Abstract
Resistant hypertension, defined as blood pressure (BP) remaining above goal despite the use of ≥3 antihypertensive medications at maximally tolerated doses (one ideally being a diuretic) or BP that requires ≥4 agents to achieve control, has received more attention with increased efforts to improve BP control rates and the emergence of device-based therapies for hypertension. This classically defined resistant group consists of patients with true resistant hypertension, controlled resistant hypertension and pseudo-resistant hypertension. In studies where pseudo-resistant hypertension cannot be excluded (for example, 24-h ambulatory BP not obtained), the term apparent resistant hypertension has been used to identify ‘apparent’ lack of control on ≥3 medications. Large, well-designed studies have recently reported the prevalence of resistant hypertension. Pooling prevalence data from these studies and others within North America and Europe with a combined sample size of >600 000 hypertensive participants, the prevalence of resistant hypertension is 14.8% of treated hypertensive patients and 12.5% of all hypertensives. However, the prevalence of true resistant hypertension, defined as uncontrolled both by office and 24-h ambulatory BP monitoring with confirmed medication adherence, may be more meaningful in terms of identifying risk and estimating benefit from newer therapies like renal denervation. Rates of cardiovascular events and mortality follow mean 24-h ambulatory BPs in patients with resistant hypertension, and true resistant hypertension represents the highest risk. The prevalence of true resistant hypertension has not been directly measured in large trials; however, combined data from smaller studies suggest that true resistant hypertension is present in half of the patients with resistant hypertension who are uncontrolled in the office. Our pooled analysis shows prevalence rates of 10.1% and 7.9% for uncontrolled resistant hypertension among individuals treated for hypertension and all hypertensive individuals, respectively.
Keywords: resistant, hypertension, epidemiology, blood pressure, control, prevalence
INTRODUCTION
The prevalence of hypertension has been reported from survey data collected in different industrialized countries and has ranged from 22% (Canada) to 55% (Germany) in the 1990s.1,2 Results of survey data collected after 2006 are limited; however, data from the United States and England report similar prevalence rates a decade later (29% in 2007 and 30% in 2006, respectively).3,4 With fairly stable hypertension prevalence rates, focus has shifted more from hypertension awareness toward blood pressure (BP) control.
Although defining BP control, particularly in certain subpopulations of individuals with hypertension, is still debated, the cardiovascular and mortality benefits of BP reduction have been well established through hypertension outcome trials.5–11 Early data indicated overall, poor BP control, especially in Europe where ≤10% of individuals with hypertension had a BP<140/90mmHg in the 1990s.12 Control rates across Europe have since improved with recent reports indicating 19% control in 2001 (Germany), 28% in 2006 (England) and 37% in 2009 (Italy).4,13,14
Interest in the hypertension population that is resistant to medical therapy has been renewed amidst a growing focus on improving BP control and the emergence of device-based therapies for hypertension (for example, catheter-based renal denervation and carotid sinus stimulation). Until recently, the prevalence of this population was not known.15,16 In the past few years, studies designed to define the epidemiology of resistant hypertension have emerged, and clinical trials investigating hard outcomes in resistant hypertension have been performed. This article will review the current data on the prevalence and outcomes of resistant hypertension and report a pooled estimate of resistant hypertensive prevalence in North America and Europe.
DEFINING APPARENT, TRUE AND PSEUDO-RESISTANT HYPERTENSION
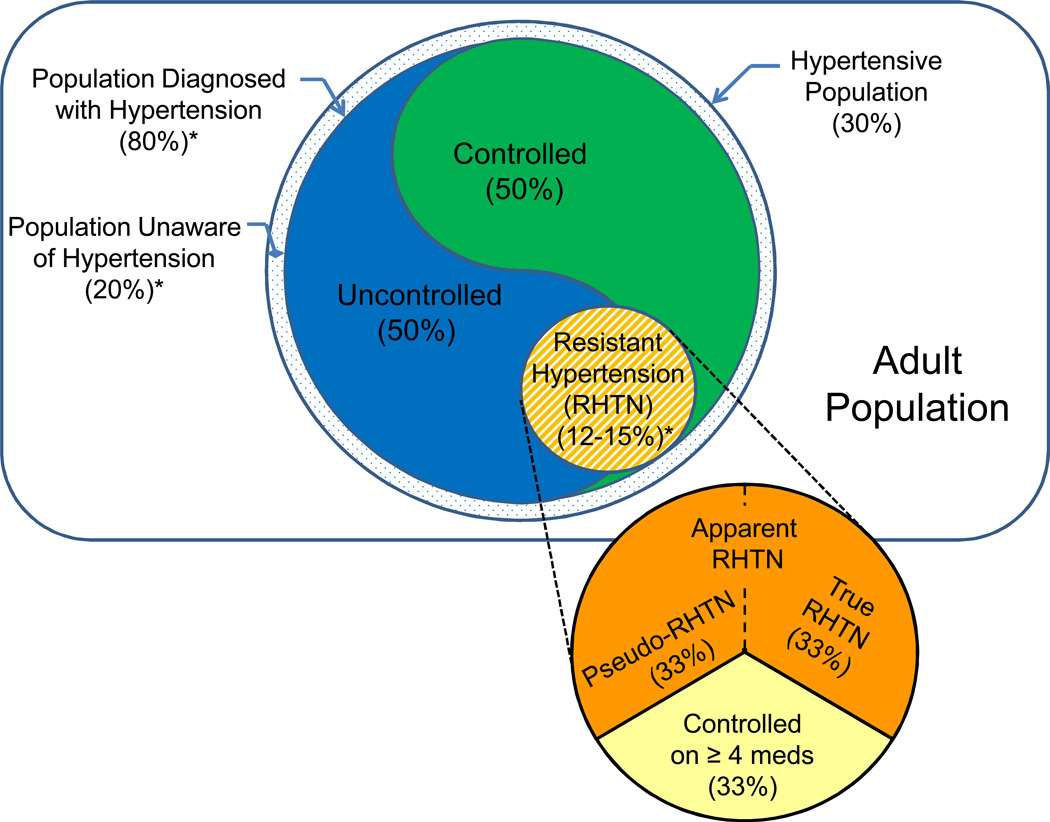
Resistant hypertension was initially defined to identify a group of high-risk patients who may benefit from specialized care, including evaluation and treatment of secondary causes of hypertension. The definition was established in an American Heart Association (AHA) scientific statement as a BP that remains above goal in spite of optimal doses of three antihypertensive agents of different classes, one ideally being a diuretic.17 By extension, if a patient achieves BP control with the addition of a fourth antihypertensive medication, he or she remains resistant. Accordingly, the resistant hypertensive population includes individuals with hypertension that are both controlled and uncontrolled by office measurement (Figure 1).
Figure 1.
Venn diagram of the prevalence of resistant hypertension. Area of subpopulations drawn to scale with estimated prevalences in percentages. Prevalences were estimated primarily from epidemiology studies performed within the United States.3,23 *Estimated prevalence among all hypertensive individuals.
The AHA definition of resistant hypertension does not make an attempt to distinguish between resistant and pseudo-resistant hypertension. Individuals with elevated office BPs due to white-coat hypertension, improper BP measurement or medication nonadherence do not have true resistant hypertension but have so-called pseudo-resistant hypertension.15,16 In order to clarify that pseudo-resistance had not been excluded, epidemiological studies adopted the term apparent resistant hypertension, when referring to the group of patients with an office BP>140/90mmHg while taking ≥3 antihypertensive medications.18 When pseudo-resistance has been excluded by 24-h ambulatory BP monitoring, proper office BP measurement technique and confirmation of medication adherence, then the distinction of true resistance can be made from apparent resistance (Figure 1). Therefore, true resistant hypertension is defined as a properly measured office BP>140/90mmHg with a mean 24-h ambulatory BP>130/80mmHg in a patient confirmed to be taking ≥3 antihypertensive medications. One of the challenges in establishing the prevalence of true resistant hypertension is excluding participants from the test population with pseudo-resistant hypertension.
PREVALENCE OF RESISTANT HYPERTENSION
The ideal study to estimate the prevalence of true resistant hypertension would require a prospective cohort study in a large hypertensive population with BP control established by forced titration up to full doses of three different classes of antihypertensive medications, including a diuretic.15 Also, pseudo-resistant hypertension would need to be excluded by an established method of establishing adequate medication adherence (that is, electronic pill bottle monitoring, pill counts or toxicology), standardized BP measurement and 24-h ambulatory BP monitoring. To date, this study has not been performed, and resistant hypertension prevalence is estimated from: (1) BP control data in population studies, (2) subpopulations of outcome-based clinical trials, (3) retrospective analyses of registry data, and (4) population studies specifically identifying resistant hypertension (Table 1).
Table 1.
Studies estimating the prevalence of resistant hypertension
| Study population | Study location | Study period (year) |
n | BP control rate (%) |
≥ 3 BP medications (n) |
Classica resistant HTN prevalenceb |
Uncontrolledc resistant HTN prevalenceb |
|
|---|---|---|---|---|---|---|---|---|
| Population Studies with BP control data | ||||||||
| Giannattasio et al.14 | Adults with HTN on stable antihypertensive treatment | Italy | 2009 | 8.3 | 22.3 | 1.86 | 17.4%d | 17.4%d |
| Falaschetti et al.4 | Persons aged ≥ 16 years and previously or newly diagnosed with HTN | England | 2006 | 1.4 | 52 | 0.28 | 12.0%d,e | 9.8%d |
| Subpopulations of outcome-based trials | ||||||||
| LIFE7 | Adults aged 55–80 years with BP >160/95mmHg and LVH by ECG | Scandinavia, United Kingdom, United States | 1995–1997 | 9.2 | 47.6 | 2.21 | 12.6%d | 12.6%d |
| INVEST20 | Adults aged ≥ 50 years with CAD and HTN | Australia, Canada, Germany, Israel, Italy, France, Mexico, New Zealand, South Africa, Spain, United States | 1997–2003 | 15.7 | 71.2 | 7.03 | 12.9%d | 12.9%d |
| ALLHAT33 | Adults aged >55 years with stage I or II HTN and at least one CV risk factor | North America | 1994–2002 | 12.2 | 65.6 | 3.33 | 14.6%d,e | 9.4%d |
| ACCOMPLISH9 | Hypertensive adults with a high risk for CV events | Scandinavia, Finland, United States | 2003–2007 | 10.4 | 73.9 | 3.36 | 8.4%d | 8.4%d |
| Retrospective analyses of registry data | ||||||||
| McAdam-Marx et al.21 | Electronic medical records (hypertensive adults) | United States | 2002–2005 | 29.4 | — | 2.67 | 13.4%e (9.7%e of all hypertensives) | 12.4% (9.1% of all hypertensives) |
| de la Sierra et al.22 | ABPM registry (treated hypertensive adults) | Spain | 2004–2009 | 68.0 | — | 10.05 | 14.8% | 12.2% (7.6% true resistant—excluding white-coat HTN) |
| Population studies specifically identifying resistant HTN | ||||||||
| Persell23 | NHANES (hypertensive adults) | United States | 2003–2008 | 6.1 | — | 0.54 | 12.8%e (8.9%e in all hypertensives) | 9.2% (6.4% in all hypertensives) |
| Egan et al.24 | NHANES (hypertensive adults) | United States | 2005–2008 | 3.6 | — | 0.42 | 16.4%e (11.8%e of all hypertensives) | 11.7% (8.4% of all hypertensives) |
| Sim et al.26 | Kaiser Permente Southern California (hypertensive adults) | United States | 2006–2007 | 470.4 | 67.2 | 60.3 | 15.3%e (12.8%e of all hypertensives) | 9.4% (7.9% of all hypertensives) |
Abbreviations: ABPM, ambulatory blood pressure monitoring; AHA, American Heart Association; BP, blood pressure; CAD, coronary artery disease; CV, cardiovascular; ECG, electrocardiogram; HCTZ, hydrochlorothiazide; HTN, hypertension; LVH, left ventricular hypertrophy; n, sample size (per 1000 participants); NHANES, National Health and Nutrition Examination Surveys.
Classic defined using AHA criteria and includes both uncontrolled and controlled (when data were available) groups.
Prevalence reported in the population of treated hypertension unless otherwise noted.
Uncontrolled defined as an office BP≥ 140/90mmHg.
Calculated assuming that BP control rates are similar in the population receiving ≥ 3 medications and the sample population.
Prevalence calculation includes the number controlled on ≥ 4 antihypertensive medications.
Population studies with BP control data
Population studies on the prevalence, treatment and control of hypertension provide indirect estimates of the prevalence of resistant hypertension. These studies often report overall BP control rates as well as the number of patients receiving ≥3 antihypertensive medications. By assuming similar control rates within the population taking at least three medications, one can estimate the percentage of patients uncontrolled on ≥3 medications and enumerate the prevalence of apparent resistant hypertension.
In an Italian study of patients being treated for hypertension by a general practitioner in 2009, 1856 of the 8299 patients were treated with at least three medications. The percentage of diuretic use and optimal medication dosing was not reported, although an angiotensin-converting enzyme inhibitor plus a diuretic was the most widely used two-drug combination. BP control rates, as defined by the European Society of Hypertension and European Society of Cardiology guidelines,19 were 22.3% in the group receiving ≥3 medications.14 Those uncontrolled on ≥3 medications in this treated hypertensive population would be estimated at 17.4%.
A study surveying the English population in 2006 identified hypertension in 30% of >7000 participants. Among those with hypertension, 1375 (54%) were treated. Two hundred and twenty of the treated participants were taking three medications, and 60 were taking four medications. Diuretic use was reported in 76% of those taking three antihypertensive medications. In the group being treated for hypertension, 52% had their BP controlled (<140/90mmHg).4 From this study, the prevalence of resistant hypertension is estimated by including both those uncontrolled on three medications plus those receiving four medications, which represented 12.0% of the treated hypertensive population. If an estimated 31 persons who were controlled on ≥4 medications were excluded, then 9.8% of the treated population consisted of persons with uncontrolled resistant hypertension (Table 1).
Subpopulations of outcome based clinical trials
Two important limitations of estimating resistant hypertension prevalence from BP control data in population studies are that the antihypertensive medication regimen cannot be confirmed to be optimal (that is, appropriate dosing of antihypertensive medications of different classes, including a diuretic) and medication adherence is unknown. Obtaining BP control data from clinical trials should lessen these limitations as antihypertensive medications are titrated by protocol, are provided without cost and adherence strictly monitored. Similar to the population studies with BP control data, outcome-based clinical trials do not report the prevalence of resistant hypertension. However, applying the published control rates to the group requiring ≥3 antihypertensive medications, an estimate of the prevalence can be calculated.
In the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT), >33 000 patients aged>55 years with hypertension and at least one additional cardiovascular risk factor were randomized to an initial antihypertensive medication (that is, chlorthalidone, amlodipine, doxazosin or lisinopril) with additional medications used if needed to obtain BP control. After 5 years of follow–up, 12 210 participants had BP control data for analysis and 65.6% achieved a BP<140/90mm Hg. At study completion, 27.3% were taking ≥3 antihypertensive medications, and approximately 8% of those with controlled BPs were receiving ≥4 medications. Diuretic use at 5 years was reported as 80.5%, 16.6% and 15.7% for the chlorthalidone, amlodipine and lisinopril arms, respectively.6,19 By calculating both the number of participants uncontrolled on ≥3 medications (n=1 147) and the number of participants controlled on ≥4 medications (n=641), the prevalence of resistant hypertension in this North American-treated hypertensive population was approximately 14.6%. When considering only those uncontrolled on ≥3 medications, the prevalence falls to 9.4% (Table 1).
In the International Verapamil-Trandolapril Study (INVEST), 22 576 patients aged ≥50 years with hypertension and coronary artery disease were randomized to sustained release verapamil or atenolol as baseline BP therapy. Trandolapril and/or hydrochlorothiazide was added to achieve a goal BP (<140/90mmHg or <130/95mmHg in those with diabetes mellitus or chronic kidney disease). After 2 years of follow-up, 44.7% (7025 out of 15 692 participants) required ≥3 antihypertensive medications and 71.2% achieved a BP<140/90mmHg.20 In this treated hypertensive population, the prevalence of resistant hypertension was 12.9%.
In the Avoiding Cardiovascular events through COMbination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial, 11 506 patients with hypertension and at high risk for cardiovascular events were randomized to benazepril plus amlodipine or benazepril plus hydrochlorothiazide with the addition of other antihypertensive agents to achieve BP control (defined as BP<140/90mmHg). At 1 year of follow-up, 32.3% of patients were receiving the maximum dosing of study medication in addition to at least one other antihypertensive medication. One-year BP control rates were 75.4% in the benazepril plus amlodipine group and 72.4% in the benazepril plus hydrochlorothiazide group.9 In the 10 400 patients with BP control data at 1 year of follow-up, the prevalence of resistant hypertension can be estimated at 8.4%.
In the Losartan Intervention For Endpoint reduction in hypertension study (LIFE), 9193 participants aged 55–80 years with a BP >160/95mmHg and evidence of left ventricular hypertrophy by electrocardiogram were randomized to losartan-based or atenolol-based therapy. After a mean follow-up of 4.8 years, 24% of participants were receiving ≥3 medications and 47.6% of participants achieved a BP<140/90mmHg.7 The estimated prevalence of resistant hypertension in this treated hypertensive population is 12.6%.
Retrospective analysis of registry data
In outcome trials, the antihypertensive medication regimen is set by protocol and may not be designed to combine three drugs of different classes or preferentially use a diuretic. Also, study populations in outcome trials were selected to meet predefined levels of cardiovascular risk, and patients with difficult to treat BP may have been disproportionately excluded or included by protocol. Hypertension registries are attractive data sets for estimating prevalence, because they may be a more representative sample of the larger hypertensive population and avoid selective exclusion or inclusion of patients.
A cross-sectional study designed to report the prevalence of resistant hypertension identified >29 000 hypertensive adults in a majority primary care research database from 2002 to 2005.21 Resistant hypertension was defined as having a BP>140/90mmHg (or>130/80mmHg in patients with kidney disease or diabetes mellitus) on ≥3 medications, one being hydrochlorothiazide. Using this definition, 2670 patients (9.1% of the total hypertensive population and 12.4% of the treated hypertensive population) were classified as resistant.21 The large hypertensive cohort allowed for stratification of BP control by the number of antihypertensive medications. Broadening the definition of resistant hypertension to include those controlled on ≥4 medication changes the prevalence only slightly (9.7% of the total hypertensive population and 13.4% of the treated hypertensive population).
Given that this analysis used one of the largest study cohorts to date to estimate the prevalence of resistant hypertension, it is worth identifying some of the study limitations. The study design attempts to select resistant patients based on a medication regimen that is close to ideal by requiring hydrochlorothiazide as one of the three or more medications. However, optimal drug dosing cannot be established retrospectively, maintaining suboptimal therapy as a cause of overestimation of true prevalence. Also, in the United States between 2002 and 2005 hydrochlorothiazide was the most commonly prescribed diuretic used to treat hypertension; however, other diuretic use, including loop diuretics in patients with chronic kidney disease, is missed when selecting patients solely by hydrochlorothiazide use. Lastly, pseudo-resistant hypertension due to medication nonadherence or white-coat hypertension could not be detected.
A study by de la Sierra et al.22 attempted to distinguish between resistant hypertension and pseudo-resistant hypertension due to white-coat hypertension. Over 68 000 treated hypertensive patients were selected from a Spanish ambulatory BP monitoring registry in 2009. Among them, 10 052 (14.8%) were identified as resistant, defined by an office BP ≥140/90mmHg while receiving ≥3 antihypertensive medications, one being a diuretic or treatment with >4 antihypertensive medications regardless of office BP. Twenty-four hour ambulatory BP monitoring data were analyzed in the subpopulation of resistant hypertension that was uncontrolled on ≥3 medications (8295 patients, 12.2%). In this uncontrolled group, 37.5% (3113 patients) were found to have white-coat hypertension, defined by a 24 h average BP<130/80mmHg.22 Therefore, the prevalence of true resistant hypertension (white-coat hypertension excluded) was estimated at 7.6% of this treated hypertensive population.
The study by de la Sierra et al.22 is the second largest study reporting resistant hypertension prevalence and the only study designed to differentiate between resistant and white-coat-related pseudo-resistant hypertension. Medication adherence and optimization of the medication regimen could not be evaluated. However, these limitations would not affect 24-h ambulatory BPs. In fact, accounting for poor medication adherence or a suboptimal medication regimen would tend to increase the percentage of white-coat related pseudo-resistant hypertension in the resistant population.
Population studies specifically identifying resistant hypertension
Data from the National Health and Nutrition Examination Survey (NHANES), which selects non-institutionalized adults in the United States through stratified, multistage probability sampling, has been used to examine hypertension awareness, treatment and control. Persell23 identified close to 6000 adults with hypertension from the 15 070 NHANES volunteers with at least one BP reading from 2003 through 2008. In this group, 539 volunteers (8.9% of all hypertensives and 12.8% of all treated hypertensives) met criteria for resistant hypertension (BP>140/90mmHg on ≥3 drugs or controlled on ≥4 drugs).23 Excluding those controlled on ≥4 medications (151 volunteers), uncontrolled resistant hypertension was seen in 9.2% of treated hypertensives and 6.4% of all hypertensives (Table 1). This definition of resistance did not include diuretic use, though the majority (85.6%) of the resistant population was on a diuretic. As with all population studies, the prevalence of resistant hypertension is underestimated by under treatment (28.3% of all treated hypertensives remained uncontrolled on≤2 antihypertensive medications). However, optimizing the medical regimen (ensuring maximum drug dosing, different drug classes and diuretic use) in patients on three medications may lead to more BP control, making suboptimal antihypertensive therapy a potential over-estimation of resistant hypertension prevalence.
In a subsequent study, Egan et al.24 subdivided the NHANES data set into time periods in order to estimate trends of resistant hypertension prevalence. Resistant hypertension (BP ≥140/90mmHg on ≥3 medications or controlled on ≥4 medications) among all hypertensives increased from 8.5% (1999–2004) to 11.8% (2005–2008).24 The rise in resistant hypertension was despite an improvement in overall BP control rates (27.3% in 1988–1994 to 50.1% in 2007–2008).3,25 Uptitration of the number of antihypertensive medications may be the main contributor to this discordance. The percentage of treated hypertensives receiving one medication fell between each of the three time periods (1988–1994, 1999–2004 and 2005–2008), while the percentages of hypertensives receiving ≥3 medications rose between the three time periods. It appears that physicians, over time, were prescribing more antihypertensive medications, explaining the increasing BP control and the shift toward more patients on ≥3 medications. With a more optimized medication regimen, the 2005–2008 prevalence of 11.8% of all hypertensive patients likely represents a truer estimate of the prevalence of AHA-defined resistant hypertension.
In the largest study to date to report a prevalence of resistant hypertension, Sim et al.26 identified >470 000 persons aged>17 years and diagnosed with hypertension in the Kaiser Permente Southern California health system. Results showed that 12.8% of all hypertensive individuals or 15.3% of all treated individuals met the classic definition of resistant hypertension, which includes those controlled on ≥4 medications.26 Notably, BP control was higher than expected both within the entire hypertensive cohort as well as within the group with resistant hypertension. In total, 23 266 out of the 60 327 resistant individuals (38%) were controlled in the office. When excluding this controlled resistant group, the prevalence of uncontrolled resistant hypertension fell to 9.4% and 7.9% in all hypertensive individuals and treated individuals, respectively (Table 1).
Although unable to directly identify poor BP measurement technique or white-coat hypertension, Sim et al.26 did estimate medication adherence. Pharmacy dispense and refill histories were accessed, and medication adherence was defined as an average of at least 80% of days covered for the prescribed medications. Adherence was high throughout the entire hypertensive cohort (90%) and unexpectedly high among those with resistant hypertension (93%). The high medication adherence as well as the use of a simplified BP treatment algorithm within the Kaiser Permente Southern California health system may explain the substantial portion of BP control within the resistant hypertension group.
OUTCOMES OF RESISTANT HYPERTENSION
An increase in cardiovascular risk for individuals with resistant hypertension compared with individuals with non-resistant hypertension has been shown in retrospective analyses of databases and confirmed in prospective studies. Daugherty et al.27 retrospectively analyzed 18 036 adults with hypertension within the United States Cardiovascular Research Network hypertension registry from 2002 to 2006. After a median of 3.8 years, resistant hypertension, defined by AHA criteria, was associated with an increased risk of adverse cardiovascular outcomes (hazard ratio, 1.47; 95% confidence interval (CI), 1.33– 1.62; P<0.001) by multivariable adjusted Cox model.27 These findings were corroborated in the large, retrospective analysis by Sim et al.27 Although not directly assessing outcomes, Sim et al.27 did use an adjusted logistic regression model to show that individuals with resistant hypertension had an increased odds of cardiovascular comorbidities, including ischemic heart disease (odds ratio, 1.34; 95% CI, 1.30–1.39), congestive heart failure (odds ratio, 1.78; 95% CI 1.72–1.86) and chronic kidney disease (odds ratio, 1.84; 95% CI, 1.78–1.90) when compared with individuals with non-resistant hypertension.26
Data from prospective studies suggest that cardiovascular events directly correlate with mean 24-h ambulatory BP in individuals with resistant hypertension, highlighting the importance of distinguishing between true resistant hypertension and pseudo-resistance due to white-coat hypertension. Pierdomenico et al.28 evaluated the occurrence of fatal and nonfatal cardiovascular events in 340 patients with a controlled clinic BP, 146 patients with white-coat hypertension and 130 patients with true resistant hypertension where white-coat hypertension was excluded. During a 4.8 year follow-up period, the event rate per 100 patient-years was a 0.87, 1.2 and 4.1 in the controlled hypertension, white-coat hypertension and true resistant hypertension groups, respectively. After covariate adjustment, individuals with true resistant hypertension had a 2.94 times greater cardiovascular risk when compared with the controlled hypertension group. Notably, in this analysis no significant difference in cardiovascular risk was detected between individuals with white-coat hypertension and those with controlled hypertension.28 A prospective follow-up study of 556 patients with resistant hypertension extended the known increased cardiovascular risk to all-cause mortality risk in individuals who were uncontrolled both in the office and by 24-h ambulatory BP monitoring. After adjustments for age, sex, body mass index, diabetes mellitus, current smoking, physical inactivity, dyslipidemia, previous cardiovascular disease, serum creatinine level and number of antihypertensive medications in use, true resistant hypertension was found to have twice the chance of dying when compared with white-coat hypertension (hazard ratio, 2.00; 95% CI, 1.12–3.55). Furthermore, in this study higher mean ambulatory BPs were independent predictors of the composite end points of fatal and nonfatal cardiovascular events.29 This association between higher mean ambulatory BPs and cardiovascular events in patients with resistant hypertension has been previously reported.30
CONCLUSIONS
Although the ideal study to estimate the prevalence of true resistant hypertension has not been performed, there exist a number of well-designed trials from which prevalence rates of resistant hypertension can be estimated (Table 1). Prevalence rates for resistant hypertension meeting the AHA criteria ranged from 8.4% to 17.4% among individuals treated for hypertension with a pooled average of 14.8%. In the four studies where resistant hypertension prevalence among all hypertensive individuals was estimated, prevalence rates ranged from 8.9% to 12.8% with an average of 12.6%.21,23,24,26 Due to the direct relationship between mean 24-h ambulatory BP and adverse cardiovascular outcomes, reporting the prevalence of uncontrolled resistant hypertension may be more important for risk assessment and estimating benefit from device-based therapies for hypertension. Pooled prevalence rates for uncontrolled resistant hypertension are 10.1% and 7.9% among treated and all hypertensive individuals, respectively.
When interpreting the results from studies discussed above, it is important to identify the potential underestimation and overestimation of prevalence rates. Failure to uptitrate the number of antihypertensive medications in those uncontrolled on 1–2 medications (that is, retrospective analyses), patient drop out and patient exclusion would all underestimate the number of resistant patients and therefore underestimate the prevalence of resistant hypertension. Pseudo-resistant hypertension from white-coat hypertension, medication nonadherence or inaccurate BP measurement and suboptimal medication regimens of ≥3 medications (duplicate drug classes, medication under dosing or failure to include a diuretic) would overestimate the number or resistant patients and therefore overestimate the prevalence of resistant hypertension.
For example, uncertainty exists when interpreting the prevalence rates from outcome-based studies like ALLHAT, LIFE, INVEST and ACCOMPLISH. In these studies, it is difficult to know if disproportionately greater or fewer individuals with resistant hypertension were included. Specifically selecting participants based on cardiovascular risk may have increased the likelihood of including individuals with resistant hypertension. Furthermore, protocols for titration of antihypertensive medication were not designed to optimize three or four drug regimens. A non-optimal multi-drug regimen would falsely inflate resistant hypertension prevalence by worsening BP control. Of the four outcome trials, ACCOMPLISH showed the highest control rate by adding spironolactone, alpha-blockers or beta-blockers to amlodipine plus benazepril versus hydrochlorothiazide plus benazepril. The resulting number of individuals uncontrolled on ≥3 medications was low (8.4% among treated hypertensive individuals).
Some of the studies provide insight into the amount of underestimation or overestimation associated with each cause. For example, de la Sierra et al.22 found that 35.7% of the uncontrolled resistant population had white-coat hypertension. Daugherty et al.27 found that 12.4% of the uncontrolled resistant population was nonadherent to their medical regimens, as defined by pharmacy refill rates. Data from Egan et al.24 suggest that with uptitration in the number of antihypertensive medications, the prevalence of resistant hypertension increases up to 12.1%. In addition, a study designed to identify the degree of medicine adherence within the resistant hypertension population showed that 53% of the uncontrolled individuals were nonadherent by urine toxicology.31 Among those who were nonadherent, 30% were taking no antihypertensive medication. Taken together, these studies suggest that pseudo-resistant hypertension due to nonadherence or white-coat hypertension likely make up half of all uncontrolled resistant hypertensive individuals. Therefore, the prevalence of true resistant hypertension would be estimated at 5.0% and 4.0% among treated and all hypertensive individuals, respectively.
This small group of true resistant individuals, who stand to gain the most from early identification and aggressive treatment, is expected to shrink further over time. With concerted efforts on improving BP control assisted by the dissemination of strategies to simplify and optimize multiple medication regimens, BP control rates will likely continue to rise. This effect was seen in the study by Sim et al.22 The use of a simplified BP treatment algorithm resulted in BP control rates of ~67% in 2006–2007, which were higher than the 50% reported using the NHANES data for the same time period.24 Notably, the Kaiser Permente BP treatment algorithm was updated to include mineralocorticoid antagonists in 2009 after the study period. Employing optimal dosing of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker with a dihydropyridine calcium channel blocker and a diuretic, appropriately screening for secondary causes of hypertension and adding a mineralocorticoid receptor antagonist, BP control rates in individuals with resistant hypertensive can reach 91.5%.32 Given that a small proportion of hypertensive patients fail optimal medication therapy, well-designed efficacy studies are needed to define the role of device-based therapies for treatment of resistant, both controlled and uncontrolled. Certainly, patients failing effective antihypertensive regimens warrant consideration for device-based treatments, but even controlled patients may be appropriate for such therapies if their quality of life can be substantially improved by reducing their pill burden and/or associated medication-related adverse events.
ACKNOWLEDGEMENTS
This review was supported by the National Institutes of Health Grant T32 HL007457 (to EJ).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22:11–19. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension and blood pressure levels in 6 European countries, Canada, and the US. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 3.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 4.Falaschetti E, Chaudhury M, Mindell J, Poulter N. Continued improvement in hypertension management in England: results from the Health Survey for England 2006. Hypertension. 2009;53:480–486. doi: 10.1161/HYPERTENSIONAHA.108.125617. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Gentile G, Angeli F, Mazzotta G, Mancia G, Reboldi G. Influence of blood pressure reduction on composite cardiovascular endpoints in clinical trials. J Hypertens. 2010;28:1356–1365. doi: 10.1097/HJH.0b013e328338e2bb. [DOI] [PubMed] [Google Scholar]
- 6.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 7.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 8.Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356:366–372. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 9.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, et al. ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, Barzi F, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336:1121–1123. doi: 10.1136/bmj.39548.738368.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert PR, Moser M, Mayer J, Glynn RJ, Hennekens CH. Recent evidence on drug therapy of mild to moderate hypertension and decreased risk of coronary heart disease. Arch Intern Med. 1993;153:578–581. [PubMed] [Google Scholar]
- 12.Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 13.Sharma AM, Wittchen HU, Kirch W, Pittrow D, Ritz E, Göke B, et al. HYDRA Study Group. High prevalence and poor control of hypertension in primary care: cross-sectional study. J Hypertens. 2004;22:479–486. doi: 10.1097/00004872-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Giannattasio C, Cairo M, Cesana F, Alloni M, Sormani P, Colombo G, et al. Blood pressure control in Italian essential hypertensives treated by general practitioners. Am J Hypertens. 2012;25:1182–1187. doi: 10.1038/ajh.2012.108. [DOI] [PubMed] [Google Scholar]
- 15.Sarafidis PA. Epidemiology of resistant hypertension. J Clin Hypertens (Greenwich) 2011;13:523–528. doi: 10.1111/j.1751-7176.2011.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension—its identification and epidemiology. Nat Rev Nephrol. 2013;9:51–58. doi: 10.1038/nrneph.2012.260. [DOI] [PubMed] [Google Scholar]
- 17.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. American Heart Association Scientific Statement. Resistant hypertension: diagnosis, evaluation, and treatment. Hypertension. 2008;51:1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun DA. Apparent and true resistant hypertension: why not the same? J Am Soc Hypertens. 2013;7(6):509–511. doi: 10.1016/j.jash.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 20.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease: the International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 21.McAdam-Marx C, Ye X, Sung JC, Brixner DI, Kahler KH. Results of a retrospective, observational pilot study using electronic medical records to assess the prevalence and characteristics of patients with resistant hypertension in an ambulatory care setting. Clin Ther. 2009;31:1116–1123. doi: 10.1016/j.clinthera.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 22.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Amario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 23.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 24.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988–2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 26.Sim JJ, Bhandari SK, Shi J, Liu IL, Calhoun DA, McGlynn EA, et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88:1099–1107. doi: 10.1016/j.mayocp.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–1428. doi: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 30.Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension. 1998;31:712–718. doi: 10.1161/01.hyp.31.2.712. [DOI] [PubMed] [Google Scholar]
- 31.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–774. doi: 10.1097/HJH.0b013e32835e2286. [DOI] [PubMed] [Google Scholar]
- 32.Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, et al. Refractory hypertension: definition, prevalence, and patient characteristics. J Clin Hypertens (Greenwich) 2012;14:7–12. doi: 10.1111/j.1751-7176.2011.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, et al. The ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid- Lowering and Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]