE-corrected level.
To further probe hormone-brain-behavior relationships as a set of exploratory analyses, we examined whether ratings of hunger and desire to eat a favorite food were similarly related to psc values in the ROI clusters identified in the ghrelin–brain activation analyses described above. With the exception of three correlations [HC group: desire to eat a favorite food and: psc in the anterior insula in response to high-calorie foods (r=0.62, P=0.033), psc in the OFC in response to low-calorie foods (r= –0.60, P=0.041); AN-WR group: hunger and psc in the OFC in response to low-calorie foods (r=0.72, P=0.030)], correlations were not significant (|r|=0.01 to 0.49; P=0.985 to 0.105, respectively). Finally, because the small sample size might have masked the ability to detect significant relationships in women with AN, all analyses were repeated using a pooled grouping of AN women (i.e., AN+AN-WR) compared with the HC group. Combining the AN and AN-WR groups did not alter any of the within- or between-group results reported above.
4. Discussion
Secretion of ghrelin, a critical hormone in the complex circuitry responsible for signaling human appetite, is elevated in AN (Germain et al., 2007, 2010; Koyama et al., 2010). Ghrelin signaling is involved in both homeostatic and hedonic pathways, both of which have been shown to be disrupted in individuals with AN (Ellison et al., 1998; Wagner et al., 2007, 2008; Fladung et al., 2010; Gizewski et al., 2010; Frank et al., 2012; Holsen et al., 2012). Results of the current study provide a critical step in understanding the link between these neural and neuroendocrine findings, suggesting the absence of or abnormality in the typical associations between peripheral plasma fasting acylated ghrelin levels and (a) self-reported homeostatic (hunger) ratings at a trend level, and (b) BOLD response to visual food cues in the amygdala, hippocampus, insula, and OFC in women with active AN and AN-WR women. The breakdown in these relationships occurred despite significantly elevated acylated ghrelin levels in these two groups, both of which also demonstrated substantial variability in ghrelin levels. This suggests that resistance to ghrelin in AN and those who have weight-recovered from AN may lead to reduced appetitive drive and restrictive eating. Although preliminary, these data offer new insight into the pathophysiology of appetite dysregulation in AN and point to potential neurobiological targets for treatment for this disorder.
Consistent with previous investigations (Germain et al., 2007, 2010; Koyama et al., 2010), we found elevated fasting acylated ghrelin in women with AN. Importantly, our data in AN-WR women, who showed statistically similar acylated ghrelin levels to AN women (both raw values and values after log transformation), indicate persistent ghrelin abnormalities even after long-term weight gain to a healthy level, suggestive of a trait characteristic of AN. Although cross-sectional, these data are in line with prior reports of decreases in ghrelin, but not to the level of healthy-weight controls, following (incomplete) weight restoration in AN (Nakahara et al., 2007), and to our knowledge the first report of elevated acylated ghrelin levels following long-term recovery. Further, although the healthy-weight control group exhibited significant positive relationships between ghrelin levels and homeostatic hunger ratings (at a trend level), replicating previous findings (Malik et al., 2008; Kroemer et al., 2012), women with active AN and AN-WR women failed to demonstrate these relationships. This pattern of results may reflect the disconnect between the body's adaptation to a state of starvation (i.e., increased ghrelin levels) and ability to recognize and interpret internal appetitive signaling in women with AN (Huse and Lucas, 1984; Robinson, 1989). It may also be suggestive of other neuroendocrine signals that may outweigh the effects of ghrelin. Alternatively, this absence of the typical ghrelin–hunger rating association might be indicative of the biased nature of such ratings in women with AN (Robinson et al., 1983; Hetherington and Rolls, 1991). As such, delineation of ghrelin–brain relationships in AN represents an opportunity to examine important mechanistic pathways free from the potential bias of self-reported appetite ratings.
In a state of high appetitive motivation, such as a prolonged fast, ghrelin acts on the hypothalamus to prompt food seeking and meal initiation (Cummings et al., 2001; Nakazato et al., 2001), while simultaneously enhancing drive for foods of higher reward value (i.e., high fat, high sugar) through action in mesolimbic dopamine circuitry (Zigman et al., 2006; Perello et al., 2010; Skibicka et al., 2011, 2012a, 2012b). Our findings in the healthy-weight control group of positive relationships between fasting ghrelin and fasting BOLD response to high-calorie foods in the amygdala, hippocampus, insula, and OFC provide evidence of the hedonic role of ghrelin in human appetite, replicating previous findings (Malik et al., 2008; Kroemer et al., 2012; Li et al., 2012) and extending them to suggest distinct coordinated biasing towards intake of high-calorie food through action of ghrelin on these regions during a fasting state. Although we measured peripheral acylated ghrelin levels and results are correlational in nature, these data are in line with findings from studies demonstrating activation of the amygdala (Malik et al., 2008), insula (Malik et al., 2008), and OFC (Malik et al., 2008) following peripheral administration of ghrelin in humans (Malik et al., 2008) and animals (Abizaid et al., 2006; Jerlhag et al., 2006).
Of particular importance, though we hypothesized generally abnormal relationships in AN and AN-WR, the absence of associations between ghrelin and BOLD response to high-calorie foods in the amygdala, hippocampus, anterior insula, and OFC in these groups is striking. We previously reported decreased mean BOLD response in these regions (Holsen et al., 2012) in the AN and AN-WR groups, despite a moderate level of variability in the BOLD response, which we tentatively predicted would be at least partially explained by ghrelin levels, especially in light of the highly variable ghrelin levels in these groups. Further, the presence of a positive association between ghrelin and BOLD response to low-calorie foods in the OFC may reflect the tight coupling between ghrelin and the reversed hedonic coding of low-calorie foods as highly rewarding in active anorexia nervosa (but of less reward value in HC women). The opposing direction of ghrelin and activity in the left hippocampus in response to high-calorie foods in women with AN-WR (vs. HC women) may indicate persistence in abnormal ghrelin signaling in this region with dense expression of GHS-R1a receptors (Guan et al., 1997) even after weight recovery, and perhaps serve as a biomarker for vulnerability to relapse.
As reviewed by Mequinion et al. (2013), several routes exist through which peripheral ghrelin may cross the blood–brain barrier to act centrally, and though the integrity of these routes has not been examined in the context of starvation, even if disrupted, this would not explain the absence of or disparate direction of the associations in the AN-WR group. Although speculative, potential mechanisms underlying resistance to ghrelin in anorexia nervosa could include disruption of ghrelin-related genes. Genetic studies focused on these potential loss-of-function mutations in AN subtypes have mixed results. However, initial evidence supports an association between restrictive AN and polymorphisms on the gene coding for ghrelin O-acyltransferase (GOAT), which is necessary for acylation of des-acylated ghrelin to its biologically active form (acylated ghrelin) (Muller et al., 2011). Elevated transmission of the preproghrelin/obestatin Gln90Leu72 polymorphism in genetic susceptibility for AN appears to be stronger in the binge-purge subtype than the restrictive subtype (Dardennes et al., 2007).
Further, in a pilot study, AN patients who received 2-week treatment with twice-daily intravenous infusions of ghrelin in AN during inpatient treatment showed improvement in gastrointestinal symptoms and increased hunger ratings, suggesting that ghrelin resistance may be overcome with supraphysiological administration (Hotta et al., 2009), although conclusions are limited by the small sample size (n=5) and lack of a control group. Additional genetic and pharmacologic studies [for example, determination of GHS-R1a binding capacity in these neural regions using positron emission tomography radiotracers for ghrelin agonists (Chollet et al., 2012)] are clearly needed and have the potential to reveal significant insight into the pathophysiology of ghrelin resistance in AN.
Current findings therefore offer a bridge between neuroendocrine dysregulation and neural circuitry disruption as related to appetite in anorexia nervosa. However, the generalizability of our results is limited by the small sample size; thus, findings should be considered preliminary in nature. In particular, the trend-level significance for the relationship between hunger and acylated ghrelin in the HC group may have been due to reduced power owing to small sample size. Further, we used a cross-sectional design and the relationships between ghrelin and brain activity are based on correlational analyses, preventing conclusions regarding causality. Some of our subjects were on psychoactive medications, which may have affected brain activity and ghrelin levels. However, we found no difference in ghrelin levels in those currently on medication compared with unmedicated subjects. Future studies should attempt to recruit larger samples of medication-free women.
In conclusion, we found a significant and striking abnormalities in the associations between circulating levels of acylated ghrelin and brain activity in response to food cues in hedonic food motivation regions in women with active AN and AN-WR women in contrast to healthy-weight control subjects. This atypical pattern of results was seen across food cues of varying hedonic value and is consistent with the disconnection between hedonic appetite ratings and ghrelin levels in women with anorexia nervosa. Our results offer preliminary evidence of disruption in the interaction of neuroendocrine and neural circuitry functioning in AN and provide a framework for future studies to examine the pharmacogenetics of appetite dysregulation in eating disorders.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. Journal of Clinical Investigation. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Crespo M, Skibicka KP, Farkas I, Molnar CS, Egecioglu E, Hrabovszky E, Liposits Z, Dickson SL. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS One. 2012;7:e46321. doi: 10.1371/journal.pone.0046321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet C, Bergmann R, Pietzsch J, Beck-Sickinger AG. Design, evaluation, and comparison of ghrelin receptor agonists and inverse agonists as suitable radiotracers for PET imaging. Bioconjugate Chemistry. 2012;23:771–784. doi: 10.1021/bc2005889. [DOI] [PubMed] [Google Scholar]
- Cowdrey FA, Park RJ, Harmer CJ, McCabe C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biological Psychiatry. 2011;70:736–743. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Dardennes RM, Zizzari P, Tolle V, Foulon C, Kipman A, Romo L, Iancu-Gontard D, Boni C, Sinet PM, Therese Bluet M, Estour B, Mouren MC, Guelfi JD, Rouillon F, Gorwood P, Epelbaum J. Family trios analysis of common polymorphisms in the obestatin/ghrelin, BDNF and AGRP genes in patients with Anorexia nervosa: association with subtype, body-mass index, severity and age of onset. Psychoneuroendocrinology. 2007;32:106–113. doi: 10.1016/j.psyneuen.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ellison Z, Foong J, Howard R, Bullmore E, Williams S, Treasure J. Functional anatomy of calorie fear in anorexia nervosa. Lancet. 1998;352:1192. doi: 10.1016/S0140-6736(05)60529-6. [DOI] [PubMed] [Google Scholar]
- Fladung AK, Gron G, Grammer K, Herrnberger B, Schilly E, Grasteit S, Wolf RC, Walter H, von Wietersheim J. A neural signature of anorexia nervosa in the ventral striatal reward system. American Journal of Psychiatry. 2010;167:206–212. doi: 10.1176/appi.ajp.2009.09010071. [DOI] [PubMed] [Google Scholar]
- Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O'Reilly RC. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37:2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain N, Galusca B, Grouselle D, Frere D, Billard S, Epelbaum J, Estour B. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. The Journal of Clinical Endocrinology and Metabolism. 2010;95:3057–3062. doi: 10.1210/jc.2009-2196. [DOI] [PubMed] [Google Scholar]
- Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, Bloom SR, Estour B. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. American Journal of Clinical Nutrition. 2007;85:967–971. doi: 10.1093/ajcn/85.4.967. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Rosenberger C, de Greiff A, Moll A, Senf W, Wanke I, Forsting M, Herpertz S. Influence of satiety and subjective valence rating on cerebral activation patterns in response to visual stimulation with high-calorie stimuli among restrictive anorectic and control women. Neuropsychobiology. 2010;62:182–192. doi: 10.1159/000319360. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Research. Molecular Brain Research. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hetherington MM, Rolls BJ. Eating behavior in eating disorders: response to preloads. Physiology and Behavior. 1991;50:101–108. doi: 10.1016/0031-9384(91)90505-i. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lawson EA, Blum J, Ko E, Makris N, Fazeli PK, Klibanski A, Goldstein JM. Food motivation circuitry hypoactivation related to hedonic and non-hedonic aspects of hunger and satiety in women with active and weight-restored anorexia nervosa. Journal of Psychiatry and Neuroscience. 2012;37:322–332. doi: 10.1503/jpn.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. NeuroImage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. Journal of Pharmacological Sciences. 2006;100:398–410. doi: 10.1254/jphs.crj06002x. [DOI] [PubMed] [Google Scholar]
- Hotta M, Ohwada R, Akamizu T, Shibasaki T, Takano K, Kangawa K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. Endocrine Journal. 2009;56:1119–1128. doi: 10.1507/endocrj.k09e-168. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Huse DM, Lucas AR. Dietary patterns in anorexia nervosa. American Journal of Clinical Nutrition. 1984;40:251–254. doi: 10.1093/ajcn/40.2.251. [DOI] [PubMed] [Google Scholar]
- Janas-Kozik M, Krupka-Matuszczyk I, Malinowska-Kolodziej I, Lewin-Kowalik J. Total ghrelin plasma level in patients with the restrictive type of anorexia nervosa. Regulatory Peptides. 2007;140:43–46. doi: 10.1016/j.regpep.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addiction Biology. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Joos AA, Saum B, van Elst LT, Perlov E, Glauche V, Hartmann A, Freyer T, Tuscher O, Zeeck A. Amygdala hyperreactivity in restrictive anorexia nervosa. Psychiatry Research. 2011;191:189–195. doi: 10.1016/j.pscychresns.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Karczewska-Kupczewska M, Straczkowski M, Adamska A, Nikolajuk A, Otziomek E, Gorska M, Kowalska I. Increased suppression of serum ghrelin concentration by hyperinsulinemia in women with anorexia nervosa. European Journal of Endocrinology/European Federation of Endocrine Societies. 2010;162:235–239. doi: 10.1530/EJE-09-0832. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Koyama KI, Yasuhara D, Nakahara T, Harada T, Uehara M, Ushikai M, Asakawa A, Inui A. Changes in acyl ghrelin, des-acyl ghrelin, and ratio of acyl ghrelin to total ghrelin with short-term refeeding in female inpatients with restricting-type anorexia nervosa. Hormone and Metabolic Research. 2010;42:595–598. doi: 10.1055/s-0030-1252017. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M, Zimmermann US, Smolka MN. Fasting levels of ghrelin covary with the brain response to food pictures. Addiction Biology. 2012;18:855–862. doi: 10.1111/j.1369-1600.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- Lawson EA, Holsen LM, Desanti R, Santin M, Meenaghan E, Herzog DB, Goldstein JM, Klibanski A. Increased hypothalamic-pituitary-adrenal drive is associated with decreased appetite and hypoactivation of food-motivation neurocircuitry in anorexia nervosa. European Journal of Endocrinology/European Federation of Endocrine Societies. 2013;169:639–647. doi: 10.1530/EJE-13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Holsen LM, Santin M, Meenaghan E, Eddy KT, Becker AE, Herzog DB, Goldstein JM, Klibanski A. Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1898–1908. doi: 10.1210/jc.2012-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, An R, Zhang Y, Li X, Wang S. Correlations of macronutrient-induced functional magnetic resonance imaging signal changes in human brain and gut hormone responses. American Journal of Clinical Nutrition. 2012;96:275–282. doi: 10.3945/ajcn.112.037440. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metabolism. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mequinion M, Langlet F, Zgheib S, Dickson S, Dehouck B, Chauveau C, Viltart O. Ghrelin: central and peripheral implications in anorexia nervosa. Frontiers in Endocrinology. 2013;4:15. doi: 10.3389/fendo.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metropolitan Life Foundation Metropolitan height and weight tables. Statistical Bulletin. 1983;64:2–9. [PubMed] [Google Scholar]
- Misra M, Miller KK, Herzog DB, Ramaswamy K, Aggarwal A, Almazan C, Neubauer G, Breu J, Klibanski A. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. Journal of Clinical Endocrinology and Metabolism. 2004;89:1605–1612. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. American Journal of Physiology, Endocrinology and Metabolism. 2005;289:E347–356. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Serritella C, Martiadis V, Scognamiglio P, Maj M. Plasma obestatin, ghrelin, and ghrelin/obestatin ratio are increased in underweight patients with anorexia nervosa but not in symptomatic patients with bulimia nervosa. Journal of Clinical Endocrinology and Metabolism. 2008;93:4418–4421. doi: 10.1210/jc.2008-1138. [DOI] [PubMed] [Google Scholar]
- Muller TD, Tschop MH, Jarick I, Ehrlich S, Scherag S, Herpertz-Dahlmann B, Zipfel S, Herzog W, de Zwaan M, Burghardt R, Fleischhaker C, Klampfl K, Wewetzer C, Herpertz S, Zeeck A, Tagay S, Burgmer M, Pfluger PT, Scherag A, Hebebrand J, Hinney A. Genetic variation of the ghrelin activator gene ghrelin O-acyltransferase (GOAT) is associated with anorexia nervosa. Journal of Psychiatric Research. 2011;45:706–711. doi: 10.1016/j.jpsychires.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Kojima S, Tanaka M, Yasuhara D, Harada T, Sagiyama K, Muranaga T, Nagai N, Nakazato M, Nozoe S, Naruo T, Inui A. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. Journal of Psychiatric Research. 2007;41:814–820. doi: 10.1016/j.jpsychires.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Ogiso K, Asakawa A, Amitani H, Inui A. Ghrelin and anorexia nervosa: a psychosomatic perspective. Nutrition. 2011;27:988–993. doi: 10.1016/j.nut.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: effect on feeding and c-Fos immunoreactivity. Peptides. 2003a;24:919–923. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003b;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- Otto B, Tschop M, Fruhauf E, Heldwein W, Fichter M, Otto C, Cuntz U. Postprandial ghrelin release in anorectic patients before and after weight gain. Psychoneuroendocrinology. 2005;30:577–581. doi: 10.1016/j.psyneuen.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biological Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AC, Brooks SJ, Stahl D, Treasure J. Systematic review and meta-analysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. American Journal of Clinical Nutrition. 2009;89:755–765. doi: 10.3945/ajcn.2008.27056. [DOI] [PubMed] [Google Scholar]
- Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochemistry International. 2009;54:89–94. doi: 10.1016/j.neuint.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Robinson PH. Perceptivity and paraceptivity during measurement of gastric emptying in anorexia and bulimia nervosa. British Journal of Psychiatry. 1989;154:400–405. doi: 10.1192/bjp.154.3.400. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Tortosa M, Sullivan J, Buchanan E, Andersen AE, Folstein MF. Quantitative assessment of psychologic state of patients with anorexia nervosa or bulimia: response to caloric stimulus. Psychosomatic Medicine. 1983;45:283–292. doi: 10.1097/00006842-198308000-00003. [DOI] [PubMed] [Google Scholar]
- Santel S, Baving L, Krauel K, Munte TF, Rotte M. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Research. 2006;1114:138–148. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Sedlackova D, Kopeckova J, Papezova H, Hainer V, Kvasnickova H, Hill M, Nedvidkova J. Comparison of a high-carbohydrate and high-protein breakfast effect on plasma ghrelin, obestatin, NPY and PYY levels in women with anorexia and bulimia nervosa. Nutrition and Metabolism. 2012;9:52. doi: 10.1186/1743-7075-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlackova D, Kopeckova J, Papezova H, Vybiral S, Kvasnickova H, Hill M, Nedvidkova J. Changes of plasma obestatin, ghrelin and NPY in anorexia and bulimia nervosa patients before and after a high-carbohydrate breakfast. Physiological Research. 2011;60:165–173. doi: 10.33549/physiolres.931952. [DOI] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. Journal of Clinical Endocrinology and Metabolism. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Egecioglu E, Dickson SL. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addiction Biology. 2012a;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Hansson C, Dickson SL. Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology. 2012b;153:1194–1205. doi: 10.1210/en.2011-1606. [DOI] [PubMed] [Google Scholar]
- Soares JB, Leite-Moreira AF. Ghrelin, des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides. 2008;29:1255–1270. doi: 10.1016/j.peptides.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Tolle V, Bassant MH, Zizzari P, Poindessous-Jazat F, Tomasetto C, Epelbaum J, Bluet-Pajot MT. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143:1353–1361. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- Uher R, Brammer MJ, Murphy T, Campbell IC, Ng VW, Williams SC, Treasure J. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biological Psychiatry. 2003;54:934–942. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, Andrew CM, Williams SC, Campbell IC, Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. American Journal of Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, Bailer UF, Fischer L, Kaye WH. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–523. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, Frank GK, Bailer UF, Fischer L, Nguyen V, Carter C, Putnam K, Kaye WH. Altered reward processing in women recovered from anorexia nervosa. American Journal of Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. Region of Interest Extraction (REX) Toolbox. Boston, MA.: 2009. [Google Scholar]
- Yi CX, Heppner K, Tschop MH. Ghrelin in eating disorders. Molecular and Cellular Endocrinology. 2011;340:29–34. doi: 10.1016/j.mce.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. Journal of Comparative Neurology. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]