Abstract
Nanocomposites consisting of oblong ultrathin plate shaped calcium phosphate nanoparticles and graphene oxide microflakes were synthesized and have demonstrated markedly synergistic effect in accelerating stem cell differentiation to osteoblasts.
Stem cells (SCs) including mesenchymal, embryonic and induced pluripotent SCs possess the potential to differentiate toward multiple lineages such as bone, fat and muscle, and therefore offer promising opportunities for developing SCs-based regenerative therapy and tissue engineering. However, to fully exploit the application of SCs in tissue regeneration, it is critical to develop biochemical, chemical, or physical factors that can control the self-renewal and differentiation of SCs toward the desired lineage. Recently, graphene family nanomaterials such as graphene (G) and graphene oxide (GO) attracted substantial interest as new platforms for SCs-based biomedical applications, because of their physicochemical properties, distinctive nanostructure and outstanding mechanical properties. Both materials, especially GO with more hydrophilic surface, promote cell adhesion, proliferation, and differentiation.1 Substrates coated with G or GO demonstrated the capability to facilitate SCs differentiation toward multiple specific lineages.2 Enhanced osteogenesis, adipogenesis, and epithelial genesis in various SCs has been reported on GO coated surface,3 suggesting G and GO as promising base materials for building scaffolds and composites for SCs-based tissue engineering.
Here we hypothesized that incorporating GO with an osteoinductive material could synergistically direct the differentiation of human mesenchymal stem cells (hMSCs) toward osteogenic lineage. Calcium phosphates (CaP) such as hydroxyapatite (HAp) are biomimetic biomaterials that are well-recognized for their osteoconductivity (facilitating bone formation) and osteoinductivity (facilitating the osteogenic differentiation of hMSCs).4 To validate the above hypothesis, we synthesized a novel biocompatible GO-CaP nanocomposite and evaluated its capability of inducing the osteogenic differentiation in hMSCs. The GO-CaP nanocomposite was fabricated using GO microflakes, uniquely structured highly osteoinductive CaP nanoparticles, and Pluronic® polymeric coating. The osteoinductive properties of GO microflakes, CaP, and GO-CaP on hMSCs were evaluated by quantitative measurements on bone nodule formation and the immunofluorescence imaging of osteoblast biomarkers. GO-CaP exhibited osteogenic capability that was superior to individual or combined effects of GO and CaP.
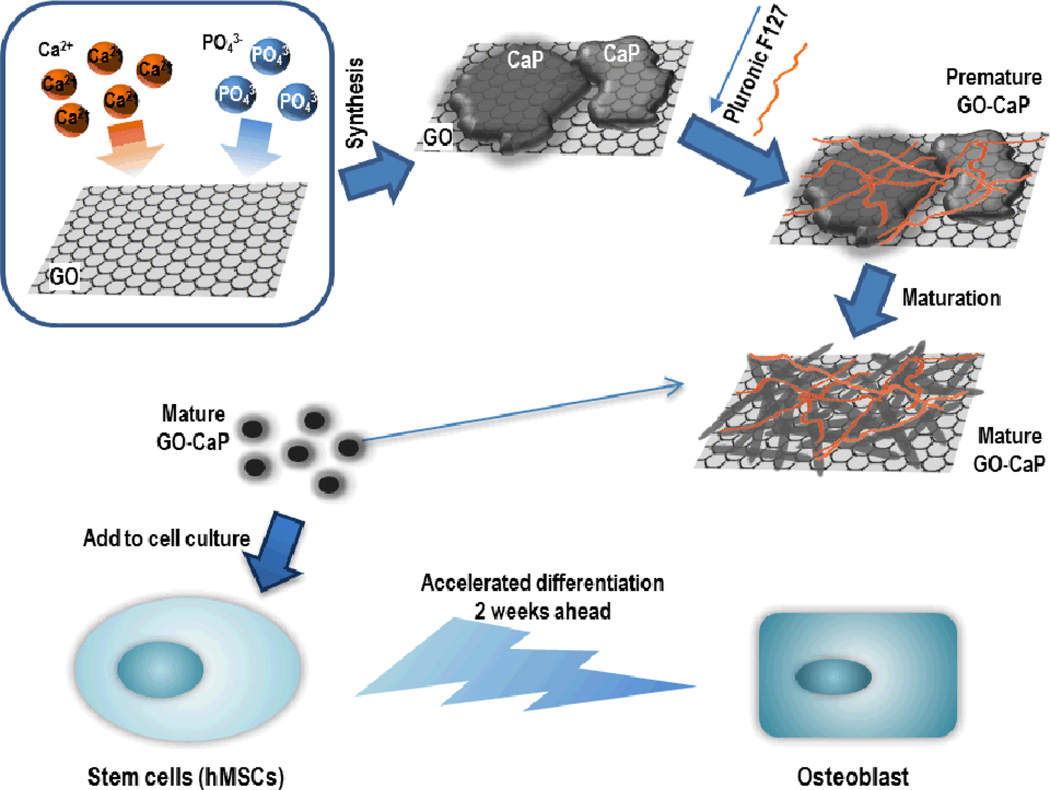
The fabrication process of GO-CaP nanocomposite is illustrated in Fig 1. Pluronic® F127 coated GO-CaP and CaP (referred as GO-CaP and CaP in the following texts) were synthesized via a double-reverse microemulsion method modified from previous report.5 Detailed procedures are described in ESI†. Once fabricated, the particles are stored in deionized water for 2 to 3 weeks to allow for crystal maturation. The maturation process is an essential procedure to obtain the highly osteoinductive nanoparticles in this study.
Fig. 1.
Schematic illustration of fabrication procedure for GO-CaP nanocomposites, and subsequent synergistic acceleration of osteogenesis in hMSCs by GO-CaP.
The relative weight percentage of GO flakes and CaP nanoparticles in the recipe of the nanocomposites was determined by analysing the cytotoxicity of each individual component. The cytotoxicity of GO and CaP was evaluated by measuring the cell viability of hMSCs after 3 days of incubation with different particle dosages using the colorimetric MTT assay (Fig. S1 in ESI†). With neutralized GO, the cell viability of hMSCs maintained above 60% when the final concentration of GO in the culture medium was ≤ 1 µg/ml, and fell sharply to < 20% at concentration above 10 µg/ml (Fig. S1.A). CaP nanoparticles demonstrated remarkable biocompatibility with > 75% viability when final concentration is ≤ 50 µg/ml (Fig. S1.B). Based on these outcomes, the optimal recipe for GO-CaP nanocomposite was determined to be 0.5 µg/ml GO and 10 µg/ml calcium phosphate, or a weight ratio of 1:20. Cell viability with GO-CaP treatment was above 80% with 10.5 µg/ml GO-CaP particles (Fig. S1.C). To achieve the highest osteogenesis while maintaining the most cell viability, 10.5 µg/ml GO-CaP was determined for the differentiation of hMSCs in the present study. To achieve valid comparison, we used GO and CaP at the same concentration as their presence in GO-CaP, or 0.5 µg/ml GO and 10 µg/ml CaP for all the following tests.
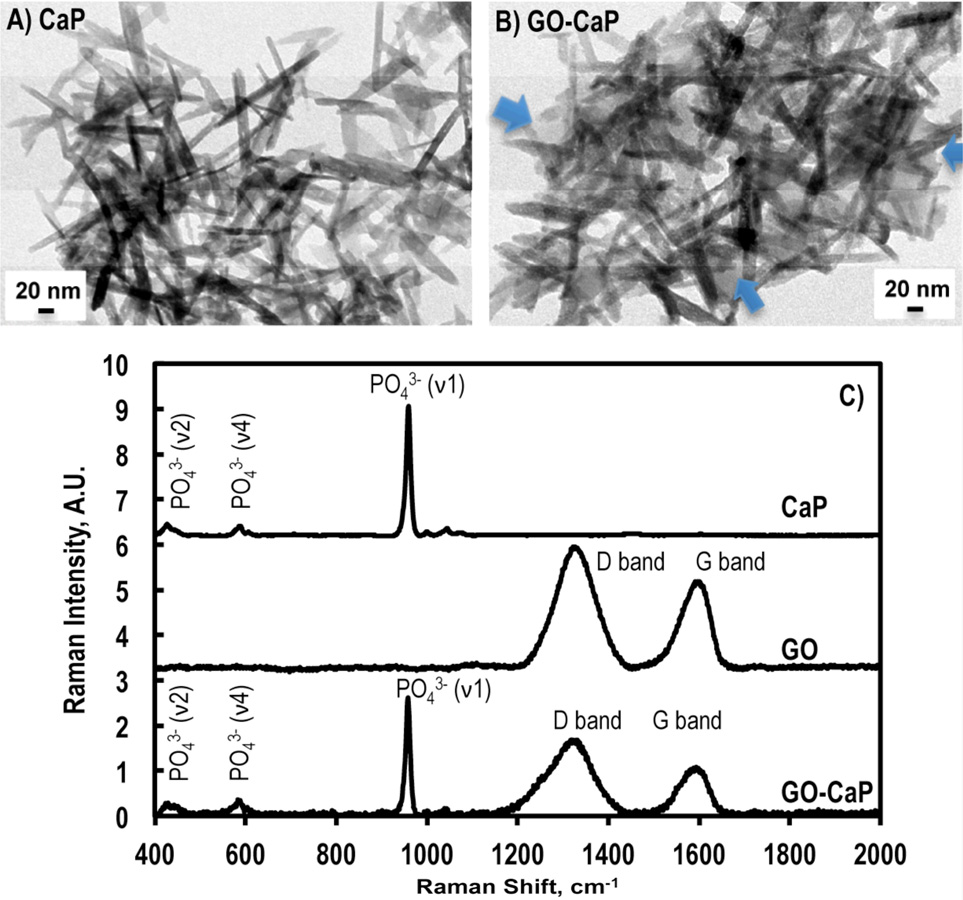
The structural morphology of GO, CaP and GO-CaP was interrogated using transmission electron microscopy (TEM). The GO microflakes were commercially purchased (Nanocs Inc, New York, NY) with a size of 0.5 – 5 microns. Over 80% of the flakes consist of a single atomic layer. CaP nanoparticles exhibited an irregular plate shape when freshly made (Fig. S2A-I), gradually separated and elongated to rod-shaped platelet in a week (Fig 2A-II), and stabilized in the form of ultra-thin oblong plate shape after two-three weeks with a dimension of 128 ± 17 nm (length) × 14 ± 3 nm (width) and a thickness on the order of a few nanometers upon maturation (Fig. 2A and Fig. S2A-III). In GO-CaP, the layer of GO microflakes was convolutely surrounded by CaP nanoparticles that experienced the same morphological elongation with maturation (Fig. 2B and Fig S2B-I-III). The freshly made or premature CaP and GO-CaP do not possess the enhancement effects on hMSC differentiation in subsequent experiments (data not shown).
Fig. 2.
TEM images of mature (A) CaP nanoparticles and (B) GO-CaP nanocomposites at 2 weeks after fabrication. Blue arrows on (B) point to the edge of underlying GO sheet. Scale bars indicate 20 nm. C) Raman spectra from CaP, GO, and GO-CaP.
Fig. 2C showed the representative Raman spectra from CaP, GO and GO-CaP. Raman spectrum from CaP indicated that the main component in CaP is hydroxyapatite with major Raman bands at 960 cm−1 (PO43− ν1), 430 cm−1 (PO43− ν2), and 589 cm−1 (PO43− ν4).6 The GO-CaP spectra revealed the co-existence of GO and CaP, showing strong peaks from phosphate (430, 589, and 960 cm−1) as well as the D band (1330 cm−1) and G band (1597 cm−1) from GO (Fig. 2C). The Ca/P ratio in the CaP molecules has been determined to be 1.20 ± 0.05 by inductively coupled plasma mass spectroscopy (ICP-MS), which is in agreement with the composition of calcium-deficient nonstoichiometric HAp.
To evaluate the materials’ osteoinductive capability, GO, CaP and GO-CaP were introduced to hMSCs in osteogenic medium (OM) with a concentration of 0.5 µg/ml, 10 µg/ml, and 10.5 µg/ml, respectively. During the osteogenic differentiation of hMSCs, osteoblasts are generated and start to form bone nodules by producing extracellular calcium deposits. Mineralization was characterized using Alizarin red staining (ARS) after 2, 3, and 4 weeks of treatments. Cells in basic growth medium (GM) with treatments at all the time points were also tested as negative controls. The presence of calcium is indicated by red colour on the images (Fig. S3 in ESI†) and quantified by quantitative ARS assay (Fig 3A). Calcium concentration was normalized by total protein content to account for possible variation in cell growth and proliferation.
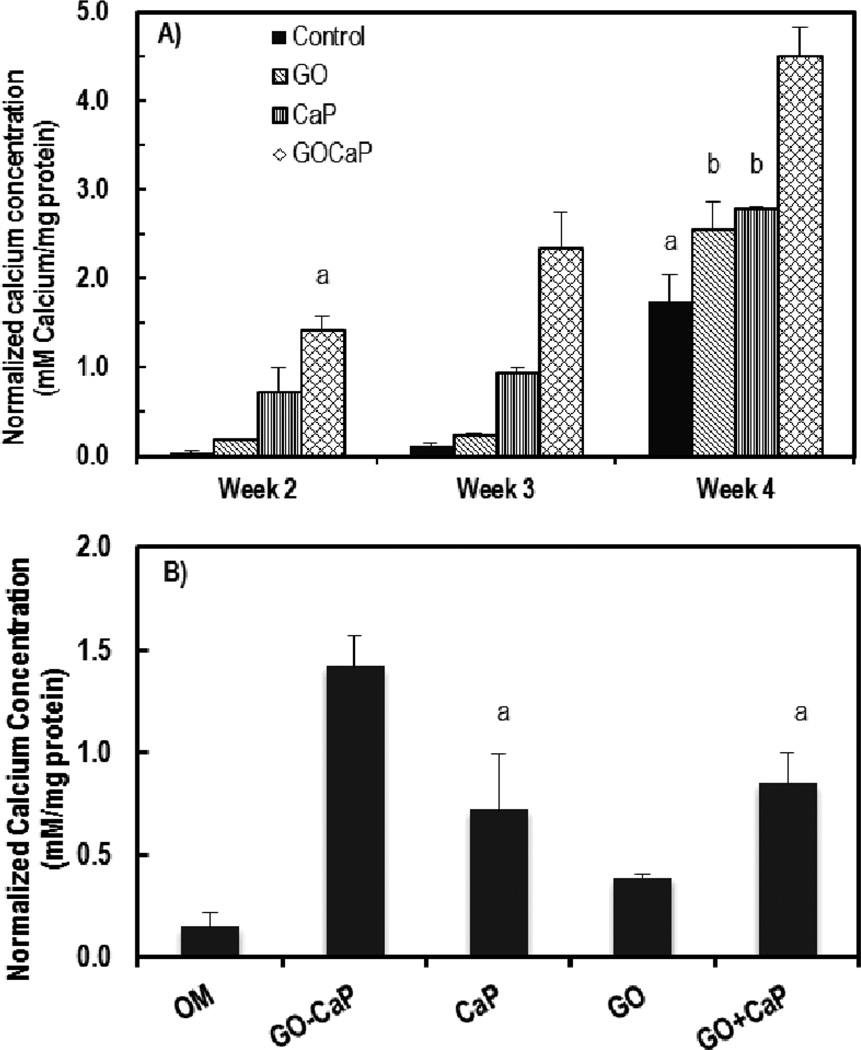
Fig. 3.
A) Normalized calcium concentrations determined by quantitative ARS assay of hMSCs after 2, 3 and 4 weeks of incubation with 0.5 µg/ml GO microflakes, 10 µg/ml CaP and 10.5 µg/ml GO-CaP. B) Normalized calcium concentrations after 2 weeks treatment with 10.5 µg/ml GO-CaP, 10 µg/ml CaP, 0.5 µg/ml GO, and 0.5 µg/ml GO+10 µg/ml CaP. Bars with the same top symbols indicate not being statistically significant (p > 0.05).
Treatments with GO, CaP and GO-CaP in OM all induced significantly larger quantity of calcium than control at all the time points (Fig. 3A), while no calcification observed in negative controls (with GM) (Fig. S3 in ESI†). GO-CaP nanocomposites exhibited superior osteoinductivity to CaP or GO, inducing much larger amount of mineralization than control. After 2 and 3 weeks, GO-CaP increased calcium deposition to levels more than 2× and 7× fold above those produced by CaP and GO, respectively. By 4 weeks, GO-CaP’s calcium deposition exceeded that from other materials by ~80%. In addition, the mineralization level with GO-CaP at 2 weeks was comparable to the control level at 4 weeks (p > 0.05), indicating GO-CaP significantly accelerated osteogenesis by two weeks. Phosphate assay was also performed from the deposition in parallel plates after 2 and 3 weeks of treatment. The amount of phosphate among all the groups follow the sequence of GO-CaP > CaP >> GO > control (Fig. S4 in ESI†), consistent with the outcome from calcium quantification.
Surprisingly, GO microflakes at this low concentration (0.5 µg/ml) increased calcification up to 50% more than the control at 3 and 4 weeks. At the late stage (4 weeks), the osteoinductivity of GO was comparable to the effect of CaP (p > 0.05). Such enhancement suggested that the GO flakes as ‘free particles’ preserved the osteoinductivity of GO coated supporting surface.
We further investigated whether the increased mineralization by GO-CaP is a simple addition effect of two individual osteoinductive compounds. First, the level of calcification in GO-CaP treated samples greatly exceeded the sum of those treated with GO and CaP individually (Fig. 3A). Next, separate experiments were carried out where hMSCs were incubated with the presence of both GO (0.5 µg/ml) and CaP (10 µg/ml) (GO+CaP) at the same concentration of individual components in 10.5 µg/ml GO-CaP in OM (Fig. 3B). GO+CaP induced increased calcification to the same extent as the effects of CaP (p > 0.05, Fig 3B), which was still markedly outperformed by GO-CaP nanocomposites. These results indicate that GO-CaP as a new composite material possesses synergistic enhancement effect in osteogenesis compared to GO or CaP alone.
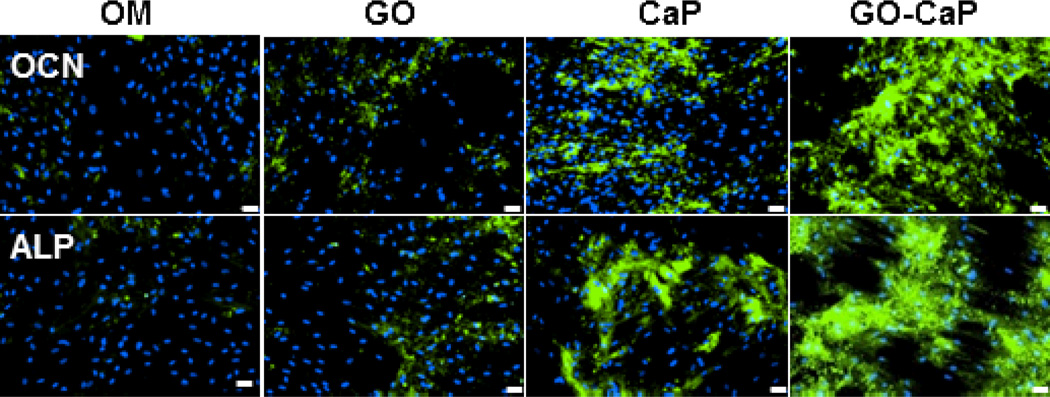
The osteogenic differentiation of hMSCs was verified through immunofluorescence staining of osteoblast markers alkaline phosphatase (ALP) and osteocalcin (OCN) after 2 weeks of treatments (Fig. 4). In good agreements with ARS assay, ALP activities and OCN expression level followed the sequence of GO-CaP > CaP >> GO > control, affirming the potential of GO-CaP in directing hMSCs differentiation toward osteogenic lineage.
Fig. 4.
Immunofluorescence staining of hMSC cell culture with FITC labeled (green) osteocalcin (OCN) antibody and DAPI (blue), and Alexa 488 (green) labeled alkaline phosphatase (ALP) antibody and DAPI (blue) after incubation with control osteogenic medium, GO, CaP, and GO-CaP for two weeks. Scale bars represent 20 µm.
The exact mechanism of GO and the derived composites in SCs differentiation is still unresolved. It has been generally hypothesized that the surface characteristics of graphene family nanomaterials such as nanotopography, surface stiffness, and large absorption capacity influence the molecular pathways that control the fate of stem cells.7 Both G and GO were reported acting as pre-concentrators for chemicals, proteins and growth factors on their surface to promote cell differentiation.8 In GO-CaP, the surface of GO flakes was mostly covered by CaP nanoparticles, and thus was inaccessible for direct absorption of molecules. The enhanced differentiation may in part arise from the increased interaction between the intracellular focal adhesion complexes of the cells and the CaP structure on GO-CaP surface.9 In addition, with the incorporation of GO and CaP, GO-CaP composites exhibited superior stiffness to GO or CaP alone.10 Such increase in material stiffness could induce an enhanced mechanotransduction effect which has been recognized to regulate stem cell differentiation,11 and thus might contribute to the synergistic enhancement in osteogenesis.
Conclusions
In conclusion, the present study synthesized a novel nanocomposite GO-CaP that possesses synergistic osteoinductive effect on hMSCs. To the best of our knowledge, only a couple of limited efforts have been made to develop composites with GO and CaP.11 Besides differences in synthesis methods, nanoparticle composition and morphology, none of the earlier studies have evaluated the osteoinductivity of GO-CaP in hMSC differentiation. The GO-CaP nanocomposites fabricated in the current study significantly facilitated the osteogenesis of hMSCs and further enhanced calcium deposition by osteoblast. The exceptional osteoinductive properties of GO-CaP allow for its future application in regenerative medicine and bone tissue engineering.
Supplementary Material
Acknowledgments
The authors acknowledge National Institute of Health K25CA149194-01 (XB) for financial support.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details and supplementary figures. See DOI: 10.1039/c000000x/
Notes and references
- 1.(a) Ruiz ON, Fernando KA, Wang B, Brown NA, Luo PG, McNamara ND, Vangsness M, Sun YP, Bunker CE. ACS nano. 2011;5:8100. doi: 10.1021/nn202699t. [DOI] [PubMed] [Google Scholar]; (b) Chen GY, Pang DW, Hwang SM, Tuan HY, Hu YC. Biomaterials. 2012;33:418. doi: 10.1016/j.biomaterials.2011.09.071. [DOI] [PubMed] [Google Scholar]; (c) Ryu S, Kim BS. J Tissue Eng Regen Med. 2013;10:39. [Google Scholar]; (d) Chen GY, Pang DW, Hwang SM, Tuan HY, Hu YC. Biomaterials. 2012;33:418. doi: 10.1016/j.biomaterials.2011.09.071. [DOI] [PubMed] [Google Scholar]
- 2.(a) Nayak TR, Andersen H, Makam VS, Khaw C, Bae S, Xu X, Ee PL, Ahn JH, Hong BH, Pastorin G, Ozyilmaz B. ACS nano. 2011;5:4670. doi: 10.1021/nn200500h. [DOI] [PubMed] [Google Scholar]; (b) Park J, Park S, Ryu S, Bhang SH, Kim J, Yoon JK, Park YH, Cho SP, Lee S, Hong BH, Kim BS. Adv Healthc Mater. 2014;3:176. doi: 10.1002/adhm.201300177. [DOI] [PubMed] [Google Scholar]
- 3.(a) Tang LA, Lee WC, Shi H, Wong EY, Sadovoy A, Gorelik S, Hobley J, Lim CT, Loh KP. Small. 2012;8:423. doi: 10.1002/smll.201101690. [DOI] [PubMed] [Google Scholar]; (b) Lee WC, Lim CH, Shi H, Tang LA, Wang Y, Lim CT, Loh KP. ACS Nano. 2011;5:7334. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]; (c) Kim J, Choi KS, Kim Y, Lim KT, Seonwoo H, Park Y, Kim DH, Choung PH, Cho CS, Kim SY, Choung YH, Chung JH. J Biomed Mater Res A. 2013;101:3520. doi: 10.1002/jbm.a.34659. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wang Z, Tang Z, Qing F, Hong Y, Zhang X. Nano: Brief Reports and Reviews. 2012;7:123004. [Google Scholar]; (b) Habibovic F, de Groot K. J Tissue Eng Regen Med. 2007;1:25. doi: 10.1002/term.5. [DOI] [PubMed] [Google Scholar]; (c) Samavedi S, Whittington AR, Goldstein AS. Acta Biomater. 2013;9:8037. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Hwang ET, Tatavarty R, Chung J, Gu MB. ACS Appl Mater Interfaces. 2013;5:532. doi: 10.1021/am302580p. [DOI] [PubMed] [Google Scholar]
- 6.Bi X, Patil CA, Lynch CC, Pharr GM, Mahadevan-Jasen A, Nyman JS. J Biomech. 2011;44(2):297. doi: 10.1016/j.jbiomech.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Yang M, Yao J, Duan Y. Analyst. 2013;138:72. doi: 10.1039/c2an35744e. [DOI] [PubMed] [Google Scholar]; (b) Gu M, Liu Y, Chen T, Du F, Zhao X, Xiong C, Zhou Y. Tissue Eng Part B Rev. 2014 doi: 10.1089/ten.teb.2013.0638. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WC, Lim CH, Shi H, Tang LA, Wang Y, Lim CT, Loh KP. ACS Nano. 2011;5:7334. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- 9.(a) Müller P, Bulnheim U, Diener A, Lüthen F, Teller M, Klinkenberg ED, Neumann HG, Nebe B, Liebold A, Steinhoff G, Rychly J. J Cell Mol Med. 2008;12:281. doi: 10.1111/j.1582-4934.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Depan D, Pesacreta TC, Misra RDK. Biomater Sci. 2014;2:264. doi: 10.1039/c3bm60192g. [DOI] [PubMed] [Google Scholar]; (c) van den Dries K, Meddens MBM, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A. Nat Commun. 2013;4:1412. doi: 10.1038/ncomms2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Li M, Wang Y, Liu Q, Cheng Y, Zheng Y, Xi T, Wei S. J Mater Chem B. 2013;1:475. doi: 10.1039/c2tb00053a. [DOI] [PubMed] [Google Scholar]; (b) Liu Y, Huang J, Li H. J Mater Chem B. 2013;1:1826. doi: 10.1039/c3tb00531c. [DOI] [PubMed] [Google Scholar]
- 11.(a) Shin JW, Swift J, Ivanovska I, Spinler KR, Buxboim A, Discher DE. Differentiation. 2013;86:77. doi: 10.1016/j.diff.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Discher DE, Mooney DJ, Zandstra PW. Science. 2009;324:1673. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. Biomaterials. 2011;32:3921. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.