Abstract
Placebo and 3 doses of methylphenidate (MPH) were crossed with 3 levels of behavioral modification (no behavioral modification, NBM; low-intensity behavioral modification, LBM; and high-intensity behavior modification, HBM) in the context of a summer treatment program (STP). Participants were 48 children with ADHD, aged 5–12. Behavior was examined in a variety of social settings (sports activities, art class, lunch) that are typical of elementary school, neighborhood, and after-school settings. Children received each behavioral condition for 3 weeks, order counterbalanced across groups. Children concurrently received in random order placebo, .15 mg/kg/dose, .3 mg/kg/dose, or .6 mg/kg/dose MPH, 3 times daily with dose manipulated on a daily basis in random order for each child. Both behavioral and medication treatments produced highly significant and positive effects on children's behavior. The treatment modalities also interacted significantly. Whereas there was a linear dose-response curve for medication in NBM, the dose-response curves flattened considerably in LBM and HBM. Behavior modification produced effects as large as moderate doses, and on some measures, high doses of medication. These results replicate and extend to social-recreational settings previously reported results in a classroom setting from the same sample (Fabiano et al., 2007). Results illustrate the importance of taking dosage/intensity into account when evaluating combined treatments; there were no benefits of combined treatments when the dosage of either treatment was high but combination of the low-dose treatments produced substantial incremental improvement over unimodal treatment.
Behavioral treatments, psychostimulant medication, and their combination are the most widely studied and accepted treatments for attention deficit/hyperactivity disorder (ADHD). A number of studies, reviewed below, suggest that the most effective short-term treatment for ADHD appears to be a combination of pharmacologic and behavioral treatment (Subcommittee on ADHD, Steering Committee on Quality Improvement and Management, 2011).
Reviews of behavior therapy for ADHD have come to differing conclusions regarding its effectiveness. For example, several reviewers (Dupaul & Eckert, 1997; Pelham & Fabiano, 2008; Stage & Quiroz, 1997) have concluded that behavior therapy (BT) is effective for children with ADHD. A meta-analysis conducted by Fabiano et al. (2009) produced a between group effect size of .74 for behavioral interventions. In contrast, earlier reviews (e.g., Jadad, Boyle, Cunningham, Kim, & Schachar, 1999) and at least one recent review paper (Van der Oord, Prins, Oosterlan, & Emmelkamp, 2008) concluded that BT is less effective than medication treatment.
This discrepancy is attributable in large part to the nature of the research studies that have been carried out. Most studies of BT effects on children with ADHD have utilized crossover or single-subject designs, typically with few subjects and conducted in classroom settings. The effects of such studies are quite large (Dupaul & Eckert, 1997; Fabiano et al., 2009; Stage & Quiroz, 1997), yet they are routinely excluded from consideration in reviews that focus on clinical trials with larger sample sizes and between-group designs (e.g., Van der Oord et al., 2008). Finally, as we discuss below, reviews have not taken into account the relative intensities of the behavioral and pharmacological treatments that are being compared.
Reviews of studies involving a combination of behavioral and stimulant treatments, typically methylphenidate (MPH; Pelham & Waschbusch, 1999) have shown that a combined intervention typically resulted in greater improvement than did either treatment alone. The largest study of comparative and combined treatments for ADHD is the Multimodal Treatment Study of ADHD (MTA; MTA Cooperative Group, 1999a, 1999b, 2004, Jensen et al., 2007). MTA results differ depending on the outcome measure, informant, assessment point, and individual difference factors, illustrating that the results of combined treatments are complex and require further examination.
Notably, the MTA emphasized optimal doses of both modalities of intervention, and it did not systematically address questions regarding the respective doses of the treatments and how they interact. For example, might the MTA results have been different if lower doses of medication had been used and if lower doses of behavioral treatment had been utilized (e.g., no classroom aide, a brief course of parent training, less intensive summer program)? Under those conditions, might the combined treatment have produced similar results with less stimulant-induced growth suppression and less complex, less costly behavioral intervention? With the exception of a few case studies (Abramowitz, Eckstrand, O'Leary, & Dulcan, 1992; Hoza, Pelham, Sams, & Carlson, 1992; Hupp, Reitman, Northup, O'Callahan, & LeBlanc, 2002; Northup et al., 1999), previous investigations have not manipulated the intensity of both behavioral interventions and medication in the same study. The limited range of treatments studied can lead researchers to make conclusions that may not hold true if a wider range of treatment intensities were studied. For example, contrast Abikoff et al. (2004), which used a high dose of stimulant medication and a low-intensity clinical behavioral treatment, with Pelham et al. (2000) who used lower doses of stimulant medication and a high-intensity behavioral treatment: both studies show few differences between combined and unimodal treatments, but in the Abikoff study the combination adds little to a high dose of medication; whereas in the Pelham study, the combination adds little to a highly intensive behavioral treatment.
Several studies of combined treatments have been carried out in the context of a summer treatment program (STP) classroom (Pelham et al., 2010). Carlson, Pelham, Milich, & Dixon (1992) found that effects of a behavioral intervention and .3 mg/kg MPH were equivalent and additive; their combination was equivalent to a 0.6 mg/kg dose of MPH. There was no incremental benefit from the combined intervention at the 0.6 mg/kg dose. In a second study (Pelham et al., 1993), both treatments were effective and the effect size of the combination of .3 mg/kg MPH and BT was larger than 0.6 mg/kg alone. Fabiano et al. (2007) crossed multiple doses of behavioral treatment and stimulant medication treatments. Results indicated that the highest dose of medication was roughly equivalent to low or high intensity behavioral interventions alone. Further, a low dose of behavioral intervention combined with a very low dose of medication (.15 mg/kg of MPH) approximated the high dose of either treatment alone. A limitation of these studies is that they occurred only in a classroom setting.
Only a handful of investigations have examined treatment effects in recreational settings. Pelham, McBurnett et al. (1990) found that MPH alone had positive effects on children's behavior during baseball games. O'Connor et al. (2013) showed that the STP package alone produced improvements in sports knowledge, motor proficiency, sport skills, and sportsmanship. Pelham and colleagues used a withdrawal design to demonstrate that the STP package (the high behavior modification condition studied in the current investigation) had large effects on children's behavior regardless of their medication status (Chronis et al., 2004). In a subsequent study, the STP package was withdrawn for two, 5-day periods during a 6-week trial (Pelham et al., 2005) in which children received a simultaneous investigation of placebo vs. three doses of MPH. Results showed large effects of both behavioral treatment and medication. In the presence of the STP package, equivalent to the high dose in the current study, medication effects at the lowest dose studied were similar to the largest dose of medication alone. In contrast to these studies, Kolko, Bukstein and Barron (1999) crossed behavioral treatment (none vs. high) with medication (placebo vs. low dose vs. high dose) in a sample of children with ADHD and conduct problems. Results from recreational settings showed moderate to large effects of medication alone, small to moderate effects of behavioral treatment alone, and little incremental benefit of combined treatment. Additional research is needed to clarify these discrepant results.
It is noteworthy that Pelham et al. (2005) employed a very low dose of medication—lower than all but a handful of studies in the previous several decades (cf. Pelham, Bender, Caddell, Booth, & Moorer, 1985; Werry and Sprague, 1974). Most studies of ADHD have used a dose range equivalent to .3 to .6 mg/kg of MPH (10-20 mg per dose for a typical third grader). The effects of .15 mg/kg in Pelham et al. (2005)—equivalent to 5 mg of MPH for a typical child—were substantial and raised the possibility that combining very low doses of stimulant with behavioral treatment might produce beneficial treatment with low rates of side effects. However, the dose of behavioral treatment in that study was the standard STP package. We are not aware of any comparable dose-response studies of behavioral treatments in recreational settings.
The current study extends the previous literature in two important ways. First, it extends research conducted in the classroom setting to the social settings common to elementary students, such as sports leagues, supervised neighborhood play, and unstructured transitions. This extension is critical because peer interactions that occur primarily in these settings are important mediators of adolescent and adult outcomes in children with ADHD and other disruptive behavior problems. Second, this study extends previous studies conducted in the STP setting to include multiple doses of both medication and behavioral treatment. Findings of studies using low treatment doses of are important because they may yield clear treatment benefits without the most problematic side effect of stimulants (growth suppression) and the most problematic issues facing behavioral treatments (cost and feasibility). We hypothesized that both treatments would produce significant improvements and that combinations of low-dose treatments would approximate those of higher-dose unimodal treatments.
Methods
Design
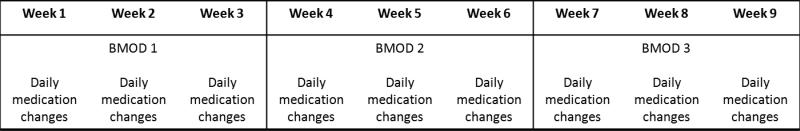
The current investigation consisted of 2 within-subjects factors: medication (placebo, .15 mg/kg/dose MPH t.i.d., .3 mg/kg/dose MPH t.i.d., and .6 mg/kg/dose MPH t.i.d.) and behavior modification (no behavior modification, NBM; low-intensity behavior modification, LBM; and high-intensity behavior modification, HBM; see Figure 1). Medication was randomly assigned within each child and varied daily. Behavioral treatment was varied in three-week blocks with order of the 3 conditions counterbalanced. Thus, over the course of the study each participant had 3-4 days in each medication X behavioral treatment condition.
Figure 1.
Study design. Each child experienced three weeks of each behavioral condition, in counterbalanced order. Within each week, each child received 4 different doses of medication with order randomized such that each condition occurred at least once during each week.
Participants
Forty-four boys and 4 girls between the ages of 5 and 12 entered the investigation (see Table 1). Participants were required to meet DSM-IV diagnostic criteria for ADHD, to have an estimated full-scale IQ of at least 80, and to have no documented adverse response to or medical conditions that would contraindicate use of MPH. Parents and children provided informed consent/assent and the University at Buffalo Health Sciences IRB approved the protocol. The sample was 79% Caucasian and 12.5% African American; one boy was Native American and the remaining participants were of mixed race. One child's parents withdrew from the study after two days because of their concerns about possible side effects of the medication. A second boy's late-afternoon dose was reduced in the .6 mg/kg condition to the .3 mg/kg dose because of evening side effects. The remainder of the participants completed the study.
Table 1.
Means and Standard Deviations for Participant Characteristics.
| Item | M | SD |
|---|---|---|
| Age in Years | 9.35 | 1.98 |
| Estimated Full Scale IQa | 106.33 | 14.61 |
| DSM IV Items Endorsed by Parents or Teachersb: | ||
| Inattention | 8.5 | 0.9 |
| Hyperactivity/Impulsivity | 7.5 | 2.0 |
| Oppositional/Defiant | 5.2 | 2.4 |
| Conduct Disorder | 1.6 | 1.6 |
| Disruptive Behavior Disorders Parent Rating Scalec | ||
| ADHD | 2.05 | 0.59 |
| Oppositional/Defiant | 1.28 | 0.61 |
| Conduct Disorder | 0.28 | 0.24 |
| Disruptive Behavior Disorders Teacher Rating Scalec | ||
| ADHD | 1.87 | 0.65 |
| Oppositional/Defiant | 0.96 | 0.69 |
IQ scores were estimated from vocabulary and block design subtests of the Wechsler Intelligence Scale for Children—3rd Ed. (1991).
Number of symptoms endorsed pretty much or very much.
Scores on the DBD Rating Scale (Pelham, Gnagy, Greenslade & Milich, 1992).
Setting
The investigation took place in the context of the STP (Pelham, Greiner, & Gnagy, 1997; Pelham et al., 2010). Children were placed in groups of 12 according to age, and supervised by five students who were trained and supervised by permanent staff members. The STP lasted 9 hours per day on weekdays, and ran for 9 weeks. Children spent 2 hours in academic settings (results from the classroom setting for this sample are presented in Fabiano et al., 2007) and the remainder of the day in group recreational activities (skill drills, games, swimming, and art). The behavioral conditions outlined below were implemented for three weeks each. In all behavioral conditions, children were suspended or sent home for severely aggressive or disruptive behavior that would endanger any child or adult.
High-Intensity Behavior Modification (HBM)
In the HBM condition, all standard STP behavioral treatments were implemented. When standard procedures were not sufficient to produce behavioral changes, individualized programs were developed in which behavioral consequences were modified or increased in intensity.
Point system
One of the primary behavioral interventions for the STP consists of a comprehensive point system with both reward and cost components, which was in place throughout the day.
Activity rules
There were standard activity rules and structure for each activity. Rules were reviewed at the beginning of the activity, and children lost points for breaking rules.
Social skills and problem solving
Counselors conducted daily social skills training sessions and incorporated social skills feedback into all daily activities and the point system. Groups conducted structured problem-solving, group contracting sessions as necessary.
Sports skills
Counselors provided intensive daily sports skills training. In addition, counselors gave immediate feedback regarding skills, sportsmanship, and sport rule violations during games, and asked game-awareness questions for which children could earn points.
Time out
Time-out procedures with escalation for inappropriate behavior, time reductions for appropriate behavior, and contingent release components were used when children exhibited aggressive, destructive, or defiant behavior.
Social reinforcement and social honors
Praise and social reinforcement were provided liberally to children who behaved appropriately. Children earned daily social rewards (buttons and accompanying privileges) for high point totals and improvements.
Daily and weekly rewards
Children received daily report cards (DRC) evaluating their performance on individualized target behaviors. DRCs were reviewed with parents at the end of the day. Children received daily and weekly rewards in the STP setting and at home for positive DRC performance. Children earned two free-play recess periods based on their DRC performance, and earned weekly field trips for meeting both individualized point goals and DRC performance goals.
Low-Intensity Behavior Modification (LBM)
In the low-intensity condition, the basic structure and treatment components remained similar to the HBM condition but they were modified to reduce their scope and frequency.
Point system
Although the same behaviors and rules applied, children received feedback about their behavior only, without earning or losing points.
Activity rules
Activity rules were reviewed at the beginning of the activity, but children received feedback without losing points for breaking activity rules.
Social skills and problem solving
Counselors conducted 60-min weekly social skills training sessions. Counselors did not incorporate social skills feedback into daily activities and groups did not conduct problem-solving training.
Sports skills
Counselors provided intensive coaching and instruction, and asked game-awareness questions without the accompanying points.
Time out
Fixed-length sit-outs (5, 10, or 15 minutes, based on age), without a contingent release component, were used rather than the time-out procedure described above.
Social reinforcement and social honors
Praise and social reinforcement were provided liberally to children who behaved appropriately. Children earned daily social rewards (buttons, but without accompanying privileges) for appropriate behavior.
Daily and weekly rewards
Children received DRCs, but parents provided rewards weekly rather than daily. Children earned two free-play recess periods based on their DRC performance, and earned weekly field trips for meeting DRC performance goals.
No Behavior Modification (NBM)
In the NBM condition, the behavior modification system was suspended. The staffing, structure and content of the activities remained the same. Staff members recorded all point system behaviors and rule violations that children exhibited, but provided feedback to children without awarding or taking away points. Children did not receive DRCs, and social skills training, intensive sports-related instruction, problem-solving discussions, and time-out procedures were not used. Social reinforcement was given less frequently, and children earned recess and field trips noncontingently.
Medication Assessment
The medication assessment procedure was a double-blind, within-subject evaluation of placebo and 3 doses of MPH: .15 mg/kg/dose, .3 mg/kg/dose, and .6 mg/kg/dose. Average doses were 5.4 mg (range = 2.5 – 10), 11 mg (range = 6.25 – 20), and 21 mg (range = 11.25 – 30), respectively. Medication was administered on a three-times-daily (t.i.d.) schedule. Conditions varied daily, and were randomized so that each child received each condition at least once each week. Because there were 15 days within each behavioral treatment condition, the placebo, .15, and .3 conditions were repeated 4 times within each behavioral condition and the .6 condition was repeated 3 times. The highest dose was repeated fewer times because previous studies have found less variability between days in the higher-dose condition (e.g., Carlson et al., 1992; Pelham et al., 1993; Pelham et al., 1999). Medication was administered by study staff at 7:45 AM, at 11:45 AM, and 3:45 PM. The children, their parents, and clinical staff members were uninformed of medication condition and only the research coordinator, pharmacist and medical director had access to the medication order. The medical director could reveal medication conditions in cases of severe side-effect reports.
Dependent Measures
Counselor-recorded measures
Indices of peer- and staff-directed social behavior were frequency counts derived from the STP point system observation code. Consistent with many previous studies (e.g., Pelham et al., 2000; Pelham et al., 2005), the following behavioral categories were derived from this system: (1) activity rule violations; (2) noncompliance; (3) interrupting; (4) complaining; (5) conduct problems (lying, stealing, intentional destruction of property, and intentional aggression); and (6) negative verbalizations (verbal abuse to staff, teasing peers, and swearing). As with previous studies (e.g., Pelham et al., 2001; Pelham et al., 2005), independent observers watched 25% of the children in a group, independently classifying and recording behaviors. These records served as both measures of validity and reliability because the observers were independent staff members who were not involved in the children's treatment. Observations were sampled across groups and days, for approximately 20% of the available observations. Reliabilities were determined by computing correlations and mean differences. Correlations averaged .87 across measures (range = .6-1.0); mean differences ranged from 0-6.87 across categories.
Ratings
Each day, counselors completed the IOWA Conners Rating Scale (Loney & Milich, 1982). Counselors also completed a modified version of the Impairment Rating Scale (IRS; Fabiano et al., 2006) on which they were instructed to rate the child's level of impairment and need for additional treatment given the treatment conditions that had been in place that day. Counselors also completed daily ratings of the stress of interacting with the children and their overall effectiveness in the treatment role. These ratings ranged from 0 (not at all, very pleasant) to 6 (very much, very unpleasant). Similar questions have been shown to discriminate between parental interactions with normal and deviant children (Pelham et al. 1998) and to detect effects of medication (Chronis, Pelham, Gnagy, Roberts, & Aronoff, 2003) and behavior modification (Chronis et al., 2004).
Side effects
Counselors completed the Pittsburgh Side Effects Rating Scale (Pelham, 1993) daily, and study staff monitored the ratings for clinically significant adverse events. The average report of moderate or severe side effects over days in each medication condition (regardless of behavior modification condition) served as a dependent measure.
Results
For the point system measures and ratings, 2 separate 4 (medication: placebo, .15 mg/kg, .3 mg/kg, .6 mg/kg) × 3 (BMOD: NBM, LBM, HBM) repeated-measures multivariate analyses of variance were performed in SPSS GLM. Linear and quadratic effects were tested to determine the dose-response effect of increasing levels of treatment. Pairwise follow-up contrasts were used to detect differences among increasing dosages of both BMOD and medication. Where significant interactions were found, simple effects tests were performed within each level of each treatment. For example, main effects of BMOD were tested at each dose of medication, and main effects of medication were tested at each level of BMOD.
Counselor-Recorded Measures
Because data were nonnormally distributed, fourth-root transformations were used on the frequency categories. There were significant multivariate main effects of BMOD, F (12, 172) = 4.86, p < .001, eta2 = .25. The linear component tests were significant (p < .01) for all measures except conduct problems (p = .07); quadratic components of the orthogonal contrasts were significant (p < .05) for noncompliance and negative verbalizations and approached significance for conduct problems (p = .06). There was also a significant multivariate main effect of medication, F(18, 395) = 7.00, p < .001, eta2 = .24. Orthogonal tests showed both linear and quadratic effects (p < .05) for all measures.
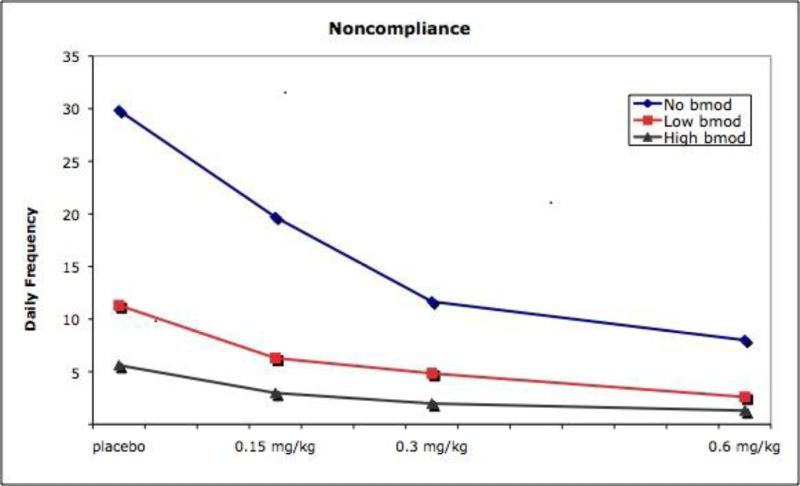
There was also a significant interaction between the two factors, F(42, 1610) = 1.94, p < .001, eta2 = .05; see Table 2. Results were similar across measures; the means for Noncompliance with adult commands is displayed in Figure 2 to illustrate the dose-response effects. Simple effects tests showed that BMOD had significant effects at all levels of drug and that drug had significant effects at all levels of BMOD. To further examine the interaction, pairwise tests of all combinations were examined. These comparisons showed that, in general, as dose of medication increased there were fewer differences among the LBM and HBM conditions (both conditions remained significantly different from NBM, however). Conversely, differences between the active medication conditions decreased in the presence of behavior modification, although they remained significantly different from placebo.
Table 2.
Medication and BMOD effects on behavioral measures in the STP.
| Placebo | .15 mg/kg MPH | .3 mg/kg MPH | .6 mg/kg MPH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NBM | LBM | HBM | NBM | LBM | HBM | NBM | LBM | HBM | NBM | LBM | HBM | |
| Rule Violations | 48.7 (41.2) | 35.8 (44.8)a | 28.4 (36.2)ab | 29.4 (31.5) | 18.6 (23.6)a | 12.2 (12.2)ab | 20.1 (15.8) | 14.2 (29.5)a | 11.0 (14.6)a | 14.4 (13.0) | 7.5 (6.3)a | 7.0 (6.3)a |
| Interruption | 18.4 (25.5) | 12.9 (16.3)a | 8.1 (9.4)ab | 11.9 (15.8) | 6.3 (7.9)a | 3.9 (4.3)ab | 6.0 (10.6) | 4.0 (6.1) | 2.9 (3.7)a | 4.0 (5.8) | 2.4 (3.7)a | 1.1 (1.3)ab |
| Noncompliance | 18.3 (30.4) | 6.3 (8.8)a | 3.5 (5.0)ab | 9.9 (18.3) | 3.0 (6.4)a | 1.5 (2.1)a | 5.7 (12.8) | 1.9 (3.7)a | 0.9 (1.3)ab | 2.7 (4.2) | 0.8 (1.3)a | 0.6 (1.1)a |
| Complaining | 21.0 (31.5) | 13.4 (25.7)a | 6.9 (13.5)ab | 11.8 (18.4) | 5.3 (8.7)a | 3.5 (4.6)a | 6.5 (9.7) | 2.7 (5.3)a | 2.5 (4.1)a | 3.8 (5.5) | 1.7 (2.8)a | 0.9 (1.3)a |
| Negative Verbalizations | 37.3 (68.8) | 13.5 (22.6)a | 7.7 (13.5)ab | 20.9 (51.8) | 6.1 (13.5)a | 3.2 (6.3)a | 14.4 (39.5) | 4.3 (12.3)a | 1.7 (5.8)a | 5.8 (13.5) | 1.7 (4.6)a | 0.8 (1.7)a |
| Conduct Problems | 7.4 (25.2) | 1.8 (5.0)a | 1.0 (2.5)a | 4.4 (17.0) | 0.7 (2.5)a | 0.3 (0.9)a | 1.8 (4.3) | 0.6 (2.8)a | 0.3 (1.0)a | 1.2 (3.7) | 0.1 (0.2)a | 0.1 (0.2)b |
Notes: Dependent measures were analyzed using fourth-root transformations to address skewness, but raw data in the form of means (standard deviations in parentheses) are presented herein for ease of interpretation. Rates are average daily frequency counts. NBM = no behavior modification, LBM = low-intensity behavior modification, HBM = high-intensity behavior modification.
mean is different from NBM at this drug level.
mean is different from LBM at this drug level.
Figure 2.
Daily rates of noncompliance as a function of medication dose and behavior modification intensity.
Individual Effect Size
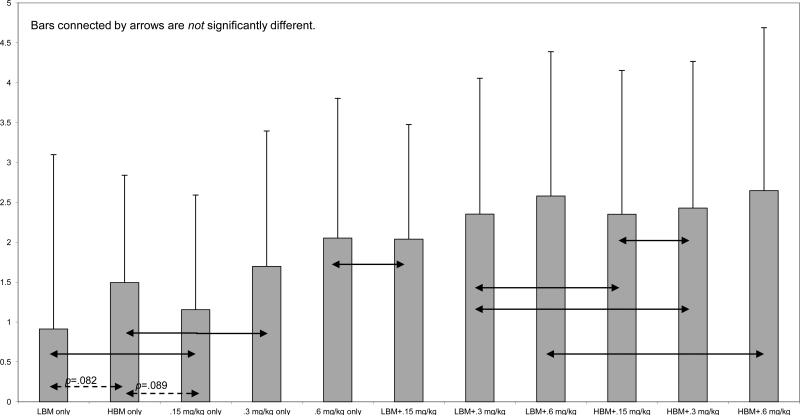
Comparisons to no-treatment
To examine the magnitude of treatment effects, effect sizes (ES) were computed for each child for a representative measure, activity rule violations. ES were computed between each of the 11 treatment combinations and the NBM-Placebo (no treatment) condition. ES were computed by taking the within-child mean difference between the two conditions and dividing by the child's no-treatment standard deviation (Pelham et al., 1993). As Figure 3 shows, all ES were in the moderate to very large range, and ES for the HBM condition are comparable to those we have previously reported in the STP classroom setting (Pelham et al., 1993). T-test comparisons were performed among pairs of ES to test differences among conditions. Most differences were significantly different (p < .01); exceptions are depicted in the Figure. Notably, LBM and .15 mg/kg were equivalent, as were HBM-only and .3 mg/kg; and .6 mg/kg was equivalent to the LBM+.15 mg/kg condition.
Figure 3.
Mean (+SD) standard effect sizes for each treatment compared with no-treatment (no behavior modification/placebo) on activity rule violations. ES were significantly different in pairwise tests with the exception of those connected by arrows.
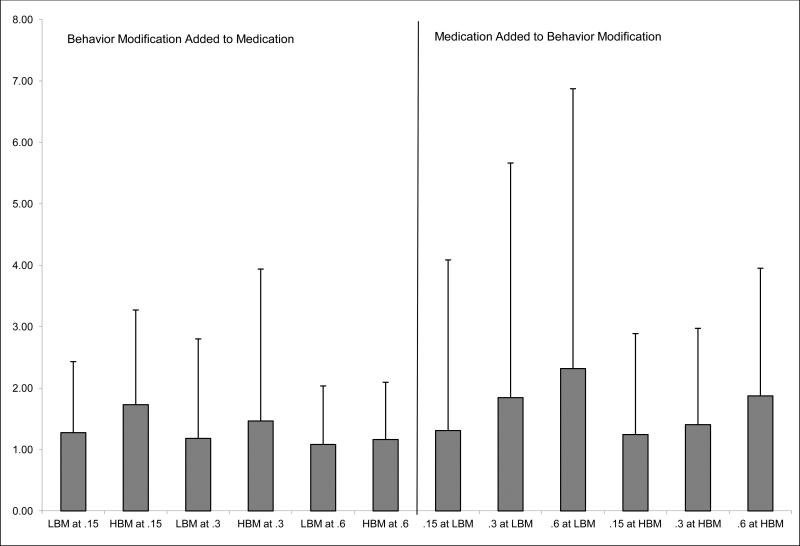
Combined relative to unimodal treatments
Figure 4 illustrates ES computed for each treatment combination relative to a baseline of one of the unimodal treatments, rather than the no-treatment baseline used above. Pairwise tests showed that at the .15 mg/kg medication dose, adding HBM produced a significantly larger ES than adding LBM; there were no differences between LBM and HBM at the other doses. Adding larger doses of medication produced significantly larger ES in the LBM condition; in the HBM condition the .15 and .3 doses did not add significantly different effects but .6 mg/kg produced a larger ES than adding .3 mg/kg.
Figure 4.
Mean (+SD) effect sizes for each combined treatment compared with baseline of the other modality (e.g., behavior modification + medication compared with behavior modification alone) on activity rule violations.
Individual differences
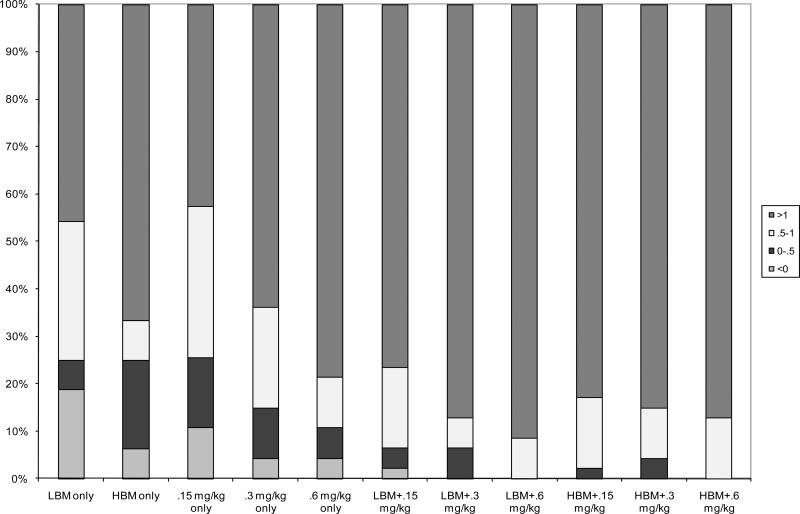
To examine individual differences in response to the treatments, the proportion of the sample that had negative, small, medium and large effect sizes was computed for each treatment combination (Figure 5). As shown, all treatments produced a large ES for the majority of the sample, with the lower-intensity unimodal treatments showing the most variability. In the LBM-only condition, 19% of the children had negative ES; that is, their behavior was worse in LBM than in NBM when they were unmedicated (average ES for LBM without these children = 1.57, equivalent to the .3 mg/kg –only condition). Six percent had negative ES in the HBM condition, 11% in the .15-only condition, and 4% in the .3 and .6 conditions, and 2% in the LBM+.15 condition. In all these cases, increasing the intensity of the treatment produced positive treatment effects.
Figure 5.
Proportion of sample experiencing low, moderate or high effects sizes by treatment condition on activity rule violations.
Ratings
On counselor ratings, there were significant main effects of medication, F(21, 402) = 6.14, p < .001; BMOD, F(14, 174) = 9.08, p < .001, and the interaction, F(42, 1650) = 1.45, p < .05 (see Table 3). The linear and quadratic components of medication were significant for all measures (p < .01); linear components of BMOD were significant (p < .01) for all measures, with quadratic effects (p > .05) for counselor ratings of effectiveness and overall child impairment. Simple effects tests showed that all BMOD had significant effects at all levels of drug and that drug had significant effects at all levels of BMOD. Counselors rated children's behavior as improved, and their own effectiveness as increased, when children received either treatment.
Table 3.
Medication and BMOD effects on counselor ratings in the STP.
| Placebo | .15 mg/kg MPH | .3 mg/kg MPH | .6 mg/kg MPH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NBM | LBM | HBM | NBM | LBM | HBM | NBM | LBM | HBM | NBM | LBM | HBM | |
| IOWA Conners Inattention/Overactivitya | 6.0 (3.6) | 5.3 (3.7)c | 4.5 (3.1)cd | 4.0 (2.7) | 3.5 (2.6)c | 2.8 (2.0)cd | 3.1 (2.5) | 2.7 (2.5) | 2.2 (1.7)cd | 2.3 (1.5) | 1.9 (2.0) | 1.7 (1.4)c |
| IOWA Conners Oppositional/Defianta | 3.3 (3.3) | 2.8 (3.0) | 1.9 (2.4)cd | 2.1 (2.4) | 1.6 (2.1)c | 1.0 (1.4)cd | 1.7 (2.5) | 1.4 (2.2) | 0.8 (1.4)cd | 1.1 (1.5) | 0.9 (2.0) | 0.5 (1.0)cd |
| Overall Impairmentb | 2.3 (1.6) | 2.2 (1.4) | 1.6 (1.3)cd | 1.7 (1.3) | 1.5 (1.2)c | 1.1 (0.9)cd | 1.4 (1.2) | 1.3 (1.1) | 0.9 (0.8)cd | 1.2 (0.9) | 1.0 (0.9) | 0.8 (0.7)cd |
| Pleasant Interactionsb | 2.2 (1.4) | 2.0 (1.2) | 1.7 (1.1)cd | 1.6 (1.0) | 1.4 (0.9)c | 1.1 (0.7)cd | 1.2 (0.9) | 1.2 (0.9) | 1.0 (0.7)cd | 1.0 (0.6) | 0.9 (0.7) | 0.8 (0.5)c |
| Stressful Interactionsb | 1.9 (1.3) | 1.7 (1.2) | 1.5 (1.1)c | 1.3 (0.9) | 1.1 (0.8)c | 0.9 (0.6)cb | 1.0 (0.9) | 1.0 (0.8) | 0.8 (0.5)c | 0.8 (0.5) | 0.7 (0.6) | 0.6 (0.4)c |
| Counselor Effectivenessb | 2.6 (1.2) | 1.8 (1.0)c | 1.6 (1.0)cd | 2.1 (0.9) | 1.3 (0.7)c | 1.0 (0.6)cd | 1.8 (0.9) | 1.3 (0.8)c | 0.9 (0.5)cd | 1.7 (0.7) | 1.0 (0.6)c | 0.8 (0.4)cd |
Notes: NBM = no behavior modification, LBM = low-intensity behavior modification, HBM = high-intensity behavior modification.
Scores on a scale from 0 (best) to 15 (worst).
Ratings on a scale from 0 (best) to 6 (worst).
different from NBM at this drug level.
different from LBM at this drug level.
Side Effects
Ratings were averaged across days within drug condition (regardless of BMOD) for the 47 children with complete data. The only side effect reported at a moderate or severe level on the average was appetite loss as measured by the amount of lunch eaten. Children were less likely to eat their lunches with increasing dose of medication: on placebo, children ate 81% of their lunches, compared with 73%, 59%, and 45% on the .15, .3 and .6 mg/kg doses, respectively.
Discussion
This study was conducted in an effort to explore more fully the efficacy of different doses of behavioral and pharmacological treatments for ADHD. This is the first controlled, large study that has manipulated the dosages of both medication and behavioral treatment in social-recreational settings. Results replicate previous studies conducted in the STP setting (Pelham et al., 2005) with the addition of a lower-intensity behavioral treatment. The results show that (1) both behavioral treatment and MPH have large effects on the social behavior of children with ADHD, (2) the combination of the low doses of the two modalities has substantial beneficial effects, and (3) the presence and dose of either treatment influences the efficacy of the other treatment in several important ways. We will discuss each of these findings below.
The current results show large effects of BMOD on child behavior in a social-recreational setting. As with the classroom setting results reported previously (Fabiano et al., 2007), the means in Tables 2 and 3 (see also Figure 1) show substantial reductions in problematic behavior in social settings with increases in the intensity of behavior modification—even when children are taking moderate to large doses of medication (e.g., Figure 4). In the absence of medication, BMOD produced reductions in negative social behaviors (e.g., noncompliance) of up to five-fold relative to NBM, with both LBM and HBM conditions producing significant improvements.
When provided as the sole treatment, higher intensity BMOD produced greater improvement than lower intensity BMOD. These results echo single-case studies that demonstrate standard behavioral treatments are sufficient for many problems, but more intensive contingency management procedures are needed for more recalcitrant problems (see Pelham & Waschbusch, 1999 and Fabiano et al., 2009 for reviews). In the presence of a very low dose of medication, the two intensities of behavior modification continued to differ for two dependent measures (rule violations and interruptions); for the other four, LBM added to the low dose of medication produced such large improvements such that further increasing the BMOD intensity did not produce incremental improvement. At the .3 and .6 doses of medication, both LBM and HBM conditions continued to be significantly superior to NBM, demonstrating additive benefit of BMOD at these doses, but there were minimal differences between the lower and higher intensity conditions. These results clearly demonstrate the interactive nature of BMOD and medication. Clinicians or researchers who fail to adequately account for the presence of BMOD when evaluating medication, or medication when evaluating BMOD, risk drawing incorrect conclusions about the utility of multimodal treatments. Figure 1 shows most clearly that the dose-response curve for medication is highly dependent on the background level of behavior modification, with the highest dose of medication producing a small incremental benefit beyond high BMOD for only one of six negative behaviors. These results replicate previous findings in the social-recreational setting (Pelham et al., 2005) and in the STP classroom setting (Fabiano et al., 2007) with the HBM condition. The current results extend the previous findings by showing that the flattening of the medication dose-response curve occurs even in the presence of a lower-intensity behavioral treatment condition (Figure 1). The fact that side effects were minimal at the very low dose relative to the two higher doses highlights the potential benefits of combining the two low dose interventions.
Although many pairwise comparisons among increasing doses are statistically significant, the relative sizes of the differences in Tables 2 and 3 decrease as (a) medication dose increases or (b) intensity of BMOD increases. We have previously argued that medication effects may be enhanced by the presence of behavioral treatment (Pelham et al., 2001), particularly with regard to the effects of low doses. The current results show this empirically. For example, Figure 1 illustrates that children's rates of noncompliance on the lowest dose of medication and in the presence of behavior modification is the same as the rate on the highest dose alone. It should be noted that the background behavioral treatment in all our previous medication trials in the STP setting were comparable to the HBM condition in this study. Thus, our previously reported medication effects (e.g., Pelham, Greenslade et al., 1990, Pelham et al., 1999), particularly at lower doses, may overstate the magnitude of the medication effect.
The effect size findings shown in Figure 2 illustrate a number of interesting results. First, the effect of all treatments is in the moderate to very large range. Furthermore, the pairwise comparisons demonstrate several points about combined treatments. For example, the ES of all combined treatments (relative to no treatment) are greater than all unimodal treatments, with the exception that .6 mg/kg-alone is equivalent to the combination of LBM+0.15 mg/kg. We have previously shown similar results of combining very low medication doses with HBM (Pelham et al., 2005); this study expands on previous research by demonstrating a similar effect with lower-intensity behavior modification. When the additive effect sizes are examined, adding behavior modification to medication produces large ES (>1); these ES are equivalent to adding a low-to-moderate dose of medication to behavior modification.
These results stand in contrast with some previous studies, which have generally shown small differences between medication-alone compared to combined treatment, and larger effects of the addition of medication to behavior modification (e.g., Klein & Abikoff, 1997; MTA Cooperative Group, 1999a; Pelham et al., 1993). As the Figures show, this discrepancy can be illustrated by examining the different treatment dose combinations (see Figure 4). For example, adding a higher dose of medication to a low-intensity behavioral program (as many previous combined treatment studies have done) produces an increase in ES that is nearly double that of adding low-intensity behavior modification to the high dose of medication. On the other hand, adding high-intensity behavior modification to a low dose of medication produces an increase in ES that is larger than adding a low or moderate dose of medication to HBM, and equivalent to adding a high dose of medication to HBM. Had previous studies examined combinations of low-dose treatments, our results suggest that they would have found a substantial benefit to combined interventions. These findings suggest that any evaluations of combined treatments should take relative doses into account at the design and evaluation phase to make accurate conclusions.
Limitations
This study was conceptualized as a well-controlled, laboratory analogue-based efficacy study, because no previous studies of this type have been conducted. Therefore, results may be limited by the controlled treatment setting. Additional research in real-world home and school settings will be necessary to extend these findings. Additionally, the treatment period in this study was only 9 weeks, further broken down by treatment conditions. It will be necessary to study longer-term intervention to determine if these acute effects will maintain.
Although medication conditions were unknown, staff members were necessarily aware of behavior modification conditions. It is possible that this knowledge influenced results. However, the long history of the measures of staff frequency counts of observed behaviors, intensive training, and reliability procedures should have minimized possible bias; the convergent validity data provided by the independent reliability observer (who was not involved in treatment) suggest that this is the case. In addition, it cannot be determined whether the order in which behavioral treatments are administered impacted response to the behavioral or combined treatments. Further examination of individual difference factors will be necessary to determine whether specific dose combinations can be matched to individual children.
The behavioral conditions included packages of multiple components designed to vary in relative intensity. Thus, the time-out programs, the point system, the frequency of rewards, and the frequency of social skills training all varied between the three conditions. We cannot say with confidence which of these components produced the obtained results, and dismantling studies would be needed to disentangle the effects of components. Finally, our sample was reflective ethnically of the population of Erie County, New York, where the study was conducted, but it was nonetheless a predominantly white, middle class sample, and generalizability to samples with other demographic and ethnic characteristics remain to be demonstrated.
Clinical Implications
These results, coupled with similar findings in classroom and home settings (Fabiano et al., 2007, Pelham, Burrows-Maclean et al., 2013) imply that the prototypic child with ADHD could be treated with the equivalent of .15 mg/kg MPH (5 mg per dose in the current sample) twice-daily—a dose lower than that used in studies of stimulant treatment in the past 30 years—if he or she is receiving a moderate to high intensity behavioral treatment. Without any concurrent behavioral intervention, the same child would need 0.6 mg/kg (20 mg per dose) twice daily to cover school hours (the equivalent dose of Concerta would be 72 mg). Thus, our data show that stimulant doses can be reduced dramatically if a child is treated with behavior modification. Given concerns about long-term side effects such as growth reduction (Poulton, 2005, Swanson et al., 2007), providing behavioral interventions would appear to minimize the need for medication and maximize response to very low doses for the majority of children with ADHD. This is important because there has been a significant increase in stimulant medication use among elementary school age children over the last decade (Scheffler, Hinshaw, Modrek, & Levine, 2007), despite the fact that a majority of parents of children with ADHD strongly favor (at least initially) non-medication treatments and family preference is an important factor in sustainability of the intervention and therefore benefits (Waschbusch et al., 2011). Finally, the individual effect size data show that all children responded to at least one treatment combination, and that either increasing the intensity of behavioral intervention, increasing the dose of medication, or combining treatment modalities resulted in improved treatment effects.
Additional research should investigate a number of questions. As mentioned above, additional investigations should evaluate what are the active components of our LBM and HBM behavioral treatment strategies. Pelham and colleagues have begun to investigate which is the best sequence in which to introduce interventions (behavioral, pharmacological, or combined) and which is the best way to enhance initial treatment for nonresponders to low-dose treatments (Pelham, Fabiano, et al., 2013), as well as the cost effectiveness of combined treatment relative to higher doses of unimodal treatment (Page et al., 2013), and what child and family characteristics influence response. Finally, will these effects be replicable in home and school settings, and can the combined low dose intervention be shown to have long-term benefits?
Acknowledgments
This study was funded by a grant from the National Institute of Mental Health (MH62946). During the conduct of this study and preparation of this report, Dr. Pelham was also funded by grants from the National Institutes of Health (MH62946, MH69614, MH53554, MH69434, MH65899, MH78051,MH062946, NS39087, AA11873, DA12414, HD42080), and the Institute of Education Sciences (L03000665A). Dr. Fabiano was supported in part by a Ruth S. Kirschstein National Research Service Award Predoctoral Fellowship (1F31MH064243-01A1) and by the Department of Education, Institute of Education Sciences (R324J06024, , R324B06045).
This research was funded by a grant from the National Institute of Mental Health (MH62946). Dr. Pelham was also supported in part by grants from the National Institute of Mental Health (MH092466, MH53554, MH065899, MH62988), the Institute of Education Sciences (R37A120169 LO30000665A, R324B06045), the National Institute of Alcohol Abuse and Alcoholism (AA11873), the National Institute on Drug Abuse (DA12414, DA12986), and the National Institute of Child Health and Human Development (HD040935).
References
- Abikoff H, Hechtman L, Klein RG, Weiss G, Fleiss K, Etcovitch J, Pollack S. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:802–811. doi: 10.1097/01.chi.0000128791.10014.ac. doi:10.1097/01.chi.0000128791.10014.ac. [DOI] [PubMed] [Google Scholar]
- Abramowitz AJ, Eckstrand D, O'Leary SG, Dulcan MK. ADHD children's responses to stimulant medication and two intensities of a behavioral intervention. Behavior Modification. 1992;16:193–203. doi: 10.1177/01454455920162003. doi: 10.1177/01454455920162003. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Pelham WE, Milich R, Dixon J. Single and combined effects of methylphenidate and behavior therapy on the classroom performance of children with ADHD. Journal of Abnormal Child Psychology. 1992;20:213–232. doi: 10.1007/BF00916549. doi: 10.1007/BF00916549. [DOI] [PubMed] [Google Scholar]
- Chronis AM, Fabiano GA, Gnagy EM, Onyango AN, Pelham WE, Williams A, Seymour K. An evaluation of the summer treatment program for children with attention-deficit/hyperactivity disorder using a treatment withdrawal design. Behavior Therapy. 2004;35:561–585. doi: 10.1016/S0005-7894(04)80032-7. [Google Scholar]
- Chronis AM, Pelham WE, Gnagy EM, Roberts JE, Aronoff HR. The impact of late-afternoon stimulant dosing for children with ADHD on parent and parent-child domains. Journal of Clinical Child & Adolescent Psychology. 2003;32:118–126. doi: 10.1207/S15374424JCCP3201_11. doi: 10.1207/S15374424JCCP3201_11. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Eckert TL. The effects of school-based interventions for attention deficit hyperactivity disorder: A meta-analysis. School Psychology Review. 1997;26:5–27. [Google Scholar]
- Fabiano GA, Pelham WE, Coles EK, Gnagy EM, Chronis AM, O'Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clinical Psychology Review. 2009;29:129–140. doi: 10.1016/j.cpr.2008.11.001. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Gnagy EM, Burrows-MacLean L, Coles EK, Chacko A, Robb JA. The single and combined effects of multiple intensities of behavior modification and multiple intensities of methylphenidate in a classroom setting. School Psychology Review. 2007;36:195–216. [Google Scholar]
- Fabiano GA, Pelham WE, Waschbusch D, Gnagy EM, Lahey BB, Chronis AM, Burrows-MacLean L. A practical impairment measure: Psychometric properties of the Impairment Rating Scale in samples of children with attention-deficit/hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. doi:10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Hoza B, Pelham WE, Sams SE, Carlson C. An examination of the “dosage” effects of both behavior therapy and methylphenidate on the classroom performance of two ADHD children. Behavior Modification. 1992;16:164–192. doi: 10.1177/01454455920162002. doi: 10.1177/01454455920162002. [DOI] [PubMed] [Google Scholar]
- Hupp SDA, Reitman D, Northup J, O'Callahan P, LeBlanc M. The effects of delayed rewards, tokens, and stimulant medication on sportsmanlike behavior with ADHD-diagnosed children. Behavior Modification. 2002;26:148–162. doi: 10.1177/0145445502026002002. doi: 10.1177/0145445502026002002. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Boyle M, Cunningham C, Kim M, Schachar R. Evidence Report/Technology Assessment No. 11. Agency for Healthcare Research and Quality; Rockville, MD: 1999. Treatment of attention-deficit hyperactivity disorder. [Google Scholar]
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, Wigal T. 3-year follow-up of the NIMH MTA study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:989–1005. doi: 10.1097/CHI.0b013e3180686d48. doi:10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- Klein RG, Abikoff H. Behavior therapy and methylphenidate in the treatment of children with ADHD. Journal of Attention Disorders. 1997;2:89–114. doi: 10.1177/108705479700200203. [Google Scholar]
- Kolko DJ, Bukstein OG, Barron J. Methylphenidate and behavior modification in children with ADHD and comorbid ODD or CD: Main and incremental effects across settings. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:578–586. doi: 10.1097/00004583-199905000-00020. doi: 10.1097/00004583-199905000-00020. [DOI] [PubMed] [Google Scholar]
- Loney J, Milich R. Hyperactivity, inattention, and aggression in clinical practice. In: Wolraich M, Routh DK, editors. Advances in Developmental and Behavioral Pediatrics. Vol. 3. JAI Press; Greenwich, CN: 1982. pp. 113–147. [Google Scholar]
- MTA Cooperative Group 14-month randomized clinical trial of treatment strategies for attention deficit hyperactivity disorder. Archives of General Psychiatry. 1999a;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. doi:10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999b;56:1088–1096. doi: 10.1001/archpsyc.56.12.1088. doi:10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group National Institute of Mental Health multimodal treatment study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder (ADHD). Pediatrics. 2004;113:754–761. doi: 10.1542/peds.113.4.754. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- Northup J, Fusilier I, Swanson V, Huerte J, Bruce T, Freeland J, Edwards S. Further analysis of the separate and interactive effects of methylphenidate and common classroom contingencies. Journal of Applied Behavior Analysis. 1999;32:35–50. doi: 10.1901/jaba.1999.32-35. doi: 10.1901/jaba.1999.32-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor B, Fabiano GA, Waschbusch DA, Belin PJ, Gnagy EM, Pelham WE, Roemmich JN. Effects of a summer treatment program on functional sports outcomes in young children with ADHD. 2013 doi: 10.1007/s10802-013-9830-0. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T, Fabiano G, Greiner A, Waxmonsky J, Pelham WE, III, Gnagy E, Pelham WE., Jr. Comparative cost analysis of sequential, adaptive, behavioral, pharmacological, and combined treatments for ADHD. 2013 doi: 10.1080/15374416.2015.1055859. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE. Pharmacotherapy for children with attention-deficit hyperactivity disorder. School Psychology Review. 1993;22:199–227. [Google Scholar]
- Pelham WE, Aronoff HR, Midlam JK, Shapiro CJ, Gnagy EM, Chronis AM, Waxmonsky J. A comparison of Ritalin and Adderall: Efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics, 103. 1999 doi: 10.1542/peds.103.4.e43. Retrieved from: http://www.pediatrics.org/cgi/content/full/103/4/e43. [DOI] [PubMed]
- Pelham WE, Bender ME, Caddell J, Booth S, Moorer SH. Methylphenidate and children with attention deficit disorder: Dose effects on classroom academic and social behavior. Archives of General Psychiatry. 1985;42:948–952. doi: 10.1001/archpsyc.1985.01790330028003. doi:10.1001/archpsyc.1985.01790330028003. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Burrows-MacLean L, Massetti G, Coles EK, Wymbs BT, Chacko A, Waschbusch DA. Behavioral and pharmacological treatment for children with ADHD in the home setting. 2013 Manuscript submitted for publication. [Google Scholar]
- Pelham WE, Burrows-MacLean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, Hoffman MT. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Experimental and Clinical Psychopharmacology. 2005;13:111–126. doi: 10.1037/1064-1297.13.2.111. doi:10.1037/1064-1297.13.2.111. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Carlson C, Sams SE, Vallano G, Dixon MJ, Hoza B. Separate and combined effects of methylphenidate and behavior modification on boys with ADHD in the classroom. Journal of Consulting and Clinical Psychology. 1993;61:506–515. doi: 10.1037/0022-006X.61.3.506. doi:10.1037/0022-006X.61.3.506. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatment for attention deficit/hyperactivity disorder: An update. Journal of Clinical Child and Adolescent Psychology. 2008;37:185–214. doi: 10.1080/15374410701818681. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Fabiano G, Waxmonsky J, Greiner A, Gnagy E, Murphy S. Treatment sequencing for ADHD: An adaptive, multiple-randomization study of medication and behavioral interventions. 2013 doi: 10.1080/15374416.2015.1105138. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-MacLean L, Williams A, Fabiano GA, Morrisey SM, Morse G. Once-a-day Concerta™ methylphenidate versus t.i.d. methylphenidate in laboratory and natural settings. Pediatrics, 107. 2001 doi: 10.1542/peds.107.6.e105. Retrieved from: http://www.pediatrics.org/cgi/content/full/107/6/e105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III R symptoms of the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greiner AR, Hoza B, Hinshaw SP, Swanson JM, McBurnett K. Behavioral vs. behavioral and pharmacological treatment in ADHD children attending a summer treatment program. Journal of Abnormal Child Psychology. 2000;28:507–526. doi: 10.1023/a:1005127030251. doi: 10.1023/A:1005127030251. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greiner AR, Waschbusch DA, Fabiano GA, Burrows-MacLean L. Summer treatment programs for Attention Deficit/Hyperactivity Disorder. In: Weisz JR, Kazdin AE, editors. Evidence-based psychotherapies for children and adolescents. 2nd ed. The Guilford Press; New York: 2010. pp. 277–292. [Google Scholar]
- Pelham WE, Greenslade KE, Vodde-Hamilton MA, Murphy DA, Greenstein JJ, Gnagy EM, Dahl RE. Relative efficacy of long-acting stimulants on children with attention deficit-hyperactivity disorder: A comparison of standard methylphenidate, sustained-release methylphenidate, sustained-release dextroamphetamine, and pemoline. Pediatrics. 1990;86:226–237. [PubMed] [Google Scholar]
- Pelham WE, Greiner AR, Gnagy EM. Summer Treatment Program Manual. Comprehensive Treatment for Attention Deficit Disorders, Inc.; Buffalo, NY: 1997. [Google Scholar]
- Pelham WE, Lang AR, Atkeson B, Murphy DA, Gnagy EM, Greiner AR, Greenslade KE. Effects of deviant child behavior on parental alcohol consumption. The American Journal on Addictions. 1998;7:103–114. doi: 10.1111/j.1521-0391.1998.tb00325.x. [PubMed] [Google Scholar]
- Pelham WE, McBurnett K, Harper GW, Murphy DA, Milich R, Clinton J, Thiele C. Methylphenidate and baseball playing in ADHD children: Who's on first? Journal of Consulting and Clinical Psychology. 1990;58:130–133. doi: 10.1037/0022-006X.58.1.130. doi:10.1037/0022-006X.58.1.130. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Waschbusch DA. Behavioral intervention in ADHD. In: Quay HC, Hogan A, editors. Handbook of disruptive behavior disorders. Plenum; New York: 1999. pp. 255–278. [Google Scholar]
- Poulton A. Growth on stimulant medication; clarifying the confusion: A review. Archives of Disease in Childhood. 2005;90:801–806. doi: 10.1136/adc.2004.056952. doi:10.1136/adc.2004.056952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler RM, Hinshaw SP, Modrek S, Levine P. The global market for ADHD medications. Health Affairs. 2007;26:450–457. doi: 10.1377/hlthaff.26.2.450. doi: 10.1377/hlthaff.26.2.450. [DOI] [PubMed] [Google Scholar]
- Stage SA, Quiroz DR. A meta-analysis of interventions to decrease disruptive classroom behavior in public education settings. School Psychology Review. 1997;26:333–368. [Google Scholar]
- Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of Attention-Deficit/Hyperactivity Disorder in children and adolescents. Pediatrics. 2011;128:2011–2654. doi: 10.1542/peds.2011-2654. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, Volkow N. Effects of stimulant medication on growth rates across 3 years in the MTA Follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1014–1026. doi: 10.1097/chi.0b013e3180686d7e. doi:10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- Van der Oord S, Prins PJM, Oosterlan J, Emmelkamp PMG. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: A meta-analysis. Clinical Psychology Review. 2008;28:783–800. doi: 10.1016/j.cpr.2007.10.007. doi: 10.1016/j.cpr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA, Cunningham CE, Pelham WE, Rimas HL, Greiner AR, Gnagy EM, Hoffman MT. A discrete choice conjoint experiment to evaluate parent preferences for treatment of young, medication naïve children with ADHD. Journal of Clinical Child and Adolescent Psychology. 2011;40(4):546–561. doi: 10.1080/15374416.2011.581617. doi: 10.1080/15374416.2011.581617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- Werry J, Sprague R. Methylphenidate in children: Effect of dosage. Australian and New Zealand Journal of Psychiatry. 1974;8:9–19. doi: 10.3109/00048677409159770. doi: 10.3109/00048677409159770. [DOI] [PubMed] [Google Scholar]