Abstract
Objective
To examine whether stroke survivors with more severe spatial neglect during their acute inpatient rehabilitation had poorer mobility after returning to their communities.
Design
A prospective observational study.
Setting
Acute inpatient rehabilitation and follow-up in the community.
Participants
Thirty-one consecutive stroke survivors with right-brain damage (women, n = 15 [48.4%]), with the mean (standard deviation) age of 60 ± 11.5 years, were included in the study if they demonstrated spatial neglect within 2 months after stroke.
Methods
Spatial neglect was assessed with the Behavioral Inattention Test (BIT) (range, 0-146 [a lower score indicates more severity]) and the Catherine Bergego Scale (range, 0-30 [a higher score indicates more severity]). A score of the Behavioral Inattention Test <129 or of the Catherine Bergego Scale >0 defined the presence of spatial neglect.
Main Outcome Measurements
The outcome measure is community mobility, defined by the extent and frequency of traveling within the home and in the community, and is assessed with the University of Alabama at Birmingham Study of Aging Life-Space Assessment (range, 0-120 [a lower score indicates less mobile]). This measure was assessed after participants returned home ≥6 months after stroke. The covariates were age, gender, functional independence at baseline; follow-up interval; and depressed mood, which may affect the relationship between spatial neglect and community mobility.
Results
A lower Behavioral Inattention Test score was a significant predictor of a lower Life-Space Assessment score after controlling for all the covariates (β = 0.009 [95% confidence interval, 0.008-0.017]); P = .020). The proportion of participants unable to travel independently beyond their homes was 0%, 27.3%, and 72.7% for those with mild, moderate, and severe acute neglect, respectively (Catherine Bergego Scale range, 1-10, 11-20, and 21-30, respectively).
Conclusions
Our result indicates that acute spatial neglect has a negative impact on regaining of functional mobility in the community. Specific screening and treatment of spatial neglect during acute stroke care may be necessary to improve long-term mobility recovery.
INTRODUCTION
Regaining mobility at the community level is a fundamental component of successful rehabilitation among stroke survivors [1]. Being able to move around in the community is multidimensional capability, reflecting not only physical function but also cognitive function, social integration, and community participation. However, current research on poststroke mobility has focused primarily on visible physical attributes, for example, gait performance [2]. The role of cognitive function has been largely overlooked, especially the domain of spatial cognition.
Spatial neglect is a cognitive disorder that affects perception and/or motor execution, and that predominantly occurs after a right hemispheric stroke [3-5]. It is a disorder of spatial attention or intention, demonstrated by a failure to attend to stimuli presented in the opposite side of space from the damaged cerebral hemisphere or a failure to act on contralesional stimuli despite preserved motor strength [4]. Stroke patients with spatial neglect usually have poor functional outcomes and prolonged hospitalizations [6-8], and impose increased burden on care-givers after discharge [9]. Spatial neglect also significantly influences mobility performance. During walking, a person computes the space around his or her body to reach a desired location. Stroke survivors with spatial neglect make errors in spatial perception of a target location and have a tendency to veer when walking [10]. Patients with spatial neglect showed 3 times more collisions during walking through doorways, although their gait velocity was similar to those of patients without neglect [11]. During wheelchair navigation, stroke patients with neglect had difficulty avoiding objects (furniture or a wall), especially on the left side, compared with those without neglect [12,13]. In addition, spatial neglect has been identified as a predictor of poor fitness to drive among stroke survivors, which affects travel in the community [14].
Despite this evidence that spatial neglect is linked to eventual functional disability, efforts to assess or treat neglect in acute care have been questioned, which may be due to the knowledge gap between research and clinical practice of spatial neglect’s prevalence, severity, and clinical significance. For example, in 1987, Sunderland et al [15] reported that spatial neglect was rarely observed by 6 months after stroke, which may be a common concept among physicians and therapists based on our communication with these clinicians. However, a recent longitudinal cohort study showed that approximately 40% of stroke patients with neglect at the acute stage showed a persistent spatial deficit more than 1 year after stroke [16]. According to the earlier view of neglect being transient [15], a consistent relationship between acute spatial neglect and poor recovery in motor functional performance at inpatient rehabilitation discharge [7] and even 3 years after stroke [17] may result from an association of spatial neglect with more severe stroke. However, this view does not consider all the skills needed for successful community mobility beyond motor performance in the clinic (eg, gait velocity) [18]. It has been reported that, despite good performance of mobility in the clinic setting, nearly one-third of stroke survivors do not get out into the community [19]. Currently, several measures are used to project and estimate community mobility among stroke survivors, including gait speed or distance, functional mobility scale (eg, Functional Independence Measure [Uniform Data System for Medical Rehabilitation, Amherst, NY], and the Barthel Index), and self-reported surveys of how much a person travels [2,19]. With increasing global acknowledgment of the environmental factors important to functioning and the relationship between environment and participation [20], self reports of mobility that extend from home to a more challenging environment may be considered the most useful stroke outcome for current clinical use [2].
One of the self-reported measures is an estimation of the spatial extent of an individual’s whole-body movement within his or her own environment [21,22]. This construct, life space, is quantified by a self report of how far and how frequently a person travels in the community setting and takes into account the amount of help needed [21]. Life space differs from conventional assessments of mobility, which is often focused on the perceived ability to move around in the environment (ie, what one thinks that he or she is capable of achieving); rather, life space assesses the spatial extent of mobility (ie, where one has been) in daily life [21]. It has emerged as an alternative and complementary approach to traditional measures of physical function and mobility (eg, gait velocity) among older adults and individuals with various illnesses [21,23,24], because life space is a report of an individual’s actual whereabouts, which suggests functional mobility and the level of social participation. The measure of life space has most often been used, as shown in the gerontology and geriatric literature, by behavioral psychologists, movement scientists, and gerontologists [21-23,25]. However, when considering that life space is a multidimensional construct that not only reflects motor skill but also cognitive and psychological well-being, resources available for the patient, and social integration [21], it may be a valuable outcome measure among stroke survivors. A small life space has been reported to be associated with increased risk of mortality [25,26] and cognitive decline [27] among older adults, even after taking into account traditional measures of motor function (eg, gait) and disability. Because of the evidence that life space may measure mobility beyond motor and gait impairment, assessing life-space recovery may allow more specific evaluation of the long-term impact of spatial neglect. Because spatial neglect affects stroke survivors’ ability to navigate in their environment [28,29], the extent and frequency of travel in the community may be reduced by this disorder even with a preserved level of functional ability. Thus, in this study, we examined whether severity of spatial neglect during inpatient rehabilitation independently predicts mobility later, back in the community, among a group of stroke survivors with right-brain damage and with spatial neglect.
METHODS
Participants
Right-handed survivors of right-brain stroke were recruited based on referrals from clinicians in an acute inpatient rehabilitation hospital. Clinicians, including physicians, physical therapists, and occupational therapists, referred patients to the research team when patients met 3 pre-screening criteria: age between 18 and 100 years; having had a right-brain stroke within the past 2 months; and being able to give informed consent and having no serious brain conditions other than a stroke (eg, seizure disorder, dementia, or Parkinson disease), brain lesions that involve the left hemisphere, a history of psychiatric hospitalization, or being blind in 1 or both eyes. When following up with the referral, the research staff screened the patients for spatial neglect. This referral pathway has been established since 2007, with daily communication between the research assistants and the clinicians in an inpatient rehabilitation unit. Thirty-one consecutive stroke survivors with spatial neglect and in acute inpatient rehabilitation were included in the study. The protocol was approved by the local institutional review board, and all the participants provided informed consent.
Assessment of Spatial Neglect
The presence of spatial neglect was determined by the Behavioral Inattention Test (BIT) score [30-32] or the Catherine Bergego Scale (CBS) score [33,34]. The BIT includes 3 target-cancellation subtests (line crossing, letter cancellation, and star cancellation) and 3 other subtests for figure and shape copying, line bisection, and representational drawing. Cancellation subtests account for 130 of the maximal 146 points for the BIT score. A score lower than 129 is considered consistent with spatial neglect, with lower scores indicating more severe neglect [32]. It takes approximately 15-30 minutes to complete the BIT with good reliability and validity [31,35]. The CBS is an ecologically valid screening tool for spatial neglect, with excellent reliability and validity [36]. Its psychometric properties allow it to capture motor-exploratory as well as perceptual-attentional spatial neglect symptoms [37]. The CBS has been used among therapists and rehabilitation researchers, and has been used as a main assessment tool for spatial neglect in the previous literature [38-42]. Specifically, the CBS assessment occurred via the Kessler Foundation Neglect Assessment Process [34]. The Kessler Foundation Neglect Assessment Process standardizes and strictly defines administration of the 10 CBS items: limb awareness, personal belongings, dressing, grooming, gaze orientation, auditory attention, navigation, collisions, eating, and cleaning after a meal [34]. For each item, a score of 0 (no neglect) to 3 (severe neglect) is given, with a total possible score of 0-30. Thus, higher scores on the CBS indicate poorer function. Patients with a score of 1 or higher were considered to have spatial neglect. Mild, moderate, and severe neglect were defined based on the CBS score of 1-10, 11-20, and 21-30, respectively [34]. A score consistent with neglect on either the BIT or the CBS was used to determine the presence of spatial neglect to increase the sensitivity and generalizability of the study.
Assessment of Mobility in the Community
Mobility in the community was assessed with the University of Alabama at Birmingham Study of Aging Life-Space Assessment (LSA) [22,43], 6 months or later after stroke, via telephone interview with participants or their live-in caregivers. The LSA has been reported to have excellent reliability and validity [22,44]. The LSA has been most used by gerontologists, physical therapists, and public health researchers in evaluating mobility status and change in aging populations [22,44-47]. It includes 6 life-space zones: (1) bedroom (zone 0), (2) home (zone 1), (3) immediately outside home (zone 2), (4) neighborhood (zone 3), (5) town (zone 4), and (6) beyond town (zone 5). The score accounts for an individual’s frequency of movement and level of assistance (ie, equipment or personal assistance) at each zone, with a total score range of 0-120. At the score extremes, 0 represents no movement beyond the bedroom at any time, and 120 represents daily, out-of-town, independent travel [22]. We also used an additional definition for restricted life space if the largest zone of travel without personal assistance was zone 1 (home). This definition of restricted life space is adapted for stroke patients from that of community-dwelling older adults, which is defined as the largest zone of independent travel being zone 3 (neighborhood) or smaller [44].
Covariates
We collected additional information as covariates, including age, gender, follow-up time, and level of functional independence and depressive symptoms. Follow-up time was the number of months between the assessment of acute neglect and the telephone interview for the LSA. The level of functional independence was assessed with the Barthel Index, scored 0-100, with higher scores indicating better function in everyday activities [48,49]. The presence of depressive symptoms was assessed with the Geriatric Depression Scale, scored 0-30, with a higher score indicating a more depressed state [50].
Statistical Analysis
We used the Pearson correlation to assess the correlation between the scores of the BIT and the LSA. The proportion of the participants with restricted life space was compared among those with mild, moderate, and severe neglect based on the CBS score with χ2 analysis. Two separate multivariate linear regression models were built, by using the BIT or the CBS scores, to predict the LSA scores. We used a stepwise backward elimination method and included age, gender, follow-up time, the Geriatric Depression Scale score, and the Barthel Index score in the final model using the BIT scores. For the final model, when using the CBS score, the Barthel Index score was not included due to collinearity between the CBS score and the Barthel Index. All statistical analyses were performed by using STATA version 12 (StataCorp LP, College Station, TX).
RESULTS
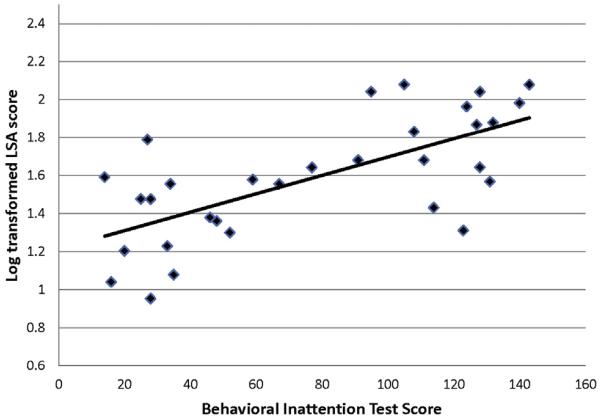
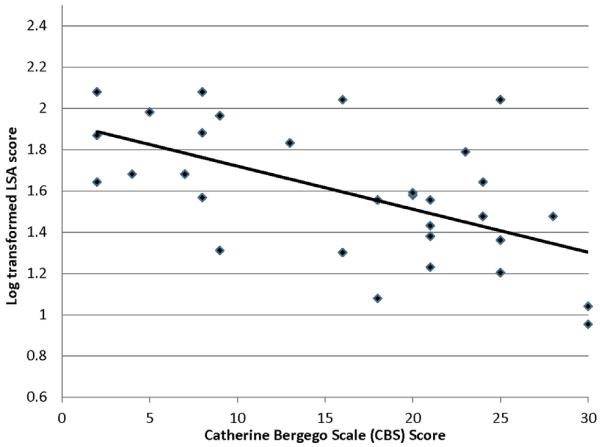
Participant characteristics and assessment scores are presented in Table 1. All participants with mild spatial neglect were traveling beyond their homes without personal assistance. However, the proportion of the individuals whose independent mobility was limited to their homes and could not travel outside by themselves was highest among those with severe neglect (72.7%) and was 27.3% in participants with moderate spatial neglect (χ2 = 10.12, P = .007). The scores of the LSA were skewed to the lower values (P = .014 for skewness); therefore, the LSA scores were log=transformed to improve normality (P = .699 for skewness). The BIT scores showed a positive correlation with the LSA scores (Pearson correlation coefficient r = .68, P < .001) (Figure 1), and the CBS scores showed a negative correlation with the LSA scores (r = −.58; P < .001) (Figure 2), which indicated that participants who had had more severe neglect at the acute phase had poorer community mobility, even 6 months after they returned home.
Table 1. Summary of participant characteristics and assessment scores.
| Severity of Neglect |
|||||
|---|---|---|---|---|---|
| Variables | Total (N = 31) |
Mild (n = 11) |
Moderate (n = 7) |
Severe (n = 13) |
P Value |
| Age, mean ± SD (y) | 60.0 ± 11.5 | 56.8 ± 16.2 | 61.8 ± 9.8 | 61.9 ± 7.1 | .788 |
| Women, n (%) | 15 (48.4) | 4 (36.4) | 3 (42.9) | 8 (61.5) | .444 |
| Follow-up time, mean ± SD (mo) | 20.8 ± 10.1 | 19.2 ± 9.8 | 22.3 ± 10.8 | 21.5 ± 10.7 | .841 |
| Life space assessment (range, 0-120), mean ± SD | 49.6 ± 34.1 | 70.5 ± 33.8 | 46.1 ± 33.3 | 33.7 ± 26.9 | .011 |
| Behavioral Inattention Test (range, 0-146), mean ± SD | 77.7 ± 44.9 | 60.5 ± 23.8 | 56.7 ± 34.0 | 50.5 ± 36.1 | <.001 |
| Catherine Bergego Scale (range, 0-30), mean ± SD | 16.2 ± 8.8 | 5.8 ± 2.9 | 17.3 ± 2.5 | 24.5 ± 3.2 | N/A |
| Barthel Index (range, 0-100), mean ± SD | 35.2 ± 27.6 | 60.5 ± 23.8 | 27.9 ± 19.8 | 17.7 ± 16.8 | <.001 |
| Geriatric Depression Scale (range, 0-30), mean ± SD | 5.1 ± 3.9 | 4.5 ± 3.8 | 5.3 ± 5.1 | 5.5 ± 3.5 | .795 |
SD = standard deviation; N/A = not available.
Kruskal-Wallis test was used to compare the values of all continuous variables. The proportion of the women was compared using chi-square test.
Figure 1.
Correlation between Behavioral Inattention Test scores and log-transformed scores of the life-space assessment (LSA).
Figure 2.
Correlation between the Catherine Bergego Scale scores and log-transformed scores of the life-space assessment (LSA).
In the multivariate linear regression analysis that included the BIT, the final model explained approximately 55.6% of the variance in the LSA score (R2 = .56). Importantly, after controlling for age, follow-up time, Barthel Index score, and depression score (Table 2), the BIT score was still significantly associated with the LSA, (β = 0.009 [95% confidence interval, 0.008-0.171]; P = 0.032). Postestimation analysis showed that a 1-point increase (less severe neglect) in the BIT score was associated with a 1-point increase in the LSA score. In the other model, controlling age, female gender, follow-up time, and depressive symptoms, the CBS score was also significantly associated with the LSA score (β = −0.453 ; [95% confidence interval, −0.074 to −0.017]; P = .003) (Table 3). This model with CBS explained approximately 39% of the variance in the LSA score (R2 = .39). Postestimation analysis showed that 1 score increase in the CBS (more severe neglect) was associated with a score decrease of approximately 0.9 in the LSA. We were unable to find that any other factors that were significantly and independently associated with life space in these analyses.
Table 2. Results of multivariate regression analysis with BIT scores.
| Predictor Variables | Estimates of Coefficient (95% CI) |
P Value |
|---|---|---|
| Age | 0.010 (−0.014 to 0.035) | .393 |
| Female gender | 0.002 (−0.452 to 0.457) | .992 |
| Follow-up time | −0.011 (−0.033 to 0.010) | .290 |
| Geriatric Depression Scale score |
−0.036 (−0.090 to 0.018) | .461 |
| Barthel Index score | 0.006 (−0.010 to 0.021) | .183 |
| BIT score | 0.009 (0.008-0.017) | .020 |
Life-space assessment (LSA) scores are log transformed.
BIT = Behavioral Inattention Test; CI = confidence interval.
Table 3. Results of multivariate regression analysis with CBS scores.
| Predictor Variables | Estimates of Coefficient (95% CI) |
P Value |
|---|---|---|
| Age | 0.003 (−0.018 to −0.024) | .756 |
| Female gender | −0.084 (−0.575 to 0.406) | .727 |
| Follow-up time | −0.012 (−0.036 to 0.011) | .294 |
| Geriatric Depression Scale score |
−0.021 (−0.082 to 0.041) | .489 |
| CBS score | −0.045 (−0.074 to −0.017) | .003 |
Life-space assessment (LSA) scores are log transformed.
CBS = Catherine Bergego Scale; CI = confidence interval.
DISCUSSION
Our findings indicate that greater severity of spatial neglect acutely after stroke independently predicts the extent of community mobility of chronic stage in stroke survivors with right-brain stroke. Specifically, better performance on paper-and-pencil tests of acute spatial neglect (the BIT) was positively associated with a community mobility measure (the LSA), even after controlling for age, gender, follow-up interval, depressive state, and basic function in daily life (ie, Barthel Index). Similarly, poorer performance on a functional assessment for acute spatial neglect (the CBS) predicted poorer mobility in the community. Based on the CBS score, the patients with moderate-to-severe spatial neglect in the acute phase had more difficulty traveling outside their homes once they returned to being community dwelling, in comparison with patients with mild neglect.
Regaining the ability to go wherever they wish at home and in the community after stroke is 1 of the top priorities among stroke survivors. Contributors to community mobility in stroke survivors have been reported primarily in the physical domain, such as cardiovascular reserve, balance, and ability to negotiate stairs [51-54]. However, the extent and frequency of mobility activities (ie, life space) has also been shown to be associated with factors other than physical function, including visual function [21], psychological well-being [55], and social involvement [21]. To move around safely at home or outdoors, individuals require spatial perception of the body, the environment, and the relative locations and dynamic relations between the two. Dysfunction in any level of this spatial cognition may limit one’s ability to move around in the environment for specific activities or purposes. So far, much work related to this construct of life space was done among older adults without regard for the special problems of stroke survivors. Our study may be the first to assess life space as a long-term outcome after acute spatial neglect after stroke.
It is intriguing that the manifestation of spatial neglect at a relatively early stage after stroke predicts functional mobility more than 6 months later, even after accounting for the ability to perform everyday activities. These findings highlight a potential long-term impact of spatial neglect on mobility, and stand in contrast to the widely accepted belief that spatial neglect is a temporary deficit after stroke [15,56]. When following no specific treatment for spatial neglect, approximately 40% of stroke survivors with spatial neglect acutely still show clinical signs of spatial neglect 1 year after stroke [16]. However, patients with spatial neglect in the acute phase, even when they have recovered from spatial neglect, are likely to have poor functional recovery in the chronic phase in various aspects of life, including personal care, social interactions, and leisure activities [17].
Spatial neglect may be manifest as perceptual deficits of the external environment (ie, space), loss of space-related memory (eg, an inability to describe the left side of the map), or an impairment in directional motor processing (eg, infrequent movement toward the left side) [57]. Any of these deficits may affect a person’s navigation ability and potentially limit his or her travel in daily life. In addition, if caregivers or patients themselves recognize that spatial problems could increase safety risk (eg, postural bias that increases the risk of falls when ambulating), they might voluntarily limit community activities to ensure their own safety.
Our current study enrolled a small patient group, and this limits our ability to generalize findings to other stroke survivors. Our study did not specifically compare those patients with persistent spatial neglect symptoms with those who rapidly improved. Future studies examining how transient versus chronic symptoms adversely affect daily life mobility will help to determine what aspects of acute spatial neglect most affect later activities in the home and the community. Also, we did not collect information related to socioeconomic status, health conditions other than stroke, the level of involvement in the patient care by live-in care-givers, personal leisure preference, or neighborhood and/or community support, all of which may be significant factors that influence mobility in the community. Future research on the impact of spatial neglect on life space should evaluate the relationship in stroke survivors of diverse ethnic, cultural, socioeconomic, and age groups. Larger-scale studies could also control for additional demographic factors, along with the key covariates we included in the present research.
CONCLUSION
When stroke survivors have symptoms of spatial neglect in the relatively early phase after stroke, this may impede their later mobility in the community. We already are aware that motor function is not the only determinant of functional stroke recovery: this study highlights the role of spatial cognition in the recovery of mobility in the community among stroke survivors. Early detection of spatial neglect may be 1 way to determine which stroke survivors are vulnerable to adverse long-term outcomes. Further studies are required to evaluate early detection, and treatment of spatial neglect could improve mobility in the community in the long term.
Acknowledgments
Disclosure related to this publication: grant, NIH (money to institution); research support R01NS055808 (PI from National Institute of Neurological Disorders and Stroke; research support, Department of Education/National Institute of Disability and Rehabilitation Research H133 G120203 (PI). Study contents do not necessarily represent the policy of the Department of Education, and one should not assume endorsement by the federal government.
Footnotes
Disclosure: nothing to disclose
This research has been presented in preliminary form at the Association of Academic Physiatrist Annual Meeting March 7-10, 2013, in New Orleans, LA.
The author receives no financial benefit from this publication.
Contributor Information
Mooyeon Oh-Park, Department of Physical Medicine and Rehabilitation, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ; Kessler Institute for Rehabilitation, West Orange, NJ; Stroke Rehabilitation Research Laboratory, Kessler Foundation, 1199 Pleasant Valley Way, West Orange, NJ 07052.
Cynthia Hung, Department of Physical Medicine and Rehabilitation, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ.
Peii Chen, Stroke Rehabilitation Research Laboratory, Kessler Foundation, West Orange, NJ; Department of Physical Medicine and Rehabilitation, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ.
A.M. Barrett, Stroke Rehabilitation Research Laboratory, Kessler Foundation, West Orange, NJ; Department of Physical Medicine and Rehabilitation, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ; Kessler Institute for Rehabilitation, West Orange, NJ.
REFERENCES
- 1.Stav WB, Pierce S, Wheatley CJ, Davis ES. Driving and community mobility. Am J Occup Ther. 2005;59:666–670. doi: 10.5014/ajot.59.6.666. [DOI] [PubMed] [Google Scholar]
- 2.Lord SE, Rochester L. Measurement of community ambulation after stroke: Current status and future developments. Stroke. 2005;36:1457–1461. doi: 10.1161/01.STR.0000170698.20376.2e. [DOI] [PubMed] [Google Scholar]
- 3.Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63:468–474. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- 4.Heilman KM, Watson RT, Valenstein E. Clinical Neuropsychology. 5th ed. Oxford University Press; New York, NY: 2011. [Google Scholar]
- 5.Stone SP, Wilson B, Wroot A, et al. The assessment of visuo-spatial neglect after acute stroke. J Neurol Neurosurg Psychiatry. 1991;54:345–350. doi: 10.1136/jnnp.54.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelros P. Prediction of length of stay for stroke patients. Acta Neurol Scand. 2007;116:15–19. doi: 10.1111/j.1600-0404.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 7.Gillen R, Tennen H, McKee T. Unilateral spatial neglect: relation to rehabilitation outcomes in patients with right hemisphere stroke. Arch Phys Med Rehabil. 2005;86:763–767. doi: 10.1016/j.apmr.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: A 2-year follow-up study. Neuropsychology. 1997;11:290–295. doi: 10.1037//0894-4105.11.2.290. [DOI] [PubMed] [Google Scholar]
- 9.Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004;62:749–756. doi: 10.1212/01.wnl.0000113730.73031.f4. [DOI] [PubMed] [Google Scholar]
- 10.Berti A, Smania N, Rabuffetti M, et al. Coding of far and near space during walking in neglect patients. Neuropsychology. 2002;16:390–399. doi: 10.1037//0894-4105.16.3.390. [DOI] [PubMed] [Google Scholar]
- 11.Tromp E, Dinkla A, Mulder T. Walking through doorways: An analysis of navigation skills in patients with neglect. Neuropsychol Rehabil. 1995;5:319–331. [Google Scholar]
- 12.Huitema RB, Brouwer WH, Hof AL, Dekker R, Mulder T, Postema K. Walking trajectory in neglect patients. Gait Posture. 2006;23:200–205. doi: 10.1016/j.gaitpost.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Webster JS, Rapport LJ, Godlewski MC, Abadee PS. Effect of attentional bias to right space on wheelchair mobility. J Clin Exp Neuropsychol. 1994;16:129–137. doi: 10.1080/01688639408402623. [DOI] [PubMed] [Google Scholar]
- 14.Akinwuntan AE, Feys H, De Weerdt W, Baten G, Arno P, Kiekens C. Prediction of driving after stroke: A prospective study. Neurorehabil Neural Repair. 2006;20:417–423. doi: 10.1177/1545968306287157. [DOI] [PubMed] [Google Scholar]
- 15.Sunderland A, Wade DT, Langton Hewer R. The natural history of visual neglect after stroke. Indications from two methods of assessment. Int Disabil Stud. 1987;9:55–59. doi: 10.3109/03790798709166235. [DOI] [PubMed] [Google Scholar]
- 16.Nijboer TC, Kollen BJ, Kwakkel G. Time course of visuospatial neglect early after stroke: A longitudinal cohort study. Cortex. 2013;49:2021–2027. doi: 10.1016/j.cortex.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Nijboer T, van de Port I, Schepers V, Post M, Visser-Meily A. Predicting functional outcome after stroke: The influence of neglect on basic activities in daily living. Front Hum Neurosci. 2013;7:182. doi: 10.3389/fnhum.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental demands associated with community mobility in older adults with and without mobility disabilities. Phys Ther. 2002;82:670–681. [PubMed] [Google Scholar]
- 19.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: How important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004;85:234–239. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . Towards a Common Language for Functioning, Disability and Health, The International Classification of Functioning, Disability and Health. WHO; Geneva: [Accessed September 29, 2013]. 2002. Available at http://www.who.int/classifications/icf/training/icfbeginnersguide.pdf. [Google Scholar]
- 21.Barnes LL, Wilson RS, Bienias JL, et al. Correlates of life space in a volunteer cohort of older adults. Exp Aging Res. 2007;33:77–93. doi: 10.1080/03610730601006420. [DOI] [PubMed] [Google Scholar]
- 22.Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner EV, Allman RM. Assessing mobility in older adults: The UAB Study of Aging Life-Space Assessment. Phys Ther. 2005;85:1008–1119. [PubMed] [Google Scholar]
- 23.May D, Nayak US, Isaacs B. The life-space diary: A measure of mobility in old people at home. Int Rehabil Med. 1985;7:182–186. doi: 10.3109/03790798509165993. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb ER, Smith EC, Wolfenden LL, Allman RM, Tangpricha V. Life-space mobility is associated with frequency of hospitalization in adults with cystic fibrosis. Clin Respir J. 2011;5:245–251. doi: 10.1111/j.1752-699X.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle PA, Buchman AS, Barnes LL, James BD, Bennett DA. Association between life space and risk of mortality in advanced age. J Am Geriatr Soc. 2010;58:1925–1930. doi: 10.1111/j.1532-5415.2010.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue QL, Fried LP, Glass TA, Laffan A, Chaves PH. Life-space constriction, development of frailty, and the competing risk of mortality: The Women’s Health and Aging Study I. Am J Epidemiol. 2008;167:240–248. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 27.Crowe M, Andel R, Wadley VG, Okonkwo OC, Sawyer P, Allman RM. Life-space and cognitive decline in a community-based sample of African American and Caucasian older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1241–1245. doi: 10.1093/gerona/63.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turton AJ, Dewar SJ, Lievesley A, O’Leary K, Gabb J, Gilchrist ID. Walking and wheelchair navigation in patients with left visual neglect. Neuropsychol Rehabil. 2009;19:274–290. doi: 10.1080/09602010802106478. [DOI] [PubMed] [Google Scholar]
- 29.Perennou D. Postural disorders and spatial neglect in stroke patients: A strong association. Restor Neurol Neurosci. 2006;24:319–334. [PubMed] [Google Scholar]
- 30.Wilson B, Cockburn J, Halligan PW. Behavioural Inattention Test. Thames Valley Test; London: 1987. [Google Scholar]
- 31.Wilson B, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Arch Phys Med Rehabil. 1987;68:98–102. [PubMed] [Google Scholar]
- 32.Halligan PW, Cockburn J, Wilson BA. The behavioural assessment of visual neglect. Neuropsychol Rehabil. 1991;1:5–32. [Google Scholar]
- 33.Bergego C, Azouvi P, Samuel C. Validation d’une echelle d’evaluation fonctionnelle de l’heminegligence dans la vie quotidienne: l’echelle C.B. Ann Readapt Med Phys. 1995;38:183–189. [Google Scholar]
- 34.Chen P, Hreha K, Fortis P, Goedert KM, Barrett AM. Functional assessment of spatial neglect: A review of the Catherine Bergego scale and an introduction of the Kessler Foundation neglect assessment process. Top Stroke Rehabil. 2012;19:423–435. doi: 10.1310/tsr1905-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartman-Maeir A, Katz N. Validity of the Behavioral Inattention Test (BIT): Relationships with functional tasks. Am J Occup Ther. 1995;49:507–516. doi: 10.5014/ajot.49.6.507. [DOI] [PubMed] [Google Scholar]
- 36.Azouvi P, Samuel C, Louis-Dreyfus A, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002;73:160–166. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goedert KM, Chen P, Botticello A, Masmela JR, Adler U, Barrett AM. Psychometric evaluation of neglect assessment reveals motor-exploratory predictor of functional disability in acute-stage spatial neglect. Arch Phys Med Rehabil. 2012;93:137–142. doi: 10.1016/j.apmr.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azouvi P, Olivier S, de Montety G, Samuel C, Louis-Dreyfus A, Tesio L. Behavioral assessment of unilateral neglect: Study of the psychometric properties of the Catherine Bergego Scale. Arch Phys Med Rehabil. 2003;84:51–57. doi: 10.1053/apmr.2003.50062. [DOI] [PubMed] [Google Scholar]
- 39.Kim BR, Chun MH, Kim DY, Lee SJ. Effect of high- and low-frequency repetitive transcranial magnetic stimulation on visuospatial neglect in patients with acute stroke: A double-blind, sham-controlled trial. Arch Phys Med Rehabil. 2013;94:803–807. doi: 10.1016/j.apmr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Luukkainen-Markkula R, Tarkka IM, Pitkanen K, Sivenius J, Hamalainen H. Comparison of the Behavioural Inattention Test and the Catherine Bergego Scale in assessment of hemispatial neglect. Neuropsychol Rehabil. 2011;21:103–116. doi: 10.1080/09602011.2010.531619. [DOI] [PubMed] [Google Scholar]
- 41.Qiang W, Sonoda S, Suzuki M, Okamoto S, Saitoh E. Reliability and validity of a wheelchair collision test for screening behavioral assessment of unilateral neglect after stroke. Am J Phys Med Rehabil. 2005;84:161–166. doi: 10.1097/01.phm.0000154902.79990.12. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Sonoda S, Hanamura M, Okazaki H, Saitoh E. Line bisection and rebisection: The crossover effect of space location. Neurorehabil Neural Repair. 2005;19:84–92. doi: 10.1177/1545968305274661. [DOI] [PubMed] [Google Scholar]
- 43.Parker M, Baker PS, Allman RM. A life-space approach to functional assessment of mobility in the elderly. J Gerontol Soc Work. 2002;35:35–55. [Google Scholar]
- 44.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 45.Brown CJ, Roth DL, Allman RM, Sawyer P, Ritchie CS, Roseman JM. Trajectories of life-space mobility after hospitalization. Ann Intern Med. 2009;150:372–378. doi: 10.7326/0003-4819-150-6-200903170-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rantanen T, Portegijs E, Viljanen A, et al. Individual and environmental factors underlying life space of older people: Study protocol and design of a cohort study on life-space mobility in old age (LISPE) BMC Public Health. 2012;12:1018. doi: 10.1186/1471-2458-12-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritchie CS, Locher JL, Roth DL, McVie T, Sawyer P, Allman R. Unintentional weight loss predicts decline in activities of daily living function and life-space mobility over 4 years among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63:67–75. doi: 10.1093/gerona/63.1.67. [DOI] [PubMed] [Google Scholar]
- 48.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. MD State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 49.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: A reliability study. Int Disabil Stud. 1988;10:61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 50.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 51.Alzahrani MA, Dean CM, Ada L. Ability to negotiate stairs predicts free-living physical activity in community-dwelling people with stroke: An observational study. Aust J Physiother. 2009;55:277–281. doi: 10.1016/s0004-9514(09)70008-x. [DOI] [PubMed] [Google Scholar]
- 52.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: The role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86:1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 53.Robinson CA, Shumway-Cook A, Matsuda PN, Ciol MA. Understanding physical factors associated with participation in community ambulation following stroke. Disabil Rehabil. 2011;33:1033–1042. doi: 10.3109/09638288.2010.520803. [DOI] [PubMed] [Google Scholar]
- 54.Vahlberg B, Cederholm T, Lindmark B, Zetterberg L, Hellstrom K. Factors related to performance-based mobility and self-reported physical activity in individuals 1-3 years after stroke: A cross-sectional cohort study. J Stroke Cerebrovasc Dis. 2013;22:e426–e434. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Murata C, Kondo T, Tamakoshi K, Yatsuya H, Toyoshima H. Factors associated with life space among community-living rural elders in Japan. Public Health Nurs. 2006;23:324–331. doi: 10.1111/j.1525-1446.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 56.Stone SP, Patel P, Greenwood RJ, Halligan PW. Measuring visual neglect in acute stroke and predicting its recovery: The Visual Neglect Recovery Index. J Neurol Neurosurg Psychiatry. 1992;55:431–436. doi: 10.1136/jnnp.55.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adair JC, Barrett AM. Spatial neglect: clinical and neuroscience review: A wealth of information on the poverty of spatial attention. Ann N Y Acad Sci. 2008;1142:21–43. doi: 10.1196/annals.1444.008. [DOI] [PMC free article] [PubMed] [Google Scholar]