Abstract
Background
Chronic-pancreatitis is a debilitating-disease resulting from many etiologies. The-subset with hereditary/genetic defects (HGP) not only has chronic-pain, but also an increased-risk for pancreatic-cancer. The long-term-outcomes of TP-IAT for chronic pancreatitis due-to-HGP are not clear.
Study Design
Review of a prospectively-maintained-database of 484 TP-IAT-from-1977-2012 at a single-center. The-outcomes (pain-relief, narcotic-use, β cell-function, health-related quality of-life-measures of patients-that-received TP-IAT for hereditary/genetic-defects (PRSS1 (n=38), SPINK1 (n=9), CFTR (n=14) and Familial (n=19) were-evaluated-and-compared to those with non-hereditary/genetic-etiology.
Results
All 80 patients with HGP were narcotic-dependent and failed-endoscopic-management or direct-pancreatic-surgery. Post TP-IAT, 90% of the patients-were-pancreatitis-pain-free with sustained-pain-relief; over 65% had partial or full β-cell-function.-Compared to non-hereditary etiologies, HGP were-younger (22 yrs vs.38 yrs p=<0.001), had-pancreatitis-pain of longer-duration (11.6±1.1 vs. 9.0±0.4 yrs p=0.016), had a higher-pancreas-fibrosis-score (7±0.2 vs. 4.8±0.1 p=<0.001), and-trended-toward-lower-Islet-yield (3,435 ± 361 IEQ vs. 3850± 128 IEQ p=0.28). Using-multivariate-logistic-regression, (1) non-HGP-etiology (p value=0.019) (2) lower severity-of-pancreas-fibrosis (p value < 0.001), (3) shorter-duration-of-years with pancreatitis (p value = 0.008) and (4) higher-transplant IEQ per KG body-weight (p value =<0.001) were-more likely-to-achieve-insulin-independence (p value < 0.001). There was a significant-improvement in HRQoL from-baseline, by SF-36, in physical-and-mental-component HRQoL scores (p <0.001). None-of-the-patients in the entire-cohort-developed-cancer of pancreatic-origin in the liver or elsewhere during 2,936 person-years of follow-up.
Conclusions
TP-IAT in patients with chronic pancreatitis due to HGP etiology provides long-term pain relief (90%) and preservation-of-beta-cell-function. Patients with chronic-painful pancreatitis due to HGP with a high-life-time-risk of pancreatic-cancer should be considered earlier for TP-IAT before pancreatic-inflammation results in higher-degree of pancreatic-fibrosis and islet-cell-function-loss.
Keywords: islet auto transplantation, chronic pancreatitis, hereditary, genetic, total pancreatectomy
Introduction
Chronic Pancreatitis is a disease resulting in debilitating pain, narcotic dependence, diminished quality of life with patients often seeking surgical options for the relief of pain. While there are many recognized anatomic and drug induced causes of pancreatitis, often the etiology is unknown or idiopathic. There is an increasing recognition that a gene predisposition, Hereditary/Genetic pancreatitis (HGP), is associated with chronic pancreatitis. Le Bodic et al. in 1996 described a genetic mutation in a specific gene affecting 47 of 147 members across for 4 generations of a French family from Vendee.1 The first molecular definition of hereditary pancreatitis was a mutation on chromosome 7q35 locus (R122H) of the protease trypsin 1 (PRSS1, cationic trypsinogen) gene.1,2 PRSS1 gene mutations are autosomal dominant with an incomplete penetrance (80%).2,3 Since 1996, more than 30 mutations have been found.2,3 The 3 common mutations are R122H, N291 and A16V.3 Other gene mutations implicated in hereditary pancreatitis include serine protease inhibitor Kazal type 1 (SPINK 1, neither autosomal dominant nor recessive) and cystic fibrosis transmembrane conductance regulator (CFTR, autosomal recessive) gene.4,5,6 Both carry a 1% penetrance. First symptoms in patients with HGP typically begin in childhood, mainly before 10 years of age. Main symptoms are pancreatic pain and acute pancreatitis (>70%).7 The disease progresses with morphological changes of chronic pancreatitis occurring in the pancreas gland by a median age of 22–25 years.7 Exocrine and endocrine pancreatic insufficiency occurs in 34% and 26% at a median age of 29 and 38 years respectively.7 More importantly, a recent large series of patients with hereditary pancreatitis showed that, there is a 44% cumulative risk of pancreatic cancer by 70 years following the onset of symptoms.8 Furthermore, 6% of the patients in EUROPAC study and 3% of the International Hereditary Pancreatitis Study group developed pancreatic adenocarcinoma (50 times higher risk than the general population).3,8 However, the relative role of CFTR/SPINK1 mutations in carcinogenesis is not well-established compared to PRSS1 mutations.9 The former mutations are likely to serve as disease modifying genes although modern series have indirectly expanded the definition of hereditary pancreatitis to include all mutations of PRSS1, SPINK1 and CFTR.9
HGP should be excellent indication for total pancreatectomy, removing all tissue associated with the pain and decreasing risk for the development of adenocarcinoma. Islet auto transplantation before the pancreas is irreparably fibrosed should offer therapeutic benefit with preserved glycemic control and better quality of life after total extirpation of the pancreas. Few reports have focused on outcomes of Total Pancreatectomy and Islet Autotransplant (TP-IAT) in HGP group.10 This study reviews University of Minnesota experience of TP-IAT in patients with HGP related chronic pancreatitis compared to those without a recognized genetic component.
Methods
An institutional database has been maintained for all TP-IATs performed between 1977 and 2012 at the University of Minnesota. This was reviewed and 80 procedures were performed for hereditary/genetic pancreatitis. Patients were categorized as hereditary pancreatitis if there was genetic confirmation (PRSS1, SPINK1, and or CFTR) or met the family history criteria; the criteria were defined as the presence of recurrent acute pancreatitis or chronic pancreatitis in two first-degree relatives or ≥ three second-degree relatives in at least two generations without any other etiology of pancreatitis.9 The criteria for selection of patients offered TP-IAT for CP has evolved over the years, but has been standardized for the last 5 years. Currently, to qualify for TP-IAT, the patient must have had abdominal pain of > 6 months duration with impaired quality of life e.g., inability to work, inability to participate in ordinary activities, repeated hospitalizations, or constant need for narcotics, each coupled with failure to respond to maximal medical treatment or endoscopic pancreatic duct drainage procedures. In addition, there must be objective findings of CP, including at least one of the following: (1) pancreas calcifications on CT scan, or abnormal ERCP, or ≥ 6/9 criteria on endoscopic ultrasound(EUS); or (2) any two of following three: (1) ductal or parenchymal abnormalities on secretin stimulated magnetic resonance cholangiopancreatography (MRCP), EUS of pancreas with 6/9 criteria positive, or abnormal pancreatic function tests with peak bicarbonate < 80 mmol/L).; or (2) Histopathologic confirmed diagnosis of chronic pancreatitis from previous operations; or (3) Hereditary pancreatitis (PRSS1 gene mutation, SPINK1 gene mutation, CFTR gene mutations), with a compatible clinical history ; or (4) History of recurrent acute pancreatitis with > 3 episodes of pain associated with imaging diagnostic of acute pancreatitis and/or elevated serum amylase or lipase 3 times normal.11
The current study was approved by the University of Minnesota Institutional Review Board. Informed consent and assent were obtained from patients participating in quality of life assessments.
Surgical Technique
The main requirement of the surgical technique for patients receiving an Islet auto transplantation is the preservation of blood supply to the pancreas until completion of pancreatic mobilization with the minimization of warm ischemia time to maximize islet cell viability. Our Minnesota Technique is described elsewhere and has evolved over the years.11 For the procedure, either laparoscopic or midline incision is used to mobilize the pancreas. Current technique includes total pancreatectomy (along 2, 3, 4 part of duodenum) with splenectomy and pylorus preservation, duodenojejunostomy to reestablish gastrointestinal continuity and a Roux en-Y hepaticojejunostomy for the biliary reconstruction; Routine use of Gastric and jejunal tubes (either as two or single GJ tube) permits early enteral feeding and prolonged gastric decompression.
Islet Isolation and Infusion
Our islet isolation technique is described elsewhere.11–14 The most critical and immediate isolation challenge is the task of enzyme distention. Variables such as pancreas size, degree of fibrosis, presence of calcifications, and dilated pancreas ducts all influence the quality of digestion. Depending on the extent of ductal alterations, for catheterization of the main pancreatic duct, we use a large-bore, metal catheter for severely dilated ducts. In cases of extreme fibrosis and rock like tissue quality, when automated and manual ductal perfusion may achieve only a partial distention; we used interstitial injection of enzyme with a needle and syringe to further distend unreached areas. After distention, the pancreas is cut into smaller (2–3cm) 20–30 pieces before digestion in the Ricordi chamber to greatly enhance the overall exposure of pancreas to dissociation enzymes.
Liberase-HI dissociation enzyme was used in the isolations until 2007, from which point SERVA premium grade collagenase and neutral protease were used.14 Recently, we found the use of VitaCyte collagenase in combination with SERVA neutral protease (New enzyme mixture) can produce greater islet yields with more consistency than using SERVA enzymes alone.13 Since December 2009 we have utilized this new enzyme combination in specific doses based on the patient age, estimated pancreas weight, and degree of pancreatic fibrosis. Pancreatic fibrosis was rated on a scale from 1 to 10; after digestion, the islets can be purified or partially purified by gradient separation method or can be transplanted as an unpurified preparation. Islet purification is performed using density gradients in the COBE 2991 cell processor. Currently, a post digest tissue volume of > 0.25cm3/Kg is an indication to purify.11 The final islet preparation is suspended in 500 mL of CMRI culture medium (Mediatech, Inc) with human albumin added to a concentration of 2.5%. We have recently added heparin 35units/Kg into the final islet preparation to prevent any clumping of the islets.
Islet cell infusion
To diminish the risk of portal vein thrombosis after islet infusion, heparin is administered prior and during the islet infusion, The islet infusion is given intraportally through the splenic vein stump and changes in portal pressure/flow are recorded using a manometer or Transonic flow probe (Transonic Systems Inc. New York, USA). If the pressure rises to more than 25 cm saline (after waiting for 15 minutes to autoregulate), the flow diminishes to less than 100 ml/min or if a total tissue volume infused exceeds 0.25/kg, the portal infusion is stopped and the rest of the islet preparation is implanted in the peritoneal cavity as a thin-film.
Post-operative management
All patients are started on an insulin drip to maintain blood sugar control between 80 and 120 mg/dL, in the immediate post operative to relieve β-cell functional stress during islet engraftment.15–18 The heparin drip is continued at 10 units per kilogram for seven days. If the Doppler ultrasound revealed any portal vein clots, anti-coagulation with warfarin was continued for three months.
Symptom control of delayed gastric emptying is accomplished by gastric decompression via the gastric port of the gastrojejunal tube (placed in the operating room) and early enteral/jejunal feeding is initiated as soon as the small bowel ileus resolves. Oral diet is started as the delayed gastric emptying resolves and evident by low gastrostomy tube outputs.
Glycemic and diet follow-up
During the first three months post TP-IAT nearly all patients receive exogenous insulin to relieve beta cell functional stress during the engraftment (neovascularization) stage.15–18 Thereafter, insulin is weaned as long as blood glucose levels remain in a near normal target range (fasting < 125 mg/dl post prandial < 180 mg/dl and glycosolated hemoglobin ≤ 6.5 %). If these parameters are not achieved, the patient is maintained on insulin.
Diet is advanced and tube feeds are stopped when the patient was able to take adequate oral calories and protein. Exogenous pancreatic enzyme and fat soluble vitamin (ADEK) modulation is essential as food intake progresses. Patients are seen after surgery in outpatient clinics at three months, six months and one year and thereafter annually. Routine laboratory studies are obtained at these intervals, including fasting glucose and C-peptide, stimulated glucose and C-peptide, and hemoglobin A1C level for assessment of metabolic control and islet graft function
Pain Management
All patients were on narcotics before the operation. Patients are post operatively started on intravenous narcotics, and as they resume gastrointestinal function, oral narcotics are reinstituted with gastrointestinal function returns. Patients are weaned off narcotics in the outpatient clinics over a protracted period (often is excess of a year). Narcotic use and strategy is overseen by the pain management service.
Data Review
484 patients underwent TP-IAT at the University of Minnesota and 80 patients were identified as CP resulting from HCP. The medical records were reviewed and clinical data prospectively stored in the long-term auto islet database and analyzed. We compared narcotic use before and after the procedure, short and long-term success of the islet transplantation, and postoperative quality of life. Narcotic use was determined from the medical records and from self-reported survey data. Patients were classified as either being on narcotics (no matter the dose) or off narcotics, meaning they took no narcotics daily or intermittently. Patients were asked if they had any pain during follow-up and if any component of the pain was similar to what they considered pancreatic pain similar to the time before TP-IAT. For the insulin use, patients were classified as follows: (1) Insulin-independent, (2) Partial graft function (C-peptide positive defined as > 0.6ng/dl or C-peptide unknown, the ability to maintain near normal glucose and glycohemoglobin levels on once daily long acting insulin only or with only occasional supplementation (less than daily) with short acting insulin), or (3) Insulin-dependent (C-peptide < 0.6 ng/ml; or if unknown on both long and short acting insulin [basal-bolus regimen] to maintain glycemic control.11
Statistical Methods
Discrete variables were summarized as counts and percentages; continuous variables were summarized as means and standard error of means. Statistical differences between hereditary/genetic and non-hereditary/genetic groups were tested using the either a Pearson's chi-square or t statistic. Data were summarized for recipient characteristics and prior procedures. Outcomes were evaluated for (1) metabolic end-points, (2) clinical status, (3) pain, and (4) health-related quality of life (HRQoL).
Least squares linear regression was used for identifying factors independently association with islet equivalents per kilogram body weight. Forward and backward stepwise regression were used in creation of a parsimonious, reduced model.
Kaplan-Meier survival analysis was used for testing differences in patient survival between groups. Time-to-event analysis was used to evaluate the days from transplant to insulin independence.
Differences in metabolic end-points, clinical status, pain, and HRQoL between hereditary/genetic and non-hereditary/genetic groups were tested using mixed model methods. For continuous variables, such as metabolic parameters, general mixed models were employed; for categorical outcomes, such as clinical status generalized linear mixed models were used. Each of these models was blocked for hereditary etiology and time as a continuous variable. All analyses were completed using SAS STAT™. Figures were prepared using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com
Results
Patient demographics
80 patients were classified as having hereditary/genetic etiologies for chronic pancreatitis, 61 of these (76.3%) were confirmed through genetic testing. The other 19 had a strong family history of CP and antedated the ability to perform genetic testing. There were 404 TP-IAT patients with non-HGP etiologies. Table 1 shows the characteristics of TP-IAT patients by etiology. HGP appears as a diagnosis in more recent times (p value = 0.028), younger (p value < 0.001) and predominantly female (p value = 0.002). Hereditary/genetic TPIATs had statistically lower BMI (p value = 0.009), greater years with pancreatitis (p value < 0.001), and more years with pain prior to TPIAT (p value = 0.016). Table 2 gives the previous surgical interventions. The groups were similar in terms of previous surgical interventions except for a statistically higher prevalence of Puestow (p value = 0.001) and lower likelihood of stenting (p value = 0.009) in the HGP etiology group.
Table 1.
Characteristics of Total Pancreatectomy Islet Autogeneic Transplants (TPIAT) by Hereditary/Genetic and Non-Hereditary/Genetic Etiology.
| Hereditary/Genetic | Non-Hereditary | p value | |||||
|---|---|---|---|---|---|---|---|
| Number of Primary TPIAT | 80 | 404 | |||||
| Transplant Era | 0.028 | ||||||
| Before 1996 | 2 | 2.5% | 47 | 11.6% | |||
| 1996 to 2005 | 12 | 15.0% | 72 | 17.8% | |||
| 2006 to 2012 | 66 | 82.5% | 285 | 70.5% | |||
| Age (years)* | 21.9 ± 1.3 | 37.9 ± 0.6 | < 0.001 | ||||
| Female Gender | 47 | 58.8% | 307 | 76.0% | 0.002 | ||
| Etiology for Chronic Pancreatitis | |||||||
| Hereditary/Genetic | N.A. | ||||||
| PRSS1 | 38 | 47.5% | |||||
| SPINK1 | 9 | 11.3% | |||||
| CFTR | 14 | 17.5% | |||||
| Familial | 19 | 23.8% | |||||
| Alcohol | 34 | 8.4% | |||||
| Idiopathic | 266 | 65.8% | |||||
| Pancreas Divisum | 56 | 13.9% | |||||
| Other | 48 | 11.9% | |||||
| Body Mass Index* | 22.9 ± 0.7 | 24.8 ± 0.3 | 0.009 | ||||
| Years with Pancreatitis* | 10.1 ± 1.0 | 6.4 ± 0.3 | < 0.001 | ||||
| Years with Pain* | 11.6 ± 1.1 | 9.0 ± 0.4 | 0.016 | ||||
| Years Narcotic Use* | 2.6 ± 0.6 | 3.2 ± 0.2 | 0.446 |
Values are the mean ± SEM
Table 2.
Surgical Characteristics and Background for Total Pancreatectomy Islet Autogeneic Transplants (TPIAT) by Hereditary/Hereditary and Non-Hereditary/Genetic Etiology.
| Genetic / Hereditary | Non-Hereditary | p value | |||
|---|---|---|---|---|---|
| Number of Primary TPIAT | 80 | 404 | |||
| Total IEQ per KG BW* | 3,435 ± 361 | 3850 ± 128 | 0.281 | ||
| Total Pancreatectomy | 70 | 87.5% | 337 | 83.4% | 0.362 |
| Pancreas Fibrosis (0 to 10)* | 7.0 ± 0.2 | 4.8 ± 0.1 | < 0.001 | ||
| Tissue Volume (ml)* | 9.4 ± 2.4 | 15.4 ± 1.1 | 0.021 |
Values are the mean ± SEM
While nearly a one-quarter of the TP-IATs did not have confirmation through genetic testing, a separate sensitivity analysis supported the similarity of the 2 sub-groups. The adoption of the genetic testing became routine after 2005. Differences between the HGP group that were genetically tested and those not genetically tested tend to reflect variations in patient selection and management prior to surgery. Genetically confirmed patients were more likely in the more recent era (p value < 0.001), had more severe pancreas fibrosis (p value = 0.003), less likely to have direct pancreas surgery before TP-IAT (p value = 0.005), and more likely to have been stented (p value = 0.049) prior to surgery.
Surgical Outcomes
HGP pancreases were generally small (<60g) and fibrotic. Table 3 gives the surgical characteristics for the removed pancreas. TP-IATs from HGP etiology had statistically increased pancreas fibrosis (p value < 0.001) and lower tissue volumes infused at the time of transplant (p value = 0.021). Islet equivalents (IEQ) per kilogram body weight were similar across the groups.
Table 3.
Previous Interventions by Hereditary/Genetic Etiology Group.
| Genetic / Hereditary | Non-Hereditary | p value | |||
|---|---|---|---|---|---|
| Surgical Procedures | |||||
| Puestow | 17 | 21% | 30 | 7% | 0.001 |
| Whipple | 2 | 3% | 26 | 6% | 0.168 |
| Beger/Frey | 2 | 3% | 7 | 2% | 0.643 |
| Distal Pancreatectomy | 5 | 6% | 22 | 5% | 0.775 |
| Sphincteroplasty | 3 | 4% | 22 | 5% | 0.358 |
| Endoscopic Procedures | |||||
| Sphincterotomy | 30 | 38% | 126 | 31% | 0.270 |
| ERCP | 63 | 79% | 345 | 85% | 0.136 |
| Stent | 41 | 51% | 269 | 67% | 0.009 |
| Endoscopic Duct Drainage | 50 | 63% | 285 | 71% | 0.154 |
| Other | |||||
| Celiac Block | 11 | 14% | 90 | 22% | 0.086 |
With multivariate statistical adjustment, there was no association between HGP etiology and IEQ adjusting for pancreas fibrosis, transplant era, years with pancreatitis, and tissue volume. In fact, the major drivers for islet yield in order of importance were: (1) the severity of pancreas fibrosis (p value < 0.001), (2) transplant era (p value = 0.010), and (3) years with pancreatitis (p value = 0.008). Tissue volume and HGP etiology were not independently related to islet yield. Prior direct pancreas surgery was associated with a lower islet yield (Table 4)
Table 4.
Association between Prior Surgical Procedures, IEQ and Insulin Independence
| Hereditary/Genetic Group Only | ||||||
|---|---|---|---|---|---|---|
| IEQ | P Value | Ever Insulin Independent | P Value | |||
| Puestow | Yes | 1075±272 | <0.001 | Yes | 0.00% | <0.001 |
| No | 4043±415 | No | 25.40% | |||
| Whipple | Yes | 2212±1407 | 0.586 | Yes | 50.00% | 0.289 |
| No | 3466±369 | No | 19.20% | |||
| Beger/Frey | Yes | 1534±726 | 0.397 | Yes | 0.00% | <0.001 |
| No | 3485±369 | No | 20.50% | |||
Patient Survival
Using a Kaplan-Meier method, patient survival at 10-years for the entire cohort of 484 TPIATs was 84.01%. In comparing patient survival by HGP etiology, the follow-up interval was truncated to 5-years. With stratification by HGP etiology, we found no statistical differences in 5-year patient survival (hereditary/genetic – 90.27% versus non- hereditary/genetic – 89.72%; p values for Log-Rank/Wilcoxon – 0.166/0.116). There were 4 late deaths in the HGP group. One patient died of sepsis 1.8 years post TP-IAT, one due to narcotic over dose at 4.2 years post TP-IAT, one due to non-pancreas cancer- at 5.2 years post TP-IAT and one unknown >10 years post TP-IAT.
Pain after TP-IAT
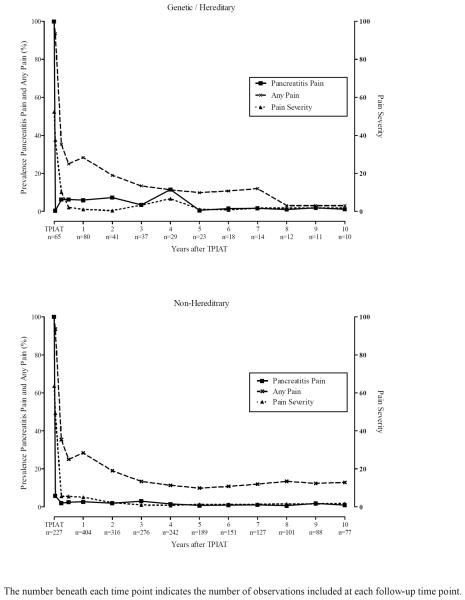
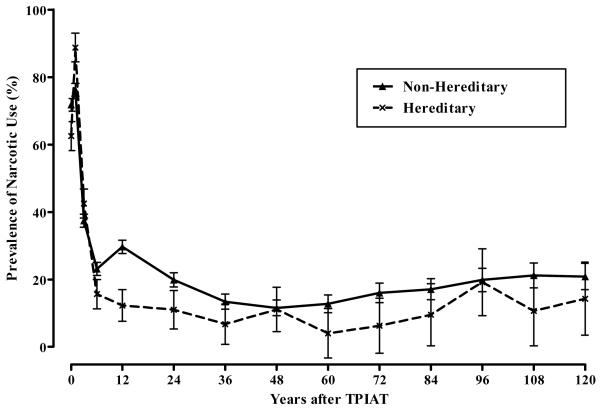
Based on generalized linear mixed model, pancreatitis pain and the prevalence of any pain were statistically improved following TP-IAT (p values < 0.001). We found no statistical differences in these outcomes between HGP and non-hereditary/genetic groups for pancreatitis pain (p value 0.227), the prevalence of any pain (p value = 0.355). Statistical tests for differences in the severity of pain used the general linear mixed model. In this analysis pain severity was statistically lower pretransplant in the HGP group (p value < 0.001) and tended to remain lower throughout the 10-years of follow-up. Overall, there was no statistical difference between the groups in pain severity. Figure 1 shows the aggregate trajectory of change for the 3 pain outcomes. Prevalence of narcotic use was tracked for HGP etiologies through 10 years after TP-IAT. Overall, the prevalence of narcotic use reduced from 70 percent pretransplant (note this is based on available data – it is likely 100%) to less than 1 in 5 at 10 years. This was a statistically significant decline based on the generalized linear mixed model (p value < 0.001). Figure 2 gives the prevalence of narcotic use and corresponding standard errors of the mean trajectory for HGP and non-hereditary etiologies. While there were no statistical differences between TP-IATs with hereditary etiology and non-hereditary etiologies (p value = 0.110), the trajectory of decline in reported narcotic use was statistically greater for the TPIATs with hereditary etiologies compared with non-hereditary etiologies (p value < 001).
Figure 1.
Prevalence of Pancreatitis Pain, Any Pain and the Severity of Pain by Hereditary/Genetic Group and Time after TPIAT.
Figure 2.
Prevalence of Narcotic Use by Hereditary/Genetic Group and Time after TPIAT.
Glycemic Control, Insulin Independence and Clinical Status
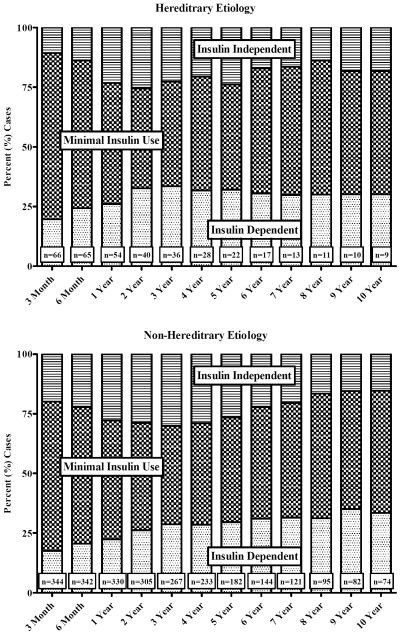
Figure 3 gives the clinical status for TP-IATs for HGP and non-hereditary etiologies by years of follow-up. Since patients did not have follow-up at each interval after TP-IAT, clinical status was ascertained using the last clinical value carried forward (LVCF). We found no statistical differences in the clinical status by etiology (p value = 0.549) or the time difference of differences (i.e., the differences in the rate of change between the two groups) by etiology for the 10-years of follow up (p value = 0.784). One-quarter of the TP-IATs were insulin dependent after TP-IAT but the greater majority of transplants had partial islet function.
Figure 3.
Clinical Status for Hereditary/Genetic and Non-Hereditary/Genetic Groups by Years of TPIAT Follow-up.
Sixteen of the 80 TP-IATs with HGP etiology (20.0%) attained complete insulin independence at some time after islet infusion. This compares with 133 of the 404 (32.9%) having a non-hereditary etiology. Since the unadjusted difference in cumulative incidence between the etiological groups was statistically significant (p value = 0.022), we examined the difference in the likelihood of ever achieving complete insulin independence using multivariate logistic regression. Forward and backward step-wise regression was used to create a parsimonious model that included: (1) HGP etiology, (2) age, (3) severity of pancreas fibrosis, (4) body mass index of the recipient at the time of transplant, and (5) IEQ per KG body weight. Table 5 summarizes the multivariate logistic regression model. With adjustment, HGP etiology was associated with a statistically reduced likelihood of insulin independence compared with non-hereditary etiology (Odds Ratio [OR] = 0.33; 95% confidence limits 0.1, 0.84; p value = 0.019). Independent risk factors for insulin independence included: (1) recipient age, (2) severity of pancreas fibrosis, (3) recipient body mass index, and (4) transplant IEQ per KG body weight. The multivariate model provided a high level of discrimination between those attaining insulin independence and those never attaining insulin independence (AUC = 80.8; 95% CL 76.0, 85.5). The number of islet equivalents was the strongest independent predictor for insulin independence with a 1,000 IEQ per body increasing the odds of insulin independence by 39% (OR = 1.39; 95% CL 1.22, 1.58; p value < 0.001). Increased severity of pancreas fibrosis (OR = 0.84; 95% CL 0.76, 0.94; p value = 0.003), advancing age (OR = 0.97; 95% CL 0.95, 0.99; p value = 0.003), and recipient body mass index at the time transplant independently decreased the likelihood of insulin independence (OR = 0.93; 95% CL 0.88, 0.98; p value = 0.005) independently decreased the likelihood of insulin independence.
Table 5.
Ever Insulin Independent Multivariate Adjusted Odds Ratios, 95% Confidence Intervals and p Values Based on the Multivariate Logistic Regression Model.
| 95% Confidence Limits | ||||
|---|---|---|---|---|
| Odds Ratio | LCL | UCL | p value | |
| Hereditary/Genetic Group | 0.33 | 0.13 | 0.84 | 0.019 |
| Age (Years) | 0.97 | 0.95 | 0.99 | 0.003 |
| Pancreas Fibrosis Severity (1 Unit on 0 to 10 Scale) | 0.84 | 0.76 | 0.94 | 0.003 |
| Body Mass Index (Weight KG/Height in Meters2) | 0.93 | 0.88 | 0.98 | 0.005 |
| Transplant IEQ per KD Body Weight (1,000 IEQ) | 1.39 | 1.22 | 1.58 | < 0.001 |
Metabolic measures
Three metabolic outcomes were looked in this analysis: (1) positive c-peptide, (2) fasting glucose, and (3) hemoglobin A1C. The unadjusted results from this analysis suggest that TPIAT recipients with HGP etiologies do slightly worse than those with non-hereditary/genetic etiologies. In this analysis, a stimulated c-peptide level ≥ 0.6 ng/ml was a considered positive c-peptide. For this analysis, positive c-peptide levels were evaluated pretransplant and at 3-months, 6-months and 12-months after TP-IAT. These time points represent most of the available data. Figure 4 shows the prevalence of c-peptide positives by hereditary/genetic etiology. Based on a generalized linear mixed model, there was a statistical decline in the prevalence of positive c-peptides with time (p value < 0.001). This occurred for the HGP and non-hereditary/genetic etiology groups. However, the prevalence of c-peptide positives was approximately 14% lower in the hereditary/genetic group than in the non-hereditary/genetic group (p value = 0.005) with the rate of decline in c-peptide positives statistically greater in the hereditary/genetic group than in the non-hereditary/genetic group (p value = 0.010).
Figure 4.
Prevalence of Positive C-Peptide (%) in the First Year after TPIAT by Hereditary/Genetic Group.
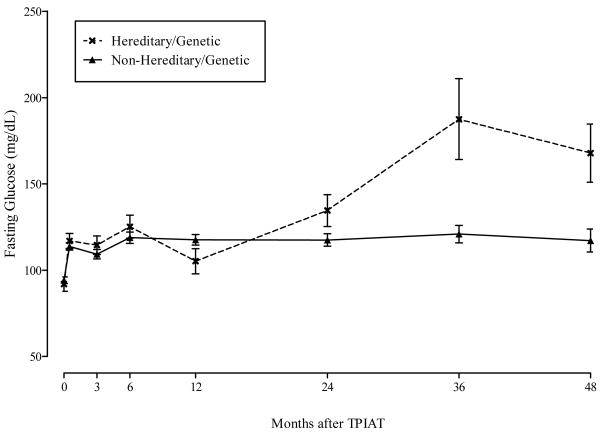
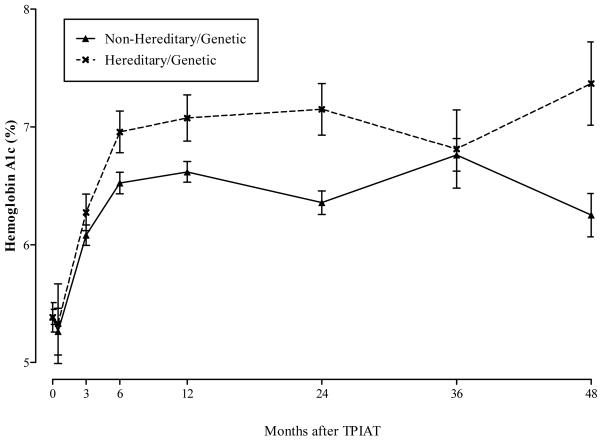
Figure 5 shows the adjusted mean fasting glucose levels in mg/dL by time after TP-IAT and hereditary/genetic group. The general mixed model results showed that fasting glucose levels were statistically higher in the HGP group after TP-IAT compared with the non-hereditary/genetic group (p value < 0.001). The trajectory of increase in fasting glucose was statistically greater for those with a HGP etiology than those with a non-hereditary/genetic etiology (p value < 0.001). Figure 6 shows similar pattern for hemoglobin A1C levels. For this metabolic measure, there was a statistical difference between hereditary/genetic and non-hereditary/genetic group (p value = 0.004) with the former group showing a poorer trajectory of change (p value = 0.014).
Figure 5.
Fasting Glucose (mg/dL) by Time after TPIAT and Hereditary/Genetic Group.
Figure 6.
Hemoglobin A1C (%) by Time after TPIAT and Hereditary/Genetic Group.
The multivariate analysis was limited to fasting glucose, since it was the most complete of the metabolic measures. For this analysis, multivariate adjustments were made for transplant era, IEQ per KG body weight, body mass index at the time of transplant, and pancreas fibrosis. With adjustment, fasting glucose remained statistically higher for the HGP group versus the non-hereditary group (p value < 0.001). In addition, the rate of increase in fasting glucose was faster for the hereditary group versus the non-hereditary group (p value 0.001).
Health-Related Quality of Life
During the past 5 years, health-related quality of life (HRQoL) was assessed using the RAND version of the 36-item short-form health survey (SF-36). For this analysis, the trajectory of change in health status was evaluated and the relationship between self-reported health and clinical status was explored. For this analysis, HRQoL pretransplant and at 3-months, 6-months, 12-months and 24-months after TP-IAT compared patients with HGP and non-hereditary/genetic etiologies.
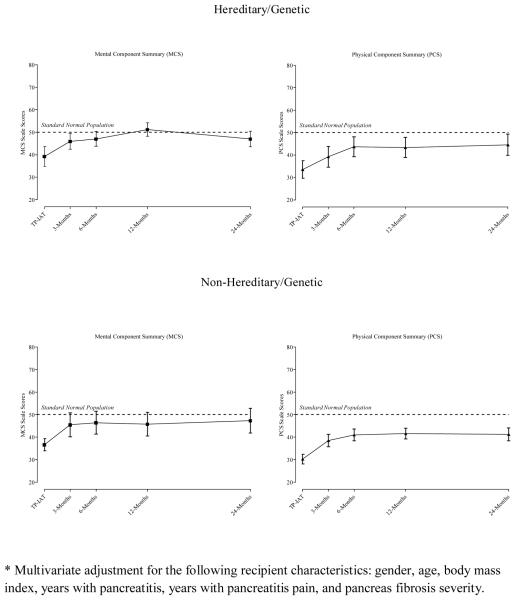
In evaluating HRQoL between the groups, adjustments were made for confounding factors. For this analysis, the Mental Component Summary (MCS) and Physical Component Summary (PCS) scale scores were adjusted for: gender of the recipient, age, body mass index at the time of transplant, the number of years with pancreatitis, the number of years with pain, and pancreas fibrosis severity. These latter factors were found associated with the health status outcomes and were baseline characteristics that distinguished HGP and non-hereditary/genetic groups. Blocking on HGP etiology and time of follow-up, an adjusted general linear mixed model was created. Figure 7 shows the trajectory of health status change for the adjusted MCS and PCS scale scores at each follow-up time point.
Figure 7.
Multivariate Adjusted Mental Component Summary (MCS) and Physical Component Summary (PCS) Scale Scores for Hereditary/Genetic and Non-Hereditary/Genetic Groups by Time of Follow-up.
The MCS scale scores statistically improved over time for both the HGP and the non-hereditary/genetic groups (p value < 0.001). There were no statistical differences between the groups (p value = 0.482) and no statistical differences in the rate of change in MCS scale scores between the groups (p value= 0.428). None of the risk factors were independently related to MCS.
The results were similar for the PCS scale scores. While physical health as measured by the PCS improved with time (p value < 0.001), there were no statistical differences between the etiological groups (p value = 0.287) and the rate of change between the groups (p value = 0.861). The principal drivers for improved physical health was the younger age (p value = 0.029) and fewer years with pancreatitis (p value = 0.389).
In further analysis, the relationship between the use of insulin and HRQoL as measured by the MCS and PCS. For this analysis, the recipient's clinical status was matched to their HRQoL for the time period during the first year following TP-IAT. For this analysis, 120 patients had corresponding a corresponding clinical and a completed SF-36 at 6 months or 12 months follow-up. Since the patient's self-reported health is heavily influenced by extraneous factors, adjustments were made for self-reported pain at that point in time, their gender and age. A multivariate mixed model was used to evaluate the association between insulin dependence and insulin independent defined as either insulin-free or partial islet function requiring some insulin. The results from this analysis are shown in Figure 8.
Figure 8.
Multivariate Adjusted* Mental Component Summary (MCS) and Physical Component Summary (PCS) Scale Scores and 95% Confidence Limits by Hereditary/Genetic Group and Time after TPIAT.
MCS as a measure of overall emotional health was statistically related to insulin independence (p value = 0.019). Overall, those patients with partial or full insulin independence were 4.23 points higher than for those, who were insulin dependent. While this was an independent factor predicting mental health score, self-reported pain at the time of the clinical assessment was by far and away the strongest driver for emotional health (p value < 0.001). As a global measure of physical health, PCS scale scores were unrelated to insulin status (p value = 0.988). Physical health tended to decline with time (p value = 0.031) and the severity of self-reported pain (p value < 0.001). In the aggregate, physical health declined more rapidly for those, who were insulin dependent compared with those less dependent on insulin (p value = 0.039). Among those that were insulin dependent, the PCS scale scores dropped by an average of 7.07 points. This is a marked decline considering that the MCS and PCS are standard normalized to have a standard deviation of 10 points.
Discussion
Hereditary/Genetic pancreatitis is increasingly being recognized as a cause of chronic pancreatitis. The Pancreatitis can be progressive, with recurrent hospitalizations, increasing pain and narcotic dependence and impaired quality of life. In these cases, progression to exocrine and endocrine insufficiency is common; in addition, lifetime risk of pancreatic adenocarcinoma is elevated.3,19 Initial treatment directed at relieving pain and restoring quality of life include: narcotic analgesics, pancreatic enzymes to reduce pancreatic stimulation and treat pancreatic exocrine insufficiency, nerve block procedures such as celiac plexus blocks, and endoscopic decompression by pancreatic sphincterotomy, stone extraction, stricture dilation and stent placement.20–22 Those who fail these medical and endoscopic interventions may be candidates for surgical intervention. A number of surgical techniques have been used in attempt to ameliorate pain and restore quality of life, including partial resection, or pancreatic duct drainage procedures such as lateral pancreaticojejunostomy (such as Puestow), or variants (such as Frey, Beger procedures).23,24 Patients often have transient pain relief, but due to the genetic defect there is diffuse involvement of the entire pancreas and pain eventually recurs in up to 50% of patients; 25–30 exocrine and endocrine insufficiency often develops over time.7,31 Total pancreatectomy (TP) eliminates the source of the pain, and potentially eliminates risk of pancreatic cancer in this HGP group patients and IAT prevents or minimizes TP- related diabetes. Based on this natural history, it has been our practice at the University of Minnesota to offer TP-IAT rather than partial resection or duct drainage procedures in this group of patients, if they meet our criteria for TP-IAT.
The primary goal of TP-IAT is the relief of pain and improved quality of life.11 This study demonstrated that the cohort with HGP had significant improvement in pain following TP IAT (p=<0.001) and that it is sustained through the 10 year follow-up. Narcotic use was significantly lower after TP-IAT in HGP group even compared the non HGP group.
The addition of Islet auto transplant to TP is to minimize the deleterious effect of brittle diabetes after pancreatic extirpation. With regards to β cell function, despite the islet yield being statistically not different between the HGP and nonhereditary group; the insulin independence rate was 20% in the HGP group. More importantly >65% of the entire cohort demonstrated partial or complete preservation of β cell function. The addition of IAT does prevent brittle diabetes in most patients.
In our study, the higher degree of pancreatic fibrosis negatively impacted the insulin independence. On further analysis, when the hereditary/genetic etiology group was separated by gene type (when it could be determined) into the following 3 groups: (1) PRSS1, (2) SPINK1, and (3) CFTR; The results from this analysis suggest an association between years with pancreatitis, pancreas fibrosis, and IEQ per KG of body weight that depends on the specific gene type in the hereditary/genetic group. In the PRSS1 group, the years with pancreatitis were independently associated with IEQs. In chronic pancreatitis patient with the PRSS1 gene, each year of pancreatitis was associated with a reduction of 163 islet cells (p value = 0.013). For CFTR gene type, the increased severity of pancreas fibrosis is strongly associated with a decline in islet yield. For this group, each unit increase in pancreas fibrosis (on a 0 to 10 scale) was associated with a decline in 1775 islet cells (p value = 0.003). The length of pancreatitis and the severity of pancreas fibrosis were unrelated to IEQ in the SPINK1. Therefore, in HGP gene types PRSS1 and CFTR, the longer duration of pancreatitis and inflammation result in a higher degree of pancreatic fibrosis and islet function loss. These data support the progressive nature of HGP group. The increased fibrosis by the time patients receive TP-IAT in our present cohort translated into lower islet yield and lower percent of patients becoming insulin independent (20% vs. 32.9% p= 0.022). Besides the timing of the TP-IAT, previous surgical procedures were an important consideration as well. Previous surgery, particularly prior Puestow operation resulted in a statistically lower islet yield and increased the risk of insulin dependence in long-term follow-up
TP-IAT is not a cure for the patient, but it does improve their quality of life. We have reported earlier that HRQoL improves after TP-IAT.11 These results from patients with HGP etiology further support our earlier findings. The pronounced relationship between partial or full insulin independence and the improved HRQoL clearly suggests that achieving partial or full insulin independence in this cohort of HGP patients is important for improving overall quality of life after TP-IAT.
Another consideration in patients with HGP is the future risk of pancreatic cancer. The risk of developing pancreatic cancer can be as great as 44% in patients with the PRSS1.8 The risk is even magnified when the abnormal gene is inherited from the paternal side and if the patient is a smoker.3,32 There is a theoretical risk that patients after TP-IAT could develop pancreatic cancer in the liver. In our entire cohort of 484 patients including 61 patients with known PRSS1 mutation and 2,936 person years of follow up, we have not seen any cancer in the liver. These findings support a rationale for not offering subtotal resections and drainage procedures for patients with known PRSS1 mutations.
As genetic testing has not been available during the time of these procedures, our entire cohort did not undergo genetic testing; Although we instituted genetic testing for patients diagnosed with chronic pancreatitis at a younger age (<25 years) since 2005, we were obtaining family history of pancreatitis since inception of the program. Among 80 patients grouped under hereditary pancreatitis, 61 had identifiable genetic mutations and 19 met the criteria for familial pancreatitis. A separate sensitivity analysis supported the similarity between those with identified genes and those with a family history.
In conclusion, we report the largest series of TP-IAT performed for patients with hereditary/genetic etiology. Hereditary/genetic TP-IATs had significantly more years with pancreatitis and pain prior to TP-IAT and had a higher degree of pancreatic fibrosis compared to patients with non-hereditary etiology. They had significantly improved pain after TP-IAT and the pain relief was sustained at 10 years of follow up. Over 65% of patients had meaningful preservation of β cell function (including 20% insulin independent). There was no incidence of pancreatic cancer in the entire cohort at 2,936 person years of follow up. Our results support offering TP-IAT earlier to patients with chronic pancreatitis from hereditary/genetic etiologies that fail medical and/or endoscopic procedures.
Acknowledgments
The authors would like to thank Louise Berry R.N., B.S., C.C.T.C. and Marie Cook R.N., C.N.P., M.P.H., C.C.T.C. for their superb care of the patients in these studies.
The authors also express appreciation to Ms. Katherine Foster for preparing the manuscript and figures for publication.
Abbreviations
- TP
Total Pancreatectomy
- IAT
Islet autotransplant
- CP
Chronic Pancreatitis
- HGP
Hereditary/Genetic Pancreatitis
- HRQoL
Health-related quality-of-life
- SF-36 RAND
36-item short form health survey
- MCS
Mental Component Summary
- PCS
Physical Component Summary
- IEQ
islet cell equivalent
- PRSS1
protease trypsin 1
- SPINK1
serine protease inhibitor Kazal type 1
- CFTR
cystic fibrosis transmembrane conductance regulator
Footnotes
Presented at the Southern Surgical Association, 125th Annual Meeting, Hot Springs, Virginia, 2013.
References
- 1.Le Bodic L, Bignon JD, Raguenes O, et al. The hereditary to long arm of chromosome 7. Hum Mol Genet. 1996;5:549–54. doi: 10.1093/hmg/5.4.549. [DOI] [PubMed] [Google Scholar]
- 2.Whitcomb DC, Preston RA, Aston CE, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996;110:1975–80. doi: 10.1053/gast.1996.v110.pm8964426. [DOI] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P, DiMagno EP. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group J Natl Cancer Inst. 1997;89:442–46. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 4.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–16. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 5.Pfutzer RH, Barmada MM, Brunskill AP, et al. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterol. 2000;119:615–23. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 6.Sharer N, Schwarz M, Malone G, et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–58. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- 7.Rebours V, Levy P. Ruszniewksi P An overview of hereditary pancreatitis Dig Liv Dis. 2012;44:8–15. doi: 10.1016/j.dld.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Howes N, Lerch MM, Greenhalf W, et al. European Registry of Hereditary Pancreatitis and Pancreatic Cancer (EUROPAC) clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252–61. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 9.Ceppa EP, Pitt HA, Hunter JL, et al. Hereditary Pancreatitis:Endoscopic and Surgical Management. J Gastrointest Surg. 2013;17:847–857. doi: 10.1007/s11605-013-2167-8. [DOI] [PubMed] [Google Scholar]
- 10.Sutton MJ, Schmulewitz N, Sussman JJ, et al. Total Pancreatectomy and islet cell auto transplantation as a means of treating patients with genetically linked pancreatitis. Surgery. 2010;148:676–86. doi: 10.1016/j.surg.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409–24. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balamurugan AN, Chang Y, Bertera S. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia. 2006;49:1845–54. doi: 10.1007/s00125-006-0318-0. [DOI] [PubMed] [Google Scholar]
- 13.Balamurugan AN, Loganathan G, Bellin MD, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93:693–702. doi: 10.1097/TP.0b013e318247281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anazawa T, Balamurugan AN, Bellin M, et al. Human islet isolation for autologous transplantation: comparison of yield and function using SERVA/Nordmark versus Roche enzymes. Am J Transplant. 2009;10:2383–91. doi: 10.1111/j.1600-6143.2009.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finzi G, Davalli A, Placidi C, Usellini L, La Rosa S, Folli F, Capella C. Morphological and ultrastructural features of human islet grafts performed in diabetic nude mice. Ultrastruct Pathol. 2005;29:525–33. doi: 10.1080/01913120500323563. [DOI] [PubMed] [Google Scholar]
- 16.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 17.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate post transplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–7. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 18.Juang JH, Bonner-Weir S, Wu YJ, Weir GC. Beneficial influence of glycemic control upon the growth and function of transplanted islets. Diabetes. 1994;43:1334–9. doi: 10.2337/diab.43.11.1334. [DOI] [PubMed] [Google Scholar]
- 19.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clinical Gastroenterol Hepatol. 2004;2(2):252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 20.Steer ML, Waxman I, Freedman S. Chronic Pancreatitis N Engl J Med. 1995;332:1482–1490. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 21.Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge Swiss Med Wkly. 2006;136:166–174. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- 22.Choudari CP, Nickl NJ, Fogel E, et al. Hereditary Pancreatitis: Clinical presentation, ERCP findings, and outcome of endoscopic therapy. Gastrointest Endosc. 2002;56:66–71. doi: 10.1067/mge.2002.125103. [DOI] [PubMed] [Google Scholar]
- 23.Clifton MS, Pelayo JC, Cortes RA, Grethel EJ, Wagner AJ, Lee H, Harrison MR, Farmer DL, Nobuhara KK. Surgical treatment of childhood recurrent pancreatitis. J Pediatr Surg. 2007;42:1203–1207. doi: 10.1016/j.jpedsurg.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal CW, Moir CR, Ishitani MB. Management of chronic pancreatitis in the pediatric patient: Endoscopic retrograde cholangiopancreatography vs operative therapy. J Pediatr Surg. 2009;44:139–143. doi: 10.1016/j.jpedsurg.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Gachago C. Draganov P Pain management in chronic pancreatitis World J Gastroenterol. 2008;14:3137–3148. doi: 10.3748/wjg.14.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmberg JT, Isaksson G, Ihse I. Long term results of pancreaticojejunostomy in chronic pancreatitis. Surg Gynecol Obstet. 1985;160:339–346. [PubMed] [Google Scholar]
- 27.Bradley EL., 3rd Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153:207–213. doi: 10.1016/0002-9610(87)90816-6. [DOI] [PubMed] [Google Scholar]
- 28.Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg. 1994;129:374–379. doi: 10.1001/archsurg.1994.01420280044006. discussion 379–380. [DOI] [PubMed] [Google Scholar]
- 29.O'Neil SJ, Aranha GV. Lateral pancreaticojejunostomy for chronic pancreatitis. World J Surg. 2003;27:1196–1202. doi: 10.1007/s00268-003-7238-7. [DOI] [PubMed] [Google Scholar]
- 30.Cahen DL, Gouma DH, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676–684. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 31.Sasikala M, Talukdar R, Pavan kumar P, et al. β-Cell dysfunction in chronic pancreatitis. Dig Dis Sci. 2012 Jul;57(7):1764–72. 2. doi: 10.1007/s10620-012-2086-7. [DOI] [PubMed] [Google Scholar]
- 32.Teich N, Mossner J. Genetic aspects of chronic pancreatitis. Med Sci Monit. 2004;10:325–8. [PubMed] [Google Scholar]