Abstract
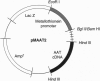
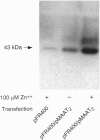
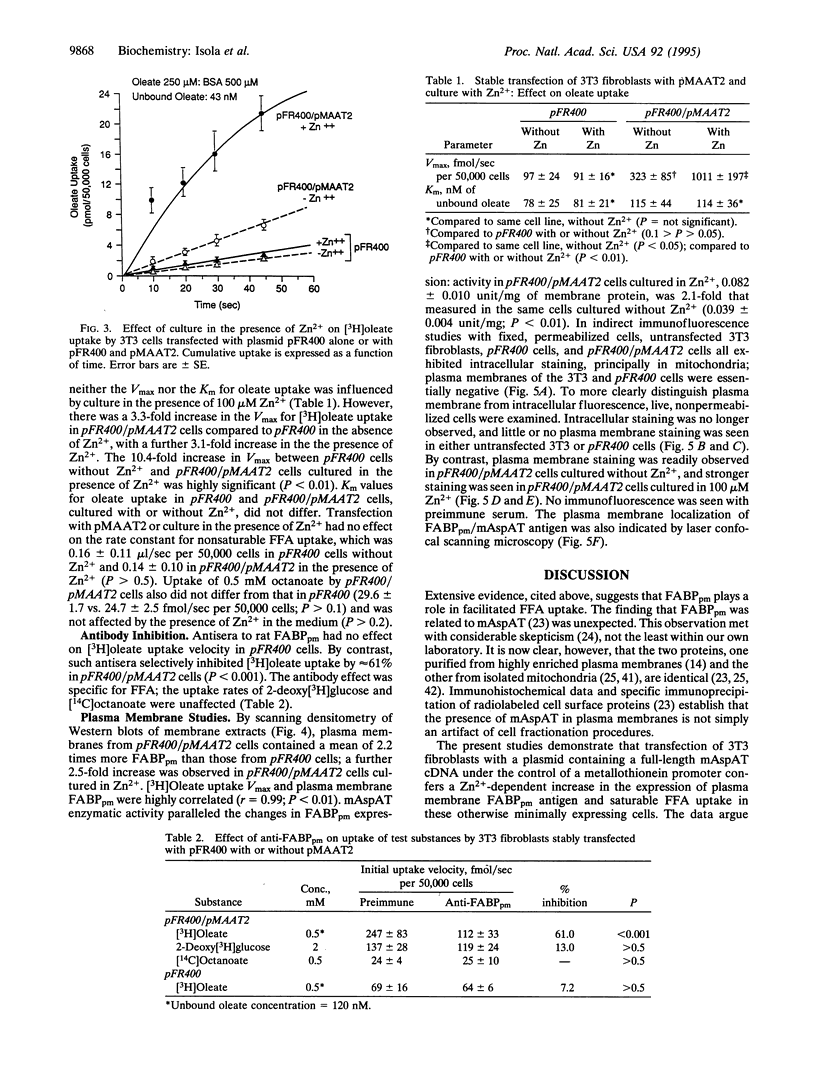
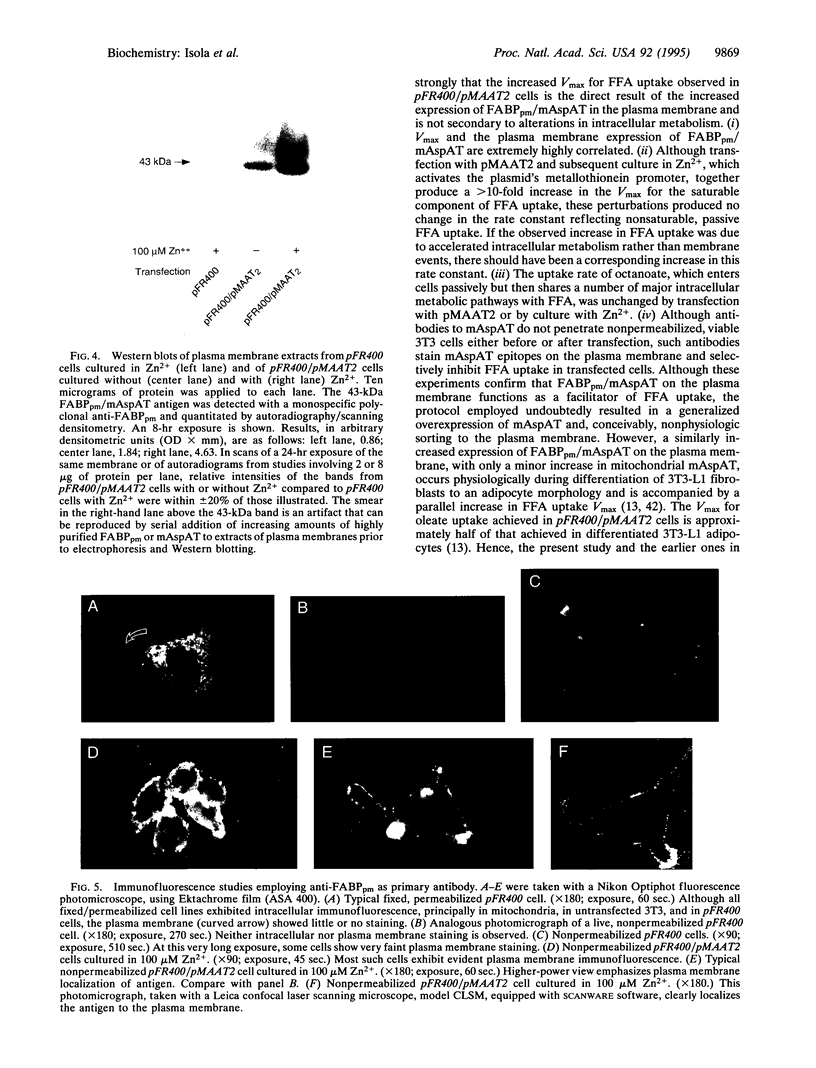
To explore the relationship between mitochondrial aspartate aminotransferase (mAspAT; EC 2.6.1.1) and plasma membrane fatty acid-binding protein (FABPpm) and their role in cellular fatty acid uptake, 3T3 fibroblasts were cotransfected with plasmid pMAAT2, containing a full-length mAspAT cDNA downstream of a Zn(2+)-inducible metallothionein promoter, and pFR400, which conveys methotrexate resistance. Transfectants were selected in methotrexate, cloned, and exposed to increasing methotrexate concentrations to induce gene amplification. Stably transfected clones were characterized by Southern blotting; those with highest copy numbers of pFR400 alone (pFR400) or pFR400 and pMAAT2 (pFR400/pMAAT2) were expanded for further study. [3H]Oleate uptake was measured in medium containing 500 microM bovine serum albumin and 125-1000 microM total oleate (unbound oleate, 18-420 nM) and consisted of saturable and nonsaturable components. pFR400/pMAAT2 cells exhibited no increase in the rate constant for nonsaturable oleate uptake or in the uptake rate of [14C]octanoate under any conditions. By contrast, Vmax (fmol/sec per 50,000 cells) of the saturable oleate uptake component increased 3.5-fold in pFR400/pMAAT2 cells compared to pFR400, with a further 3.2-fold increase in the presence of Zn2+. Zn2+ had no effect in pFR400 controls (P > 0.5). The overall increase in Vmax between pFR400 and pFR400/pMAAT2 in the presence of Zn2+ was 10.4-fold (P < 0.01) and was highly correlated (r = 0.99) with expression of FABPpm in plasma membranes as determined by Western blotting. Neither untransfected 3T3 nor pFR400 cells expressed cell surface FABPpm detectable by immunofluorescence. By contrast, plasma membrane immunofluorescence was detected in pFR400/pMAAT2 cells, especially if cultured in 100 microM Zn2+. The data support the dual hypotheses that mAspAT and FABPpm are identical and mediate saturable long-chain free fatty acid uptake.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Park J. H., Park C. R. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984 Jul 25;259(14):8945–8953. [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993 Aug 25;268(24):17665–17668. [PubMed] [Google Scholar]
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Berk P. D., Wada H., Horio Y., Potter B. J., Sorrentino D., Zhou S. L., Isola L. M., Stump D., Kiang C. L., Thung S. Plasma membrane fatty acid-binding protein and mitochondrial glutamic-oxaloacetic transaminase of rat liver are related. Proc Natl Acad Sci U S A. 1990 May;87(9):3484–3488. doi: 10.1073/pnas.87.9.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. N., DiRusso C. C. Molecular and biochemical analyses of fatty acid transport, metabolism, and gene regulation in Escherichia coli. Biochim Biophys Acta. 1994 Jan 3;1210(2):123–145. doi: 10.1016/0005-2760(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Gerber M., Price P. M., Flordellis C., Edelman J., Acs G. Amplification of expression of hepatitis B surface antigen in 3T3 cells cotransfected with a dominant-acting gene and cloned viral DNA. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1815–1819. doi: 10.1073/pnas.79.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Kawaguchi H., Yasuda H. Isolation and partial characterization of an amphiphilic 56-kDa fatty acid binding protein from rat renal basolateral membrane. J Biochem. 1987 Mar;101(3):679–684. doi: 10.1093/jb/101.3.679. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Beverley S. M., Kiely M. L., Schimke R. T. Properties of an altered dihydrofolate reductase encoded by amplified genes in cultured mouse fibroblasts. J Biol Chem. 1981 Sep 25;256(18):9501–9510. [PubMed] [Google Scholar]
- Harmon C. M., Luce P., Beth A. H., Abumrad N. A. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J Membr Biol. 1991 May;121(3):261–268. doi: 10.1007/BF01951559. [DOI] [PubMed] [Google Scholar]
- Joh T., Nomiyama H., Maeda S., Shimada K., Morino Y. Cloning and sequence analysis of a cDNA encoding porcine mitochondrial aspartate aminotransferase precursor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6065–6069. doi: 10.1073/pnas.82.18.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. R., Jr, Rodriguez-Berrocal F. J., Gordon J., Iriarte A., Martinez-Carrion M. Molecular cloning and in vivo expression of a precursor to rat mitochondrial aspartate aminotransferase. Biochem Biophys Res Commun. 1987 Dec 31;149(3):859–865. doi: 10.1016/0006-291x(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Potter B. J., Stump D., Schwieterman W., Sorrentino D., Jacobs L. N., Kiang C. L., Rand J. H., Berk P. D. Isolation and partial characterization of plasma membrane fatty acid binding proteins from myocardium and adipose tissue and their relationship to analogous proteins in liver and gut. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1370–1376. doi: 10.1016/s0006-291x(87)80283-8. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Lai E., Rosen O. M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977 May 25;252(10):3554–3557. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Determination of succinic dehydrogenase activity. Methods Biochem Anal. 1957;4:307–333. doi: 10.1002/9780470110201.ch9. [DOI] [PubMed] [Google Scholar]
- Schaffer J. E., Lodish H. F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994 Nov 4;79(3):427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Schwieterman W., Sorrentino D., Potter B. J., Rand J., Kiang C. L., Stump D., Berk P. D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988 Jan;85(2):359–363. doi: 10.1073/pnas.85.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sorrentino D., Robinson R. B., Kiang C. L., Berk P. D. At physiologic albumin/oleate concentrations oleate uptake by isolated hepatocytes, cardiac myocytes, and adipocytes is a saturable function of the unbound oleate concentration. Uptake kinetics are consistent with the conventional theory. J Clin Invest. 1989 Oct;84(4):1325–1333. doi: 10.1172/JCI114301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino D., Stump D., Potter B. J., Robinson R. B., White R., Kiang C. L., Berk P. D. Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. J Clin Invest. 1988 Sep;82(3):928–935. doi: 10.1172/JCI113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Berk P. D. Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci U S A. 1986 May;83(10):3086–3090. doi: 10.1073/pnas.83.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Diede H. E., Rodilla-Sala E., Vyska K., Schrader M., Fitscher B., Passarella S. The membrane fatty acid-binding protein is not identical to mitochondrial glutamic oxaloacetic transaminase (mGOT). 1990 Oct 15-Nov 8Mol Cell Biochem. 98(1-2):191–199. doi: 10.1007/BF00231384. [DOI] [PubMed] [Google Scholar]
- Stremmel W. Fatty acid uptake by isolated rat heart myocytes represents a carrier-mediated transport process. J Clin Invest. 1988 Mar;81(3):844–852. doi: 10.1172/JCI113393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P. D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W. Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J Clin Invest. 1988 Dec;82(6):2001–2010. doi: 10.1172/JCI113820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Palmiter R. D. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. 1985 Oct 31-Nov 6Nature. 317(6040):828–831. doi: 10.1038/317828a0. [DOI] [PubMed] [Google Scholar]
- Stump D. D., Nunes R. M., Sorrentino D., Isola L. M., Berk P. D. Characteristics of oleate binding to liver plasma membranes and its uptake by isolated hepatocytes. J Hepatol. 1992 Nov;16(3):304–315. doi: 10.1016/s0168-8278(05)80661-0. [DOI] [PubMed] [Google Scholar]
- Stump D. D., Zhou S. L., Berk P. D. Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am J Physiol. 1993 Nov;265(5 Pt 1):G894–G902. doi: 10.1152/ajpgi.1993.265.5.G894. [DOI] [PubMed] [Google Scholar]
- Stump D. D., Zhou S. L., Potter B. J., Berk P. D. Purification of rat liver mitochondrial aspartate aminotransferase and separation of its isoforms utilizing high-performance liquid chromatography. Protein Expr Purif. 1990 Sep;1(1):49–53. doi: 10.1016/1046-5928(90)90045-z. [DOI] [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigatti B. L., Mangroo D., Gerber G. E. Photoaffinity labeling and fatty acid permeation in 3T3-L1 adipocytes. J Biol Chem. 1991 Nov 25;266(33):22621–22625. [PubMed] [Google Scholar]
- Trimble M. E. Mediated transport of long-chain fatty acids by rat renal basolateral membranes. Am J Physiol. 1989 Oct;257(4 Pt 2):F539–F546. doi: 10.1152/ajprenal.1989.257.4.F539. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T., Obaru K., Setoyama C., Shimada K. Structural organization of the mouse mitochondrial aspartate aminotransferase gene. J Mol Biol. 1987 Nov 5;198(1):21–31. doi: 10.1016/0022-2836(87)90454-2. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wosilait W. D., Nagy P. A method of computing drug distribution in plasma using stepwise association constants: clofibrate acid as an illustrative example. Comput Programs Biomed. 1976 Oct;6(3):142–148. doi: 10.1016/0010-468x(76)90020-9. [DOI] [PubMed] [Google Scholar]
- Zhou S. L., Stump D., Isola L., Berk P. D. Constitutive expression of a saturable transport system for non-esterified fatty acids in Xenopus laevis oocytes. Biochem J. 1994 Jan 15;297(Pt 2):315–319. doi: 10.1042/bj2970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. L., Stump D., Kiang C. L., Isola L. M., Berk P. D. Mitochondrial aspartate aminotransferase expressed on the surface of 3T3-L1 adipocytes mediates saturable fatty acid uptake. Proc Soc Exp Biol Med. 1995 Mar;208(3):263–270. doi: 10.3181/00379727-208-43854. [DOI] [PubMed] [Google Scholar]
- Zhou S. L., Stump D., Sorrentino D., Potter B. J., Berk P. D. Adipocyte differentiation of 3T3-L1 cells involves augmented expression of a 43-kDa plasma membrane fatty acid-binding protein. J Biol Chem. 1992 Jul 15;267(20):14456–14461. [PubMed] [Google Scholar]