Abstract
Background
An aphasia treatment was designed to shift laterality from the left to right lateral frontal lobe during word production by initiating word-finding trials with complex left-hand movements. Previous findings indicated successful re-lateralization.
Objective
The current study was designed to ascertain whether the shift was attributable to the left-hand movement.
Methods
Using stratified random sampling, 14 subjects were equally divided between Intention (IT) and Control (CT) treatments. CT was identical to IT, except with no left-hand movements. Both treatments trained picture naming (phases 1, 2) and category-member generation (phase 3), each phase lasting 10 sessions. fMRI of category member generation occurred at pre-treatment, post-treatment, and 3-month follow-up.
Results
IT shifted lateral frontal activity rightward compared to pre-treatment both at post-treatment (t=−2.602, df=6, p<.05) and 3-month follow-up (t=−2.332, df=5, p<.05), but CT did not. IT and CT yielded similar changes for all picture-naming and category probes. However, IT patients showed gains for untrained category (t=3.33, df=6, p<.01) and picture-naming probes (t=3.77, df=5, p<.01), but CT patients did not.
Conclusions
The rightward shift in lateral frontal activity for IT was due to the left-hand movements. IT evoked greater generalization than CT.
Keywords: Aphasia, Magnetic Resonance Imaging, Functional, fMRI, Rehabilitation, Rehabilitation of Speech and Language Disorders, Neuronal Plasticity
Aphasia is a common aftermath of dominant-hemisphere stroke. More than half of aphasia patients surviving stroke for 6 months show significant language deficits1, causing substantial disability2,3. Classical aphasia therapies focus on retraining language skills using cognitive/behavioral methods. Recently, however, aphasia therapies have begun to target specific cortical structures for engagement or disengagement, using physiological manipulations. For example, Naeser and colleagues4 used low-frequency repetitive transcranial magnetic stimulation (rTMS) to decrease excitability in right pars triangularis, the anterior portion of right Broca's area homologue. With no other treatment, rTMS increased naming accuracy. Barwood and colleagues5 replicated this finding with a sham-rTMS control that did not show the same improvement as the real-rTMS. However, rTMS of right pars opercularis, the posterior component of Broca's area homologue, had the opposite effect of stimulating pars triangularis, it slowed naming performance6. Hence, it cannot be assumed that right-hemisphere structures do not contribute to language functions in aphasia. Studies using transcranial direct current stimulation to engage or suppress various cortical structures also have shown therapeutic effects7–11, though these studies can appear contradictory regarding underlying mechanisms.
Few studies have used behavioral manipulations to engage specific brain mechanisms. Although one interpretation of the purpose of melodic intonation therapy (MIT) is that it remaps language production to right frontal cortex, one positron emission tomography study called this explanation into question12. However, recent findings by Vines and colleagues indicated that increasing excitability of right Broca's homologue improved MIT results13. Crosson and colleagues14 used a different approach to remapping word production to right frontal cortex. They designed an Intention treatment (IT) to re-lateralize language production from the left to the right frontal lobe, using a different behavioral manipulation to accomplish this remapping. Nonfluent aphasia patients initiated picture naming trials with complex left-hand movements, putatively to activate right-hemisphere (medial frontal) intentional mechanisms that engage right lateral frontal structures during training. Moderately to severely anomic patients improved during treatment and showed generalization to untrained items. Patients re-learned words more quickly during IT than they did during an Attention control treatment.
Superficially, these data seem to conflict with rTMS data showing that reduction in right pars triangularis excitability improves naming in nonfluent aphasia4,5. However, Crosson et al.15 used fMRI of category member generation to study five patients receiving IT. Four patients who improved showed a significant rightward shift in lateral frontal activity. Their frontal activity was significantly more right lateralized than that of controls after but not before treatment. Importantly, activity was concentrated in motor/premotor cortex and right pars opercularis, posterior to the site where reducing cortical excitability with rTMS led to improved naming4–5, and closer to the site where reducing cortical excitability slowed naming in nonfluent aphasia6. The problem with the Crosson et al. study, however, was that no control treatment was imaged; so, it was not certain that the rightward frontal activity shift was specific to the intention component of IT (i.e., initiating naming trials with left-hand movements).
The purpose of the present study was to determine if the Intention component was responsible for the rightward lateral frontal activity shift during word production. In a parallel groups design, we compared IT to a Control treatment (CT) which was exactly the same as IT only without complex hand movements. Our main hypothesis was that IT would evoke a significant rightward shift in lateral frontal activity during word production, but CT would not.
Method
Subjects
Fourteen chronic (>6 months) aphasia patients participated. Subjects gave written informed consent in accordance with procedures approved by the University of Florida Health Science Institutional Review Board. Patients were premorbidly right-handed16, used English as first language, and had single or multiple left-hemisphere ischemic or hemorrhagic strokes. They had no contraindications for MRI, no central neurological disorder excepting stroke, no drug/alcohol abuse (last 12 months), no major psychiatric disorder, and no hearing loss>75 dB HL at 500–4,000 Hz. Subjects had Boston Naming Test scores between 4 and 45 correct of 60 items, Western Aphasia Battery Aphasia Quotients<94, Peabody Picture Vocabulary Test-IV<2 SD below age-appropriate mean, lesions extending frontally at least into the precentral gyrus or underlying white matter (see Supplemental Figure 1 for lesion distribution), and at least minimal evidence of nonfluent output during narrative picture description as judged by an experienced Speech/Language Pathologist (JCR). Subjects generated members to at least 8/120 categories during initial assessment.
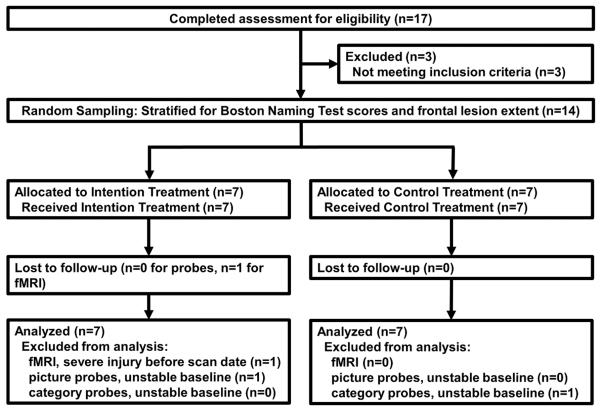
Subjects were assigned to Intention (IT) or Control (CT) treatments using stratified random sampling (see Figure 1 for consort flow diagram). Stratification equalized groups for picture-naming impairment (Boston Naming Test) and number of subjects whose frontal lesions extended anteriorly beyond the precentral sulcus. There were no significant differences between groups on any demographic or language-performance variable in Table 1 (p<.05), but the groups were marginally different (p<.10) in the gender composition (more females in Intention) and education (Intention>Control). Groups also were marginally different in repetition; 4 of 7 Intention patients had conduction aphasia, 4 of 7 Control patients had anomic aphasia (Western Aphasia Battery classifications). Although these types of aphasia normally are considered fluent, apraxia of speech (AOS) was judged to be present by consensus of three experienced Speech/Language Pathologists (LJGR, JJR, JCR) in 4 of 7 Intention patients and 5 of 6 Control patients (AOS measures were inadvertently omitted in one Control patient).
Figure 1.
Consort flow diagram. This diagram shows the parallel groups design of the study and subject progress through the trial.
Table 1.
Demographic Data by Group
| Intention (N=7) | Control (N=7) | |
|---|---|---|
| Gender (M:F) | 2:5 | 6:1 |
| Age (years) | 72.1 (10.5) | 63.0 (9.2) |
| Education level (years) | 14.9 (2.5) | 12.9 (1.1) |
| Time since stroke (months) | 37.4 (33.5) | 38.1 (37.4) |
| range (months) | 12–87 | 10–112 |
| Stroke type (ischemic:hemorrhagic) | 5:2 | 6:1 |
| WAB aphasia classification | ||
| Broca's | 2 | 1 |
| Transcortical Motor | --- | 1 |
| Conduction | 4 | 1 |
| Anomic | 1 | 4 |
| WABAQ | 65.5 (8.3) | 71.9 (11.8) |
| WAB Spontaneous Speech | 12.3 (2.5) | 12.3 (2.7) |
| WAB Comprehension | 173.1 (17.9) | 178.4 (10.0) |
| WAB Repetition | 51.4 (16.4) | 73.4 (23.4) |
| WAB Naming | 66.4 (17.0) | 74.0 (14.4) |
| BNT | 24.7 (13.4) | 30.9 (6.3) |
Procedures
Treatment and Probe Stimuli
On two separate occasions prior to baseline, patients received over 400 pictures to name and 120 categories for which to generate members. Pictures were presented via computer and monitor; categories were presented in both written and auditory formats. Sixty pictures and 40 categories were selected as probe items to track treatment change. Fifty pictures were selected for training during treatment phase 1, 50 different pictures were selected for phase 2, and 40 categories were selected for phase 3. An attempt was made to select items such that patients missed 75% of the items and obtained correct answers to 25% of the items on both administrations, though with the limited number of items, this goal was sometimes difficult to accomplish for categories. Twenty probe pictures overlapped with phase1 training items, 20 overlapped with phase 2, and 20 probe pictures were never used in treatment. Twenty probe categories overlapped with phase 3 training, and 20 were never trained.
Baseline probe sessions
Prior to treatment, picture and category probes were given in daily baseline sessions until the C statistic17 indicated no significant upward trend for eight consecutive sessions. Subsequently during treatment, half of the naming and category probes were administered prior to each treatment session. Each treatment phase consisted of 10 sessions per week; there were 5 complete administrations of both probe sets during each treatment phase.
Intention and Control treatments
IT was fully described previously14. Briefly, patients sat in front of a computer monitor, with a small box (23 × 14 × 5 cm) in their left hemispace. Stimuli were described above. To initiate treatment trials, patients lifted the lid to the box with their left hand and pushed the red button on a key pad inside the box, which triggered presentation of a treatment stimulus. These stimuli were pictures to name in phases 1 and 2. Treatment progressed to category member generation in Phase 3 because it involves selection of a single word from multiple possibilities, which more nearly parallels generating a word for a concept in every-day conversation that does picture naming. If responses to stimuli were correct, patients progressed to the next trial. If not, they repeated correct responses after therapists while making circular left-hand gestures, with stimuli remaining on the monitor. The 50 trained pictures and 40 categories were each presented once per session for respective treatment phases. CT was identical to IT except that CT trials were initiated by the therapist instead of left-hand movements, and there was no gesture during error correction.
Functional MRI (fMRI)
fMRI sessions assessed changes in laterality of frontal functions before commencement of baseline, after treatment, and 3 months after treatment termination using a Philips 3 Tesla Achieva scanner. During each of 60 trials, patients heard and read a category and attempted to generate aloud a single member. Category member generation was chosen as the fMRI task because this task was chosen as the culminating task for treatment and because it more closely parallels word selection demands in conversation, as explained above. Trial length was 6.8 sec. Patients viewed a “+” during intertrial intervals that alternated between 13.6, 15.3, and 17.0 sec. For functional images, the whole brain was imaged in 1.70 sec using a gradient echo-echo planar sequence, an 8-channel head coil, and 36 × 4 mm thick sagittal slices (TR=1700 ms; TE=30 ms; FA=70 degrees; FOV=24 cm, matrix = 64 × 64). Prior to functional images, high-resolution T1-weighted structural images were acquired for 160 × 1.0 mm thick sagittal slices, using a turbo field echo acquisition (TE=3.7 ms; TR=8.1 ms; FOV=24 cm; FA=8 degrees; matrix size=240 × 240).
Data Analyses
fMRI
Lesions were masked on T1-weighted images using ITK-Snap (http://www.itksnap.org/pmwiki/pmwiki.php), with boundaries adjusted by an operator (HP) and then warped into MNI-152 atlas space using the nonlinear FNIRT algorithm from FSL (http://www.fmrib.ox.ac.uk/fsl/). Separate deconvolution analyses (AFNI: http://afni.nimh.nih.gov/afni) were timed to stimulus onset and response initiation, respectively, yielding blood oxygenation level dependent (BOLD) hemodynamic responses (HDRs) with 16 time-points (27.2 sec) on a voxelwise basis. A threshold of R2≥0.12 (p<5×10−21) was set for correlation between the highest of the derived HDRs and the original time series. HDRs meeting this criterion were filtered with five gamma variate functions representing ideal HDRs of different width. HDRs with the highest r for the five gamma variates≥0.80 (p<.01) were considered to represent voxels with task-related activity. Three regions of interest (ROIs: medial frontal, lateral frontal, and posterior perisylvian) were constructed for each hemisphere by combining regions from the Harvard-Oxford atlas distributed with FSL (the anterior most portion of frontal polar cortex was eliminated from frontal ROIs). The volume of active cortex from each ROI was extracted, and laterality indices used the following formula (left – right) / (left + right). 1.0 represented completely left-lateralized activity, and −1.0 represented completely right-lateralized activity. We hypothesized that lateral frontal activity would become more right lateralized after treatment for IT but not for CT. Hence, post-treatment and 3-month follow-up laterality indices both were compared to pre-treatment laterality indices using repeated-measures t tests.
Aphasia treatment
A secondary hypothesis that the Intention treatment would show greater treatment response than the Control treatment was assessed by three methods. (1) The average pre-treatment baseline accuracy for picture-naming and category probes was subtracted from respective post-treatment and 3-month follow-up accuracy, and changes were compared between the Intention and Control groups using two-sample t tests. Weaknesses in this strategy are first that it relies on a single data point at post-treatment and 3-month follow-up, and single data points have an inherent variability relative to the average of multiple data points, and second that between-subject analyses have less power than within subjects, especially with small numbers. (2) Hence, the average performance on probes during Phase 3 minus the average performance at baseline served as the dependent variable for a within group t-test. This analysis was performed separately for naming and category probes, and analyses were done for trained and untrained probes combined and for untrained probes alone. (3) The C-statistic 17–18 was calculated for each individual subject. It assesses treatment gains for individual-subject time series by evaluating changes in slope from baseline to treatment relative to variability in successive data points. We have previously shown15 that the C statistic produced similar results to analysis of effect sizes and a modified Conservative Dual Criteria test19 for similar studies. Subjects had baseline performances in which the C statistic did not indicate progressive increases in performance before treatment was initiated.
Correlation of treatment outcome with lateralization
To determine the relationship between improvement during treatment and changes in laterality, Z scores for both picture-naming and category probes were correlated with laterality shifts from pre- to post-treatment using a product-moment correlation for the lateral frontal, medial frontal, and posterior perisylvian ROIs.
Results
Changes in ROI Laterality
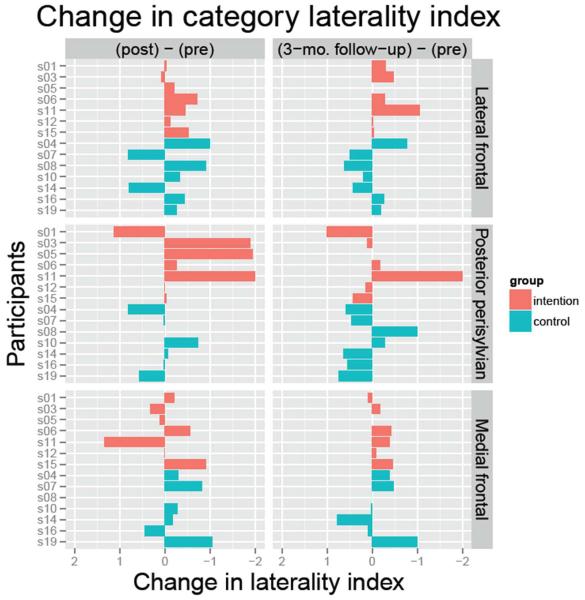
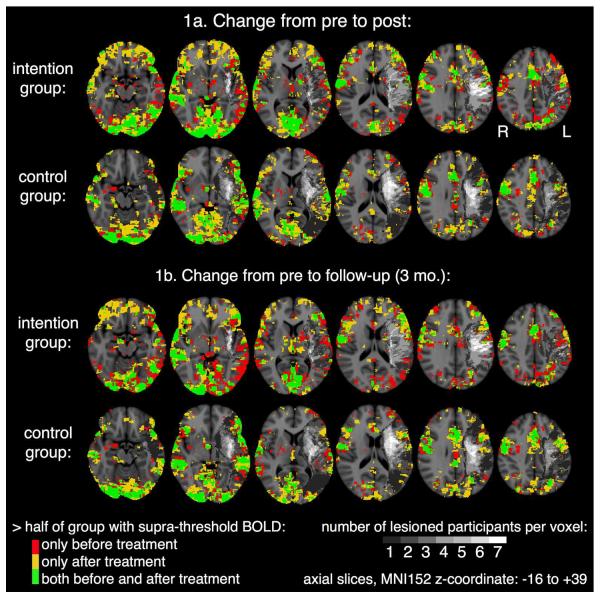
Figure 2 shows changes in laterality indices for individual subjects for post-treatment minus pre-treatment and 3-month follow-up minus pre-treatment. Red bars represent the Intention treatment (IT), blue bars the control treatment (CT). Changes to the right of zero (negative changes) represent rightward laterality shifts. One-tailed t-tests indicated that IT patients demonstrated a significant rightward shift in lateral frontal activity from pre-treatment to both post-treatment (t=−2.602, df=6, p<.05) and 3-month follow-up (t=−2.332, df=5, p<.05). One patient was lost to follow-up. CT patients did not demonstrate such a shift at either time (ps>.25). Hence, our main hypothesis was confirmed. There also was a significant rightward shift in medial frontal activity for IT at 3 months post (t=−2.615, df=5, p<.05). There were no other significant laterality shifts for either group at either post-treatment or 3-month follow-up (ps>.05). Figure 3 (top 2 rows) shows areas where more than half of the subjects showed activity at both pre- and post-treatment (green), only at pre-treatment (red), and only at post-treatment (yellow). The bottom 2 rows of the figure show similar images for activity at pre-treatment and 3-month follow-up.
Figure 2.
Change in Laterality Indices. Changes in laterality indices from the pre-treatment fMRI scans to the post-treatment or 3-month follow-up scans are shown by individual subject. Red bars represent the Intention group; blue bars represent the Control group. Negative changes (to the right of zero) represent rightward laterality shifts.
Figure 3.
Maps of Activity for Pre- and Post-Treatment (Top 2 rows) and for Pre-Treatment and 3-Month Follow-up. In green voxels, activity was present for more than half of the subjects at both Pre-Treatment and Post-Treatment (or 3-Month Follow-up); in red voxels, activity was present in more than half of subjects only at Pre-Treatment; in yellow voxels, activity was present in more than half of the subjects only at Post-Treatment (or 3-Month Follow-up). Gray to white scale represent the numbers of subjects with lesions in various left hemisphere voxels, with the darkest gray representing only 1 subject with a lesion in a voxel and white representing all seven subjects with a lesion in a voxel. Voxels with no lesion or activity are represented in the usual gray-scale for anatomy.
Treatment Gains for Probes
Patients were required to have non-improving probe baselines before treatment commenced. Originally, baseline stability was tested with the formula from Tryon17. However, when an erratum to this formula18 was discovered and applied to baselines retrospectively, one IT patient showed an improving baseline for picture-naming probes, and one CT patient showed an improving baseline for category probes. These patients' data were eliminated from analyses of treatment gains. There were no significant differences between the Intention and Control groups for change in either picture-naming or category probe accuracy from pre-treatment performance at either post-treatment or follow-up (ps>.05).
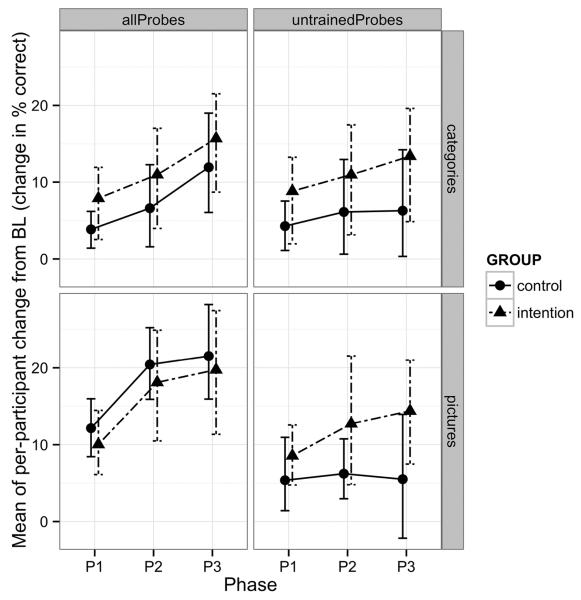
Individually, patients from both groups showed similar gains during treatment for all picture-naming probes. Five of 6 Intention patients and 6 of 7 control patients showed significant improvement. However, for the category probes, 6 of 7 Intention patients and only 3 of 6 Control patients showed significant improvement. When both trained and untrained probes were considered, both IT and the Control Treatment showed significant gains from Baseline to Phase 3 (t=4.44, df=5, p<.005 for IT on picture-naming; t=6.03, df=6, p<.0005 for Control on picture-naming; t=4.31, df=6, p<.005 for IT on categories; t=3.40, df=5, p<.01 for Control on categories).
To assess generalization, untrained probes were evaluated separately. Three of 6 Intention patients and 3 of 7 Control patients showed significant gains on untrained picture-naming probes. However, 6 of 7 Intention patients but only 1 of 6 Control patients showed significant gains on untrained category probes. Further, when untrained probes alone were considered in group analyses, only IT showed significant improvement (t=3.77, df=5, p<.01 for IT on picture-naming; t=1.20, df=6, p>.05 for CT on picture-naming; t=3.33, df=6, p<.01 for IT on categories; t=1.56, df=5, p>.05 for Control on categories). Figure 4 shows changes in accuracy from baseline at each of the treatment phases for naming and category probes, both for all probes and for only untrained probes.
Figure 4.
Changes in accuracy from baseline at each of the treatment phases for category probes (top) and naming probes (bottom), both for all probes (left) and for only untrained probes (right).
Correlations between Laterality Changes and Treatment Gains
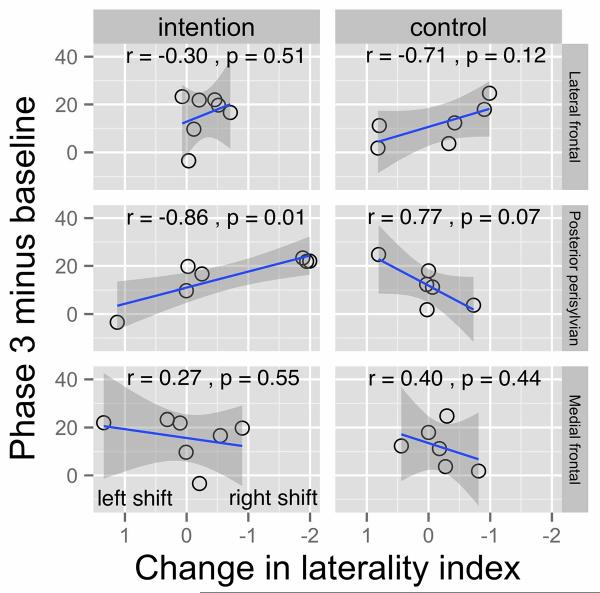
Change in laterality index from pre- to post-treatment was correlated with the change in probe performance (all probes) from Baseline to Phase 3. For category-member generation probes, neither the shift in lateral frontal activity nor shift in medial frontal activity correlated with treatment gains, ps>0.10 for either group. However, rightward shifts in posterior persylvian activity showed a significant correlation with treatment gains for IT (r=−0.86, n=7, p<.01), but for CT, treatment gains were marginally associated with a leftward shift in posterior perisylvian activity (r=0.77, n=6, p<.10). Because of the small number of subjects in the groups, the regression lines and 2-tailed confidence intervals have been plotted in Figure 5. For the Intention treatment, the removal of any one subject would not greatly alter the regression line; however, the removal of one subject at either end of the regression line for the Control group could alter the regression line, leaving some doubt that this correlation is real. There were no significant correlations between Z scores for picture-naming probes and laterality shifts in any ROI (ps>.10).
Figure 5.
Treatment Change-Laterality Index Change Correlations. Regression lines, confidence intervals, and correlations between change in laterality indices and treatment change are shown for the three ROIs in each of the groups.
Discussion
Patients in the Intention treatment (IT) initiated word-finding trials with complex left-hand movements during treatment, and, consistent with a priori hypotheses, engagement of right relative to left lateral frontal cortex during word generation increased immediately post-treatment. This laterality shift persisted for three months post-treatment. No such shift occurred for the Control treatment (CT), whose treatment was identical to that of IT, except that there was no left-hand movement to initiate word-finding trials or to accompany error correction. Hence, it was not simply the word-finding treatment, but the use of the left-hand movement that led to the shift in lateral frontal laterality for IT. Medial frontal cortex also showed a laterality shift for IT, but only at 3-month follow-up.
These findings are important for treatment of language or cognitive deficits due to stroke or other brain damage. They indicate that behavioral manipulations can be designed to engage specific cortical mechanisms during treatment. This approach adds a new method to the toolbox for aphasia treatment. To engage specific cortical mechanisms in rehabilitation, investigators must know what mechanisms to engage and how to engage them. Such decisions can be made on the basis of theoretical constructs regarding the specific deficits being addressed and the mechanisms that can be engaged to mitigate them. Originally, Crosson et al.14 hypothesized that rightward shifts in lateral frontal cortex would be driven by increased activity in right medial frontal cortex. Current findings indicate that this scenario is unlikely because laterality shifts in medial frontal cortex followed those of lateral frontal cortex, occurring only at 3-month follow-up. Rizzolatti and Arbib21,22 have hypothesized a close link between hand movements and the development of language phylogenetically through mirror neurons in pars opercularis. Given the current data, maintenance of a residual relationship between hand movements and language seems like a more plausible explanation for the rightward laterality shift due to IT. Behaviorally, IT and CT both yielded gains for responses to all picture-naming and category probes. However, when only untrained probes were analyzed only IT showed significant gains from Baseline to Phase 3. This latter finding for the untrained probes indicates that effects of IT generalized to untrained items but effects of CT did not. Generalization IT may reflect a general shift of word production to more capable substrates while CT may have relied on training of specific pictures or categories using existing substrates. Analysis of discourse production from these treatments is being presented in a separate paper, but indicates greater generalization in discourse for word-finding in IT than in CT20, consistent with generalization on category and naming probes. Hence, engaging new (right-hemisphere) substrates seems to lead to generalization, but engaging remaining left-hemisphere mechanisms does not.
One surprising finding was that a rightward increase in laterality of posterior perisylvian, not lateral frontal, activity was associated with better treatment outcome for the Intention group. No other correlations were noted for this group. This phenomenon underscores that no brain area operates in isolation to produce complex behaviors, such as word production. Apparently, treatment gains were greatest in patients for whom right lateral frontal engagement leverages a rightward shift in posterior perisylvian activity. In other words, the more patients engaged right posterior perisylvian mechanisms to replace damaged left-hemisphere mechanisms, the greater treatment gains were. Other studies have implicated posterior right-hemisphere mechanisms in aphasia treatment response23, especially in fluent aphasia24.
Indeed, the brain structures in which activity changes correlate with behavioral outcome may be specific to aphasia classification. In the current study, IT patients were dominated by patients with conduction aphasia and had significant posterior perisylvian damage (see Supplemental Figure 1). In our previous fMRI study of IT15, the sample consisted of 3 patients with Broca's aphasia and 2 patients with anomic aphasia. With the exception of one previous patient with completely lesioned posterior perisylvian cortex, frontal structures were more extensively damaged in the previous than the current study, and posterior persylvian structures were more intact in the previous than the present study. In all but the patient with completely destroyed left posterior perisylvian cortex, the posterior perisylvian ROI in the former study showed stable laterality or a leftward shift from pre- to post-treatment scans. This finding contrasts with the correlation in the current study indicating that greater gains in category member generation were associated with higher right posterior perisylvian activity in the Intention group. Hence, type of aphasia and lesion location may affect roles of left and right posterior perisylvian cortex in the Intention treatment.
While groups were stratified for degree of frontal lesion and for severity of naming deficit, the IT group had four conduction aphasias and the CT group had four anomic aphasias. A question arising from the difference in group composition is whether type of aphasia could have influenced treatment response. However, error correction relied on repetition, which would put patients with conduction aphasia in IT at a relative disadvantage compared to CT. Even with this impediment, though, findings favored the Intention group. A further facet of group composition is that while patients were required to show minimal evidence of nonfluent narrative output, the nonfluent characteristics were not substantial enough to place patients into nonfluent categories of aphasia, as both conduction and anomic aphasia were considered to be fluent. In our previous study15, patients with anomic and Broca's aphasia both showed rightward relateralization of frontal activity during word production and improvement as a result of the Intention treatment. In that study, both patients with anomic aphasia had treatment Z scores from the C statistic as high or higher than the highest Z score in the current study, suggesting that patients with anomic aphasia in CT could have responded to IT had they received it, which mitigates concerns about differences in group composition for the current study.
In summary, current findings endorse the possibility that specific neural substrates can be targeted with behavioral strategies. If it is possible in this instance, it likely is possible in other kinds of treatments for cognitive disorders. Also, while our previous study suggested that in patients with Broca's aphasia, improvement during the Intention treatment was associated with a rightward shift in lateral frontal laterality, the current study indicates that greater treatment improvement in patients with more fluent aphasias was associated with a rightward shift in posterior perisylvian laterality. Thus, the mechanisms of change in different types of aphasia may be different. The response of different kinds of aphasias to IT and the underlying mechanism of change are worth further consideration.
Supplementary Material
Acknowledgments
This research was funded by Grants R01DC007387 (BC) and K23 DC010197 (JJR) from the National Institute on Deafness and Other Communication Disorders; Senior Research Career Scientist Award B6364L (BC), Research Career Scientist Award B5083L (LJGR), and Center of Excellence Award B3149C (LJGR) from the VA Rehabilitation Research & Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The authors wish to acknowledge Floris Singletary for her assistance in recruitment of subjects for the current study and to recognize JoEllen Gilbert and Cecilia Brooks for providing the therapies to subjects in Jacksonville.
References
- 1.Pedersen PM, JørgensenH S, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38:659–666. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 2.de Haan EJ, Limburg M, Van der Meulen JHP, Jacobs HM, Aaronson NK. Quality of life after stroke. Impact of stroke type and lesion location. Stroke. 1995;26:402–408. doi: 10.1161/01.str.26.3.402. [DOI] [PubMed] [Google Scholar]
- 3.King RB. Quality of life after stroke. Stroke. 1996;27:1467–1472. doi: 10.1161/01.str.27.9.1467. [DOI] [PubMed] [Google Scholar]
- 4.Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, O'Sullivan J, Coulthard A, Wong A, Aitken P, Hall G. The effects of low frequency Repetitive Transcranial Magnetic Stimulation (rTMS) and sham condition rTMS on behavioural language in chronic non-fluent aphasia: Short term outcomes. NeuroRehabilitation. 2011;28:113–128. doi: 10.3233/NRE-2011-0640. [DOI] [PubMed] [Google Scholar]
- 6.Naeser MA, Martin PI, Theoret H, Kobayashi M, Fregni F, Nicholas M, Tormos JM, Steven MS, Baker EH, Pascual-Leone A. TMS suppression of right pars triangularis, but not pars opercularis, improves naming in aphasia. Brain Lang. 2011;119:206–213. doi: 10.1016/j.bandl.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flöel A, Meinzer M, Kirstein R, Nijhof S, Deppe M, Knecht S, Breitenstein C. Short-term anomia training and electrical brain stimulation. Stroke. 2011;42:2065–2067. doi: 10.1161/STROKEAHA.110.609032. [DOI] [PubMed] [Google Scholar]
- 9.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham controlled study. Stroke. 2011;42:819–821. doi: 10.1161/STROKEAHA.110.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Maneli F, Mrakic-Sposta S, Vergari M, Zago S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79:451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- 11.You DS, Kim DY, Chun MH, Jung SE, Park SJ. Cathodal transcranial direct current stimulation of the right Wernicke's area improves comprehension in subacute stroke patients. Brain Lang. 2011;119:1191–5. doi: 10.1016/j.bandl.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Belin P, Van Eeckhout P, Zilbovicius M, Remy P, François C, Guillaume S, Chain F, Rancurel G, Samson Y. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47:1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- 13.Vines BW, Norton AC, Schlaug G. Non-invasive brain stimulation enhances the effects of melodic intonation therapy. Front Psychol. 2011;2:230. doi: 10.3389/fpsyg.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosson B, Fabrizio KS, Singletary F, Cato MA, Wierenga CE, Parkinson RB, Sherod ME, Moore AB, Ciampitti M, Holiway B, Leon S, Rodriguez A, Kendall DL, Levy IF, Rothi LJ. Treatment of naming in nonfluent aphasia through manipulation of intention and attention: a phase 1 comparison of two novel treatments. J Int Neuropsychol Soc. 2007;13:582–594. doi: 10.1017/S1355617707070737. [DOI] [PubMed] [Google Scholar]
- 15.Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, Sherod ME, Wierenga CE, Peck KK, Briggs RW, Rothi LJ, White KD. Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain Lang. 2009;111:73–85. doi: 10.1016/j.bandl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 17.Tryon WW. A simplified time-series analysis for evaluating treatment interventions. J Appl Behav Anal. 1982;15:423–429. doi: 10.1901/jaba.1982.15-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tryon WW. A simplified time-series analysis for evaluating treatment intervention. Erratum. J Appl Behav Anal. 1983;16:250. doi: 10.1901/jaba.1982.15-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher WW, Kelly ME, Lomas JE. Visual aids and structured criteria for improving visual inspection and interpretation of single-case designs. J Appl Behav Anal. 2003;36:387–406. doi: 10.1901/jaba.2003.36-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altmann L, Hazamy AA, Carvajal PH, Benjamin M, Rosenbek J, Crosson B. Delayed stimulus-specific improvements in discourse following anomia treatment using an intentional gesture. Unpublished manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- 22.Arbib M. Broca's area in stem perspective: Language in the context of action-oriented perception. In: Grodzinsky Y, Amunts K, editors. Broca's Region. Oxford University Press; New York: 2006. pp. 153–168. [Google Scholar]
- 23.Menke R, Meinzer M, Kugel H, Deppe M, Baumgartner A, Schiffbauer H, et al. Imaging short- and long-term training success in chronic aphasia. BMC Neurosci. 2009;10:118. doi: 10.1186/1471-2202-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122:1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.