Abstract
Decreased cardiac contractility is a central feature of systolic heart failure. Existing drugs increase cardiac contractility indirectly through signaling cascades but are limited by their mechanism-related adverse effects. To avoid these limitations, we previously developed omecamtiv mecarbil, a small-molecule, direct activator of cardiac myosin. Here, we show it binds to the myosin catalytic domain and operates by an allosteric mechanism to increase the transition rate of myosin into the strongly actin-bound force-generating state. Paradoxically, it inhibits adenosine 5′-triphosphate (ATP) turnover in the absence of actin, which suggests that it stabilizes an actin-bound conformation of myosin. In animal models, omecamtiv mecarbil increases cardiac function by increasing the duration of ejection without changing the rates of contraction. Cardiac myosin activation may provide a new therapeutic approach for systolic heart failure.
Heart failure is a common human disease; after 40 years of age, the lifetime risk of developing heart failure is 20% for both women and men. Despite advances in medical therapy between 1996 and 2006, hospitalizations for heart failure rose by 25% to over 1.1 million (1). Once hospitalized for heart failure, mortality rates at 30 days, 1 year, and 5 years were as high as 10%, 22%, and 42% in patients from four United States communities (2). In its most common manifestation, heart failure is marked by a decrease in cardiac contractility and called systolic heart failure (3). To preserve cardiac output, the body increases sympathetic tone and activates neurohormonal pathways. These compensatory mechanisms can, however, accelerate the decline of cardiac systolic function (4).
Current heart failure therapies rely on two different strategies. The most successful is to block neurohormonal activation with inhibitors of the renin-angiotensin pathway, β-adrenergic blockers, and/or aldosterone receptor blockers (4). The second approach is to increase cardiac contractility, which is accomplished indirectly by activating second-messenger signaling pathways that increase cardiac myocyte intracellular calcium concentration (e.g., β-adrenergic receptor agonists or phosphodiesterase inhibitors). Unfortunately, these drugs also increase heart rate and myocardial oxygen consumption and can produce clinically significant arrhythmias and hypotension, which contributes to higher mortality (5).
We hypothesized that directly activating the contractility of the cardiac sarcomere would improve cardiac performance while avoiding the adverse effects of indirect mechanisms. The sarcomere is made up of interdigitating thin and thick filaments. Myosin, the main component of the thick filament, uses chemical energy derived from ATP hydrolysis to produce force for contraction. Myosin motors act upon thin filaments composed of actin and the troponin-tropomyosin regulatory complex. In resting muscle, the free calcium concentration is low, and the regulatory proteins prevent myosin from interacting with actin. During each heart beat, calcium is released transiently from the sarcoplasmic reticulum into the cytoplasm, where it binds to troponin and allows myosin to interact with actin filaments and to produce contraction. The muscle relaxes as calcium is removed from the cytoplasm (6).
Conceptually, the direct activation of the cardiac sarcomere can be achieved by either sensitizing the regulatory proteins to calcium or activating cardiac myosin directly. A high-throughput screen of the cardiac myosin adenosine triphosphatase (ATPase) identified a small-molecule activator of cardiac myosin that was further optimized for potency, physical properties, and pharmacokinetics, which culminated in the synthesis of omecamtiv mecarbil (molecular weight 401.43, formerly CK-1827452) (7) (Fig. 1A). When tested in a dog model of heart failure, omecamtiv mecarbil improved cardiac function in the absence of changes in myocardial oxygen consumption (8). We sought to understand the mechanistic basis for this effect on cardiac contractility.
Fig. 1.
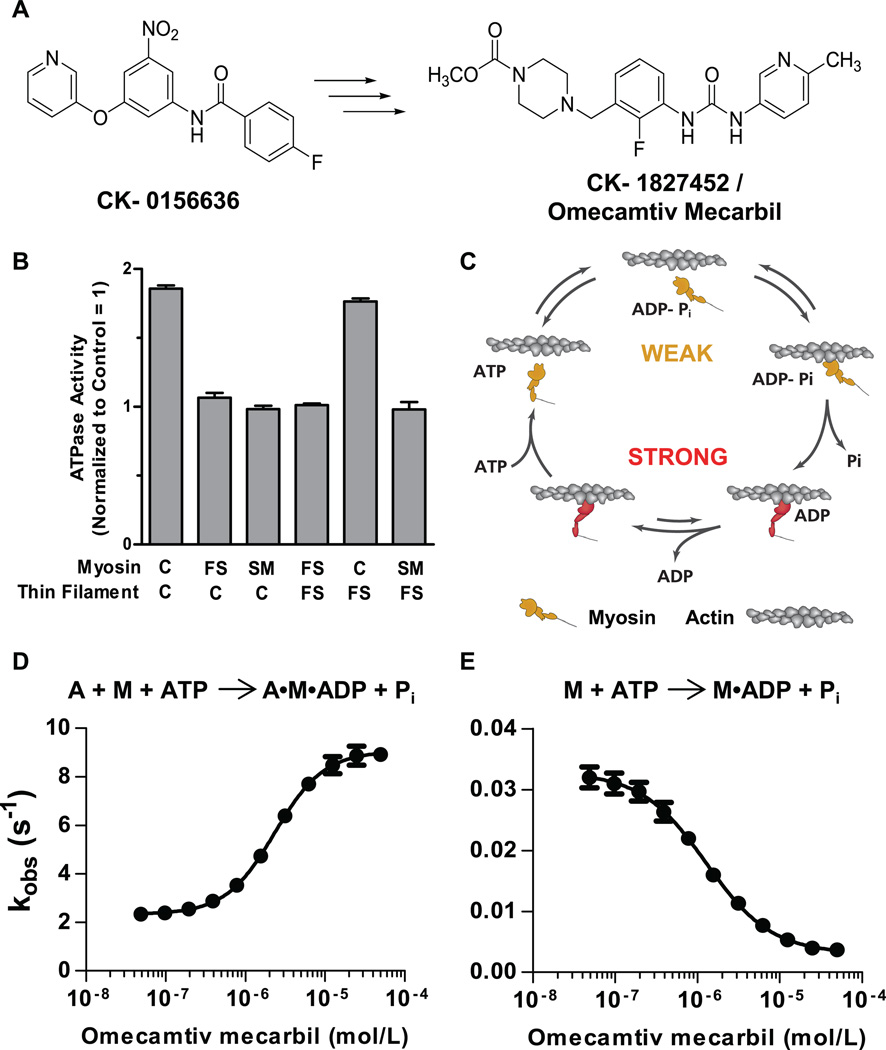
Identification and characterization of omecamtiv mecarbil. (A) The chemical structure of the original hit, CK-0156636, and the chemical structure of omecamtiv mecarbil whose optimization and synthesis have been previously reported (7). (B) Target identification of omecamtiv mecarbil using heterologous reconstituted combinations of the troponin-tropomyosin regulated actin-myosin system. Means ± SD are shown for three to five replicates at each condition. ATPase measurements were made at 25°C in 12 mM Pipes, 2 mM MgCl2, 1 mM dithiothreitol at pH 6.8 and pCa 6.75 by using a coupled enzyme system consisting of pyruvate kinase and lactate dehydrogenase and monitoring the oxidation of NADH at 340 nM (14). C, FS, and SM denote cardiac, fast skeletal, and smooth muscle myosin isoforms. Similarly, C and FS denote reconstituted cardiac and fast skeletal thin filaments, respectively. (C) The mechanochemical cycle of myosin. Yellow indicates myosin weakly bound to actin and red indicates myosin strongly bound to actin (adapted from an illustration, courtesy of J. Spudich, Stanford University). The effect of omecamtiv mecarbil on the phosphate release rate is shown for actin (A) plus myosin (M) in panel (D) and of myosin alone in panel (E). Transient-state kinetic measurements of phosphate release from cardiac myosin S1 (n = 3 for each data point, means ± SD) were carried out by rapidly mixing a solution of myosin and ATP with 7-diethylamino-3-({[(2-maleimidyl)ethyl]amino}carbonyl) coumarin–modified phosphate-binding protein (MDCC-PBP) plus or minus actin with a stopped-flow apparatus under single-turnover conditions at 25°C. Phosphate released from myosin binds rapidly to MDCC-PBP, which leads to an increase in its fluorescence (13). The rate of phosphate release is measured at various concentrations of omecamtiv mecarbil to construct a dose response.
Omecamtiv mecarbil selectively activates the S1 domain of cardiac myosin but not other muscle myosins, as demonstrated by using heterologous reconstituted versions of troponin-tropomyosin–regulated actin-myosin (9). Different myosin S1 isoforms can interact interchangeably with either the cardiac or skeletal muscle thin filaments to hydrolyze ATP (including myosins that are not derived from striated muscle, such as smooth muscle myosin). Omecamtiv mecarbil increased the rate of ATP turnover only when cardiac myosin S1 was present, irrespective of the source of thin filament (troponin-tropomyosin–regulated actin) and not when fast skeletal or smooth muscle myosin were present (Fig. 1B and fig. S1). Further, isothermal titration calorimetry showed that omecamtiv mecarbil binds to purified cardiac myosin S1 with an affinity of 1.6 ± 0.3 µM and a stoichiometry of no more than one molecule of omecamtiv mecarbil per cardiac myosin S1 but showed no significant binding to skeletal or smooth myosin (fig. S2).
The enzymatic and mechanical cycles of myosin are tightly coupled (Fig. 1C) (10, 11), and thus, analysis of the individual kinetic steps in the cardiac myosin ATPase cycle can provide insight into the mechanism of action for omecamtiv mecarbil. The myosin-ATP intermediate binds weakly to the actin filament. In concert with the hydrolysis of ATP to adenosine diphosphate and inorganic phosphate (ADP-Pi) and subsequent release of Pi from myosin, there is a transition from the weakly actin-bound state to a strongly actin-bound state accompanied by a force-producing power stroke (12). Omecamtiv mecarbil accelerated the transition rate from the weakly bound to the strongly bound force-producing state as indicated by an increase in the actin-dependent rate of phosphate release from cardiac myosin S1 [median effective concentration (EC50) = 2.3 ± 0.09 µM (SD)] (Fig. 1D and fig. S3) measured by using coumarin-modified phosphate-binding protein as a fluorescent reporter of phosphate production (13). The rate of the subsequent step, ADP release, governs the length of time in the strongly bound state and did not change nor did the rate of ATP binding and myosin release from the actin filament (fig. S4). These results explain the ability of omecamtiv mecarbil to increase the steady-state ATPase rate (fig. S5a) (14), because the weak-to-strong binding state transition is the rate-limiting step in the overall actin-myosin ATPase cycle.
To our surprise, when the rate of phosphate release was tested in the absence of actin, it was slowed by omecamtiv mecarbil, which decreased the overall ATPase rate of cardiac myosin in the absence of actin [median inhibitory concentration (IC50) = 1.3 ± 0.09 µM (SD)] (Fig. 1E and figs. S5b and S6). In the presence of actin, the release of phosphate is intimately associated with the rate-limiting transition of myosin from a weakly actin-bound state to a strongly actin-bound force-producing state (12). Because omecamtiv mecarbil accelerated phosphate release only in the presence of actin, we postulated that omecamtiv mecarbil lowers the energy barrier for the transition from the weak to strong binding state in the actomyosin cycle. In a sarcomere containing an ensemble of myosin heads, of which the majority are not tightly bound to the actin filament at any moment during muscle contraction (11, 15), omecamtiv mecarbil is expected to increase the number of myosin heads interacting with actin filaments in a force-producing state. Because each head acts as an independent force generator, this results in an increased force output from the muscle.
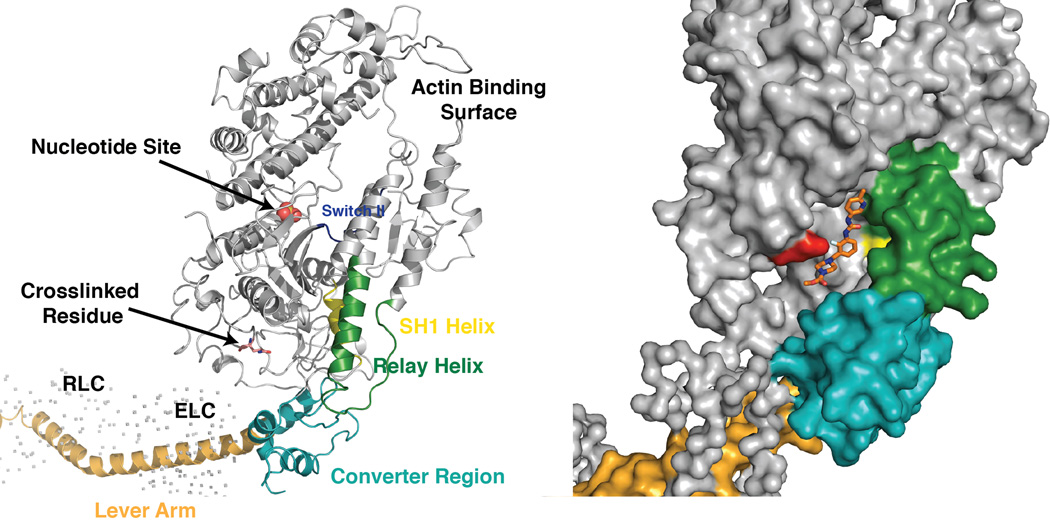
To better understand the structural basis for the action of omecamtiv mecarbil on myosin, we used a benzophenone derivative of omecamtiv mecarbil (fig. S7) as a photo-activated affinity label to identify its potential binding site to cardiac myosin S1. Tandem mass spectrometry consistently identified the same peptide (RSEAPPHIF) (16) labeled by the omecamtiv mecarbil analog at serine 148 (table S1); competition with a high-affinity analog lacking the benzophenone labeling functionality eliminated this binding. Mapped onto the structure of the myosin S1 domain (17), the labeled peptide resides in a cleft large enough to accommodate omecamtiv mecarbil; its location is about 6.5 nm from the actin-binding interface and about 3 nm from the nucleotide-binding pocket (Fig. 2). The cleft is in a region where the relay helix and the converter domain converge at the base of the lever arm. These structural elements are thought to relay conformational changes in the nucleotide-binding pocket to produce motion in the lever arm that generates the power stroke. Consistent with this, crystallographic studies suggest that the position of the converter or lever arm can affect chemical transitions in the nucleotide-binding site (11, 12). Thus, the proposed site of omecamtiv mecarbil binding could allow it to allosterically modulate both the enzymatic and mechanical properties of the cardiac myosin motor.
Fig. 2.
The proposed binding site for omecamtiv mecarbil to cardiac myosin S1. The ribbon diagram to the left shows the major features of the myosin S1 head. A space-filling model of the myosin structure showing the position of the identified peptide is shown to the right (the structure of the chicken skeletal S1 fragment, PDB ID 2MYS, was used as a model because the cardiac S1 structure has not been determined). The compound was manually fit into the cleft containing the identified labeled peptide. The red residue indicates the labeled amino acid serine 148.
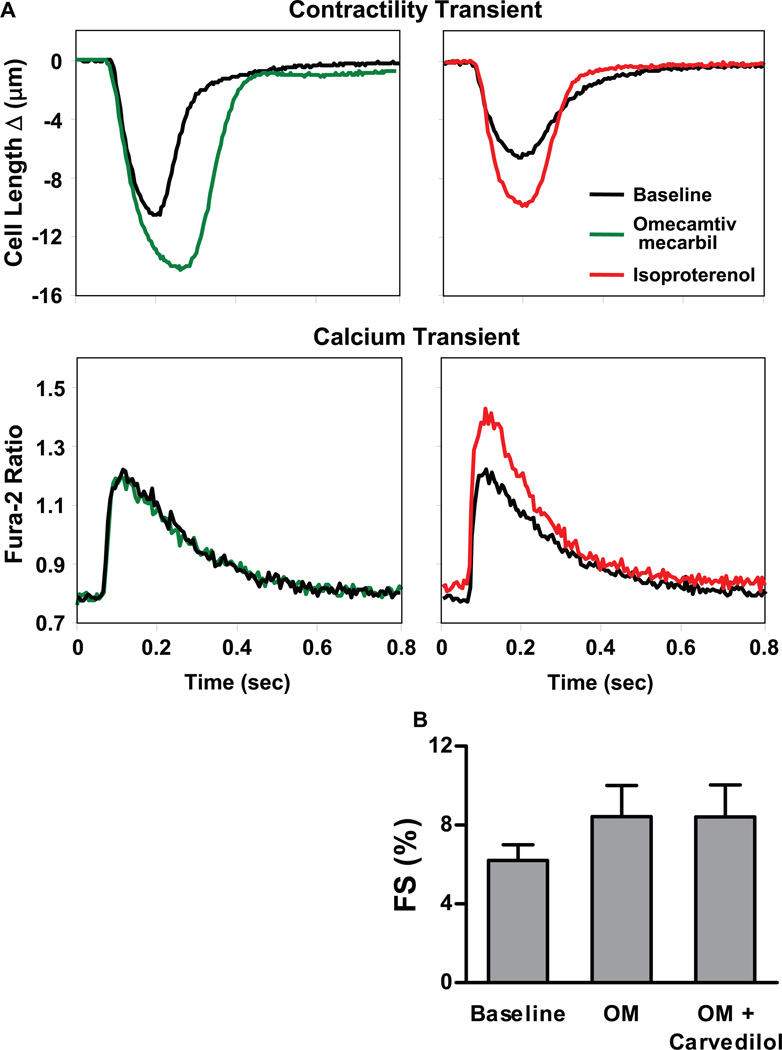
On the basis of its site of action in the sarcomere and biochemical mechanism of action, omecamtiv mecarbil should increase cardiac contractility without changes in cardiac myocyte calcium homeostasis. To test this hypothesis, we made simultaneous quantitative measurements of the contractile response and calcium transient in freshly isolated adult rat cardiac ventricular myocytes (18). Compared with control myocytes, omecamtiv mecarbil (200 nM) significantly increased the fractional shortening (percent cell length change) of adult rat cardiac myocytes (Table 1 and Fig. 3A, top left) but did not have any effect on the calcium transient as measured by the ratiometric fluorescent calcium indicator Fura-2 (Table 1 and Fig. 3A, bottom left). In contrast, the β-adrenergic agonist, isoproterenol, increased peak systolic and diastolic calcium and increased the rates of contraction and relaxation (Table 1 and Fig. 3A, right side). Omecamtiv mecarbil and isoproterenol were additive in terms of their effect on contractility, but addition of omecamtiv mecarbil to isoproterenol did not lead to any further changes in the calcium transient (Table 1). Higher concentrations of omecamtiv mecarbil (up to 10 µM) did not increase the magnitude or kinetics of the calcium transient (table S2). As evident in the individual contractile transients shown (Fig. 3A), omecamtiv mecarbil (200 nM) extended the duration of contraction, as indicated by an increase in the time to its peak (156 ± 7.4%, P < 0.05 compared with baseline) without affecting the rates of contraction or relaxation (89 ± 8.4% and 101 ± 11%, P > 0.05 compared with baseline) measured in the absence of Fura-2 (table S3). Omecamtiv mecarbil also increased myocyte contraction in the presence of carvedilol (Fig. 3B), a β-adrenergic blocker commonly used in heart failure patients (4). In summary, omecamtiv mecarbil increases the contractility of cardiac myocytes without affecting their calcium transient, in contrast to the β-adrenergic agonist isoproterenol.
Table 1.
The effects of omecamtiv mecarbil, isoproterenol (ISO), and their combination on cardiac myocyte contractility and calcium transient. The effects of omecamtiv mecarbil, isoproterenol, and the combination of the two drugs on FS, the Fura-2 ratio at the peak of contraction (systole), during relaxation (diastole), and the time to 75% decline from peak systolic calcium (T75% Ca2+). Data are means ± SEM; Statistics were performed using a one-way ANOVA with a post hoc Student-Newman-Keuls test.
| Treatment | n | FS (% baseline) |
Fura-2 ratio | T75% Ca2+ (s) |
|
|---|---|---|---|---|---|
| Systolic | Diastolic | ||||
| Control | 6 | 100 ± 7.7 | 1.23 ± 0.017 | 0.80 ± 0.005 | 0.32 ± 0.018 |
| OM (200 nM) | 6 | 146 ± 3.4* | 1.19 ± 0.015 | 0.78 ± 0.004 | 0.33 ± 0.025 |
| ISO (2 nM) | 6 | 169 ± 13* | 1.38 ± 0.017*† | 0.84 ± 0.011*† | 0.25 ± 0.009*† |
| OM (200 nM) + ISO (2 nM) | 6 | 212 ± 15*†‡ | 1.37 ± 0.013*† | 0.84 ± 0.015*† | 0.25 ± 0.011*† |
P < 0.05:
compared with control,
compared with OM,
compared with isoproterenol.
Fig. 3.
The effects of omecamtiv mecarbil in cardiac myocytes. (A) Representative tracings showing that omecamtiv mecarbil (200 nM) increases cardiac myocyte contractility without changing the Ca2+ transient. In contrast, the β-adrenergic agonist isoproterenol (2 nM) increases contractility by increasing the Ca2+ transient. (B) Treatment of myocytes (n = eight myocytes per condition) with the β-adrenergic blocker, carvedilol, at a concentration (200 nM) sufficient to completely block the effect of isoproterenol (20 nM), does not alter the effect of omecamtiv mecarbil (200 nM) on myocyte contractility (FS). Data are means ± SEM.
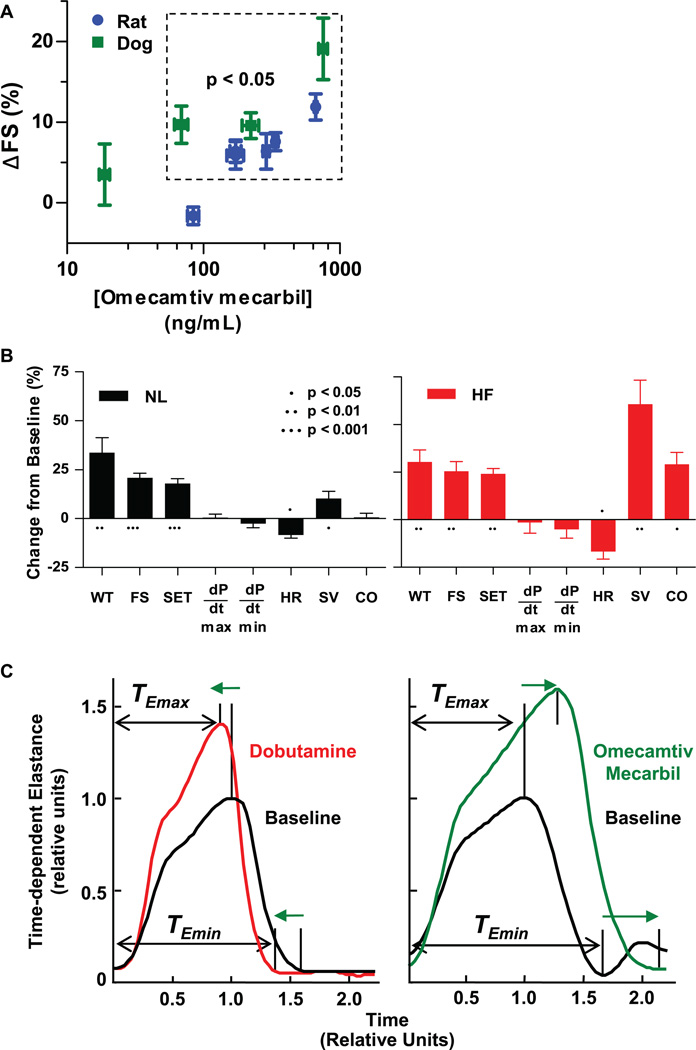
Given the findings obtained in cardiac myocytes, we sought to understand how these effects of cardiac myosin activation would affect cardiac function in the intact animal. We used echocardiography to evaluate the effect of omecamtiv mecarbil infusions on the cardiac function of Sprague-Dawley rats and beagle dogs under isoflurane anesthesia. Measurements of systolic and diastolic left ventricular (LV) dimensions in the parasternal long axis (a cross-sectional view of the heart) were used to calculate an index of cardiac function, percent fractional shortening (FS). In both species, omecamtiv mecarbil produced statistically significant, dose-dependent, concentration-dependent, and visually evident changes in cardiac contractility as indicated by increases in FS (Fig. 4A and movie S1) (19).
Fig. 4.
The effects of omecamtiv mecarbil in models of cardiac function. (A) Fractional shortening (FS) of the heart was measured by echocardiography (parasternal long-axis view) with coincident determination of omecamtiv mecarbil plasma concentration in isoflurane anesthetized Sprague-Dawley rats and beagle dogs during 30- to 60-min infusions of drug or vehicle. Placebo-corrected absolute percentage point increases of FS from baseline are plotted against the mean plasma concentration at each infusion dose. Data are means ± SEM, n = five or six rats or four dogs at each dose. (B) Cardiac function in conscious mongrel (NL) and in heart failure (HF) dogs chronically instrumented to measure LV pressure, wall thickness, dimensions, and cardiac output. In NL (n = 5) and HF (n = 5) dogs, omecamtiv mecarbil was administered as a bolus at 0.5 mg/kg of body weight, followed by infusion at 0.5 mg/kg of body weight per hour. Measurements plotted are mean ± SEM values 15 min after the start of drug administration. WT, wall thickening; FS, fractional shortening; SET, systolic ejection time; dP/dt, rate of pressure change; HR, heart rate; SV, stroke volume; CO, cardiac output. (C) Time-dependent elastance is plotted for dobutamine (10 µg/kg per min) and omecamtiv mecarbil (10 min following a 1 mg/kg bolus). The response of each dog is normalized to the magnitude and time of peak elastance at baseline to allow for comparison of the shape of the response. Statistics were performed using a one-way analysis of variance (ANOVA) with a post hoc Student-Newman-Keuls test.
Omecamtiv mecarbil improved left ventricular systolic function in a conscious canine model with chronically implanted sensors to assess LV dimensions, atrial and arterial pressures, and stroke volume (20). The direct effect on cardiac contractility was evident from the increase in myocardial wall thickening (WT) and FS (Fig. 4B) in the absence of a change in loading conditions, such as mean arterial pressure, LV end diastolic pressure, and total vascular resistance (−2.2 ± 1.3%, −7.3 ± 5.8%, and −2.4 ± 2.3%, means ± SEM, P > 0.05). In dogs with heart failure induced by chronic fast pacing of heart rate in concert with a localized myocardial infarction (21), omecamtiv mecarbil produced substantially greater and statistically significant (P < 0.01) increases in stroke volume (60.8 ± 12.5%) and cardiac output (29.1 ± 6.1%) than it did in normal dogs (10.2 ± 3.6% and 0.8 ± 2.0%, respectively). The increase in cardiac output was especially notable given the coincident lowering of heart rate (−16.7 ± 4.0%, P = 0.014) observed in the dogs with heart failure (Fig. 4B; P calculated using Student’s t test).
Underlying the effects on systolic function was an increase in systolic ejection time (SET) in the absence of changes in the rate of LV pressure development (dP/dt) (Fig. 4B). In contrast, existing drugs, such as the β-adrenergic agonist dobutamine, increase cardiac contractility by increasing dP/dt and shortening SET (22). We investigated this finding further by comparing omecamtiv mecarbil with dobutamine, using time-dependent LV end systolic elastance, a load-independent measure of cardiac contractility derived from the pressure-volume loop (23). The plots of time-dependent elastance (Fig. 4C) are illustrative of the different effects that the two drug mechanisms have on the dynamics of cardiac contractility.
Overall energy balance in the contracting heart is set by a combination of loading conditions, heart rate, membrane ion fluxes, calcium cycling, and crossbridge cycling. Although omecamtiv mecarbil might increase ATP turnover at the level of the sarcomere, on balance, myocardial energetics appear unchanged following omecamtiv mecarbil administration, as it does not increase overall myocardial oxygen consumption (8) at doses producing substantial improvements in cardiac function. However, excessive crossbridge activation at excessive doses of omecamtiv mecarbil could lead to an increase in the duration of systole to an extent where coronary blood flow during diastole is reduced, and signs and symptoms of cardiac ischemia may emerge.
As a selective, allosteric activator of cardiac myosin, omecamtiv mecarbil is a rare example of a drug mechanism whose action depends on activation rather than inhibition of an enzyme, an approach that may have broader application for therapeutic intervention (24–26). It represents a therapeutic approach to directly improve cardiac function that potentially avoids the deleterious effects limiting current indirect inotropic mechanisms (27). Further studies in patients with heart failure will eventually define the clinical benefit and risk profile of cardiac myosin activation in a condition that is still marked by substantial rates of mortality and morbidity.
Supplementary Material
Acknowledgments
We thank J. Chabala, C. Homcy, T. A. Pollard, J. H. Sabry, R. J. Solaro, J. A. Spudich, J. R. Teerlink, and R. D. Vale for their advice over the course of this work; A. Franklin and S. Hollenbach for their contributions to the conduct of some of these studies; and M. Rowley and J. Goldstein for assistance in preparing the manuscript. Y.-T.S., D.A.K., and S.F.V. are members of the Scientific Advisory Board and received research support, compensation, and stock options (D.A.K. and S.F.V.) from Cytokinetics. Cytokinetics holds a patent on omecamtiv mecarbil (U.S. Patent no. 7,507,735); Amgen is a licensee of this patent. Support provided by Small Business Innovation Research grant, NIH (1-R43-HL-66647-1) and Cytokinetics, Inc.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/VOL/ISSUE/PAGE/DC1
Materials and Methods
Figs. S1 to S9
Tables S1 to S3
References
Movie S1
References and Notes
- 1.Lloyd-Jones D, et al. Executive summary: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation. 2010;121:948. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am. J. Cardiol. 2008;101:1016. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Jr, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am. Heart J. 2005;149:209. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJV. Clinical practice. Systolic heart failure. N. Engl. J. Med. 2010;362:228. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 5.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- 6.Pollard T, Earnshaw W. Cell Biology. ed. 2. Philadelphia: Saunders/Elsevier; 2008. pp. 705–725. [Google Scholar]
- 7.Morgan BP, et al. Discovery of omecamtiv mecarbil the first, selective, small molecule activator of cardiac myosin. ACS Med Chem Lett. 2010;1:472. doi: 10.1021/ml100138q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen YT, et al. Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3:522. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- 9.Materials and methods are available as supporting material on Science Online.
- 10.Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10:4617. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu Rev Biophys. 2010;39:539. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 12.Holmes KC, Angert I, Kull FJ, Jahn W, Schröder RR. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425:423. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 13.Brune M, Hunter JL, Corrie JE, Webb MR. Direct, real-time measurement of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry. 1994;33:8262. doi: 10.1021/bi00193a013. [DOI] [PubMed] [Google Scholar]
- 14.De La Cruz EM, Ostap EM. Kinetic and equilibrium analysis of the myosin ATPase. Methods Enzymol. 2009;455:157. doi: 10.1016/S0076-6879(08)04206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki T, et al. Effects of cardiac myosin isoform variation on myofilament function and crossbridge kinetics in transgenic rabbits. Circ. Heart Fail. 2009;2:334. doi: 10.1161/CIRCHEARTFAILURE.108.802298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Single-letter abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr.
- 17.Rayment I, et al. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science. 1993;261:50. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 18.Cleemann L, Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: Evidence from Ca2+ transients and contraction. J. Physiol. 1991;432:283. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.In the online materials, a side-by-side movie shows several heart beats of a short-axis echocardiogram in an anesthetized beagle before (on the left) and after (on the right) a 1-hour infusion of omecamtiv mecarbil at 1 mg/kg of body weight per hour. In this view, the left ventricular cavity is in the middle of a ring of contracting myocardium.
- 20.Komamura K, et al. Alterations in left ventricular diastolic function in conscious dogs with pacing-induced heart failure. J. Clin. Invest. 1992;89:1825. doi: 10.1172/JCI115787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen YT, Lynch JJ, Shannon RP, Wiedmann RT. A novel heart failure model induced by sequential coronary artery occlusions and tachycardiac stress in awake pigs. Am. J. Physiol. 1999;277:H388. doi: 10.1152/ajpheart.1999.277.1.H388. [DOI] [PubMed] [Google Scholar]
- 22.Banfor PN, et al. Comparative effects of levosimendan, OR-1896, OR-1855, dobutamine, and milrinone on vascular resistance, indexes of cardiac function, and O2 consumption in dogs. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H238. doi: 10.1152/ajpheart.01181.2007. [DOI] [PubMed] [Google Scholar]
- 23.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ. Res. 1974;35:117. doi: 10.1161/01.res.35.1.117. [DOI] [PubMed] [Google Scholar]
- 24.Grimsby J, et al. Allosteric activators of glucokinase: Potential role in diabetes therapy. Science. 2003;301:370. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 25.Stasch JP, et al. NO- and haem-independent activation of soluble guanylyl cyclase: Molecular basis and cardiovascular implications of a new pharmacological principle. Br. J. Pharmacol. 2002;136:773. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorn JA, Wells JA. Turning enzymes ON with small molecules. Nat. Chem. Biol. 2010;6:179. doi: 10.1038/nchembio.318. [DOI] [PubMed] [Google Scholar]
- 27.Petersen JW, Felker GM. Inotropes in the management of acute heart failure. Crit. Care Med. 2008;36(suppl.):S106. doi: 10.1097/01.CCM.0000296273.72952.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.