Abstract
Purpose
Insulin-like growth factor 1 receptor (IGF-1R) is commonly expressed in primary breast cancers. Understanding the role of IGF-1R signaling in the different subtypes of breast cancer is important because each subtype has a different outcome and requires different treatment modalities. However, the precise biological significance of IGF-1R expression in cancer cells is still unclear. In this study, we examined the expression of IGF-1R in the different molecular subtypes of breast cancer. The effects of IGF-1R expression on the survival rates and outcomes of breast cancer were also examined.
Methods
IGF-1R expression was evaluated immunohistochemically in tissue microarray blocks constructed from 1,198 invasive breast cancer samples collected from six medical institutions. IGF-1R expression was interpreted according to the human epidermal growth factor receptor 2 (HER2)/neu immunohistochemistry scoring system. Scores of 2+ and 3+ were considered positive.
Results
Positive IGF-1R expression was observed in 65.4% of invasive breast cancer samples. IGF-1R expression was detected in all cancer subtypes (luminal A, 84.4%; luminal B, 75.9%; HER2, 21.2%; triple-negative, 46.6%) and was found to be associated with a positive hormone receptor status and the absence of HER2 amplification (p<0.001). Positive IGF-1R expression was significantly associated with high survival rates (p=0.014). However, a multivariate analysis revealed that the expression levels of IGF-1R did not achieve statistical significance. In the triple-negative cancer subtype, IGF-1R expression was found to be associated with a lower disease-free survival rate (p=0.031).
Conclusion
Positive IGF-1R expression is associated with a favorable prognosis in breast cancer. IGF-1R is frequently expressed in the luminal A/B subtypes of breast cancer, and its expression is related to the hormone receptor status.
Keywords: Breast neoplasms, Insulin-like growth factor 1 receptor, Immunohistochemistry
INTRODUCTION
Insulin-like growth factor 1 receptor (IGF-1R) is a tyrosine kinase receptor that is activated by insulin-like growth factor (IGF) types I and II. An overexpression and enhanced activation of IGF-1R has been observed in many malignant neoplasms, including breast cancer [1,2]. Nielsen et al. [3] found that, in a cohort of 930 primary breast cancer patients, IGF-1R was expressed in 87% of the cases.
The tyrosine kinase receptors of the human epidermal growth factor receptor (HER/EGFR) family (also known as the EGFR or ErbB family)-EGFR, HER2, HER3, and HER4- and IGF-1R mediate many of the malignant phenotypes of breast cancer such as increased cell proliferation, decreased apoptosis, metastasis, and resistance to chemotherapy and radiotherapy [4,5,6]. Several studies have described the signaling interactions between the members of the ErbB/HER family of tyrosine kinase receptors and have implicated the IGF-1R pathway in the development of resistance towards targeted therapies, including towards trastuzumab [7,8], EGFR tyrosine kinase inhibitors, and tamoxifen [9]. Therefore, targeting IGF-1R as well as members of the epidermal growth factor receptor family (specifically, EGFR and HER2) is an attractive therapeutic strategy for the treatment of primary breast cancer. In addition, this strategy may also be useful for the treatment of breast cancers that develop resistance towards HER2-, EGFR-, and estrogen (ER)-targeted therapies.
In breast cancer, each molecular subtype has a different outcome and requires different treatment modalities. ER-positive luminal breast cancers usually have better outcomes than the more aggressive ER-negative, HER2-positive, and triple-negative subtypes [10]. Tamoxifen or aromatase inhibitors are generally the first-line therapy for ER-positive luminal breast cancers, whereas trastuzumab is the first-line treatment for HER2-overexpressing cancers. Currently, there is no targeted treatment for triple-negative breast cancers, and patients afflicted with this subtype usually die within 2 years of diagnosis [11,12].
Recently, several studies have shown that IGF-1R is expressed in all breast cancer subtypes, including the triple-negative subtype, but the prognostic role of IGF-1R expression is still unclear. Law et al. [13] showed that the presence of phosphorylated IGF-1R is associated with a poor prognosis. Yerushalmi et al. [14] reported that IGF-1R expression is correlated with a favorable prognosis in the luminal B subtype and a poor outcome in the HER2 subtype.
An improved understanding of the role of IGF-1R signaling in the different subtypes of breast cancer is required to facilitate the development of novel therapeutic approaches.
Therefore, in this study, we examined the expression of IGF-1R in the different molecular subtypes of breast cancer by immunohistochemical analysis and evaluated its effects on survival rates and disease outcomes.
METHODS
Patients and samples
The Korean Study Group for Breast Pathology collected primary invasive breast carcinoma samples from six medical institutions representing each province in South Korea. In total, 1,198 samples were obtained from the Asan Medical Center (216 cases, 1998), Chonnam National University Hospital (204 cases, 1997-2002), Chungnam National University Hospital (200 cases, 2000-2003), Samsung Medical Center (199 cases, 2000-2001), Soonchunhyang University Hospital (157 cases, 2000-2003), and Yeungnam University Hospital (222 cases, 2000-2002). All tissues were surgically resected, fixed in 10% buffered formalin, and embedded in paraffin. A pathologist at each institution reviewed the slides and selected a representative block for each case. The tumor area on the paraffin block was marked with a pen and sent to the Asan Medical Center to construct tissue microarray blocks. Patient and tumor characteristics, including age, type of surgery, histological type, histological grade, and follow-up data were obtained from the aforementioned institutions.
The histological grade of each sample was assessed using the Bloom-Richardson grading system [15,16] with modifications.
Ethical permission
The Institutional Review Board (IRB) at the Asan Medical Center (Seoul, Korea) approved the study protocol (number, 2013-0083) and provided all the necessary ethical permissions.
Construction of tissue microarray blocks
Formalin-fixed, paraffin-embedded tissue samples were arrayed using a tissue-arraying instrument (Beecher Instruments, Silver Spring, USA). Briefly, the designated zone on each donor block was punched with a tissue cylinder (1.0 mm in diameter), and the sample was transferred to a recipient block. Each sample was arrayed in triplicate to minimize tissue loss and compensate for tumor heterogeneity.
Immunohistochemical staining and silver-enhanced in situ hybridization
Immunohistochemical staining for ER, progesterone (PR), and IGF-1R was performed in the formalin-fixed, paraffin-embedded tissue microarray (TMA) blocks using a Benchmark® automatic immunostaining device (Roche Tissue Diagnostics, Tucson, USA) and an UltraView™ Universal DAB Detection Kit (Ventana Medical Systems, Tucson, USA), according to the manufacturer's instructions. Four-micron-thick sections were immunostained with primary antibodies against ER (diluted 1:50, NCL-6F11, monoclonal; Novocastra, Newcastle, UK), PR (diluted 1:100, 1E2, rabbit monoclonal; Roche, Tucson, USA), and IGF-1R (diluted 1:100, G11; Roche). Silver-enhanced in situ hybridization (SISH) was performed with INFORM® HER2 DNA and Chromosome 17 (Chr17) probes (Ventana Medical Systems, Tucson, USA) on two consecutive TMA sections using a Benchmark® automatic immunostaining device (Ventana Medical Systems, Tucson, USA) according to the manufacturer's protocol. Both probes were labeled with dinitrophenol (DNP). The HER2 DNA probe was denatured at 95℃ for 12 minutes and hybridization was performed at 52℃ for 2 hours. After hybridization, an appropriate stringency washes were performed 3 times at 72℃. The Chr17 probe was denatured at 95℃ for 12 minutes and hybridization was performed at 44℃ for 2 hours. After hybridization, appropriate stringency washes were performed 3 times at 59℃. The HER2 and Chr17 DNP-labeled probes were visualized using rabbit anti-DNP primary antibody with an UltraView™ SISH Detection Kit (Ventana Medical Systems, Tucson, USA). Silver precipitated in the nuclei following the sequential addition of silver acetate, hydroquinone, and H2O2. The slides were counterstained with hematoxylin II (Ventana Medical Systems, Tucson, USA) to facilitate interpretation by light microscopy [17].
Interpretation of immunohistochemical staining and SISH data
SISH data for HER2 expression and immunohistochemistry (IHC) data for the expression of ER and PR from a previously reported study were used for the interpretation of data in the present study [17].
Evaluation of SISH was performed as described previously. Briefly, the SISH signals for HER2 and Chr17 were counted in more than 20 nonoverlapping nuclei per sample. Data was interpreted on the basis of the American Society of Clinical Oncology and the College of American Pathologists guidelines [18], which state that an absolute HER2 gene copy number >6 or a HER2/Chr17 ratio >2.2 indicates SISH positivity; an absolute HER2 gene copy number between 4 and 6 or a HER2/Chr17 ratio between 1.8 and 2.2 indicates an equivocal SISH result; and an absolute HER2 gene copy number <4 or a HER2/Chr17 ratio of <1.8 indicates SISH negativity.
All immunohistochemical markers were assigned a positive or negative score. ER and PR were evaluated according to the Allred score (Harvey). Briefly, the proportion score (0-5) and intensity score (0-3) were summed to give a total score that ranged between 0 and 8. A tumor was considered positive for ER or PR when the total score was >2.
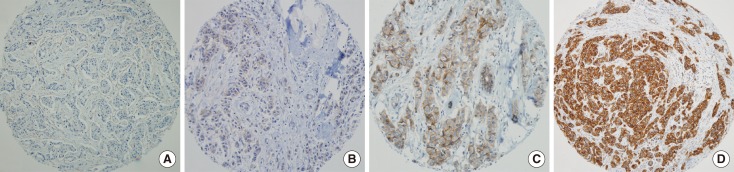
IGF-1R expression was detected as membrane staining and defined according to the intensity of the staining within the invasive tumor component in accordance with the HER2 expression scoring system described in the Hercep Test™ manual (Dako). A result was defined as positive (3+) when the tumor showed uniform, intense membrane staining in more than 30% of the invasive tumor cells. An equivocal (2+) result was defined as the presence of weak or nonuniform membrane staining in more than 10% of the tumor cells. A negative result was defined as the presence of weak/incomplete membrane staining (1+) or the complete absence of membrane staining ( 0) in any portion of the tumor cells (Figure 1). Heterogeneous staining of IGF-1R within any area of the tumor was assigned the highest score. An IGF-1R score of 2+ or 3+ was considered positive.
Figure 1.
Immunohistochemical staining of insulin-like growth factor 1 receptor (IGF-1R). IGF-1R expression was scored according to the intensity of the membrane staining in accordance with human epidermal growth factor receptor 2 expression scoring system described in the Hercep Test™ manual (Dako). (A) Score=0, (B) score=1, (C) score=2, (D) score=3 (Immunohistochemical staining, ×100).
Molecular subtypes
Breast cancer molecular subtypes were classified according to a gene expression profile-validated immunohistochemical surrogate panel (luminal A: ER+ and/or PR+ and HER2-; luminal B: ER+ and/or PR+ and HER2+; HER2: ER-, PR-, and HER2+; and triple-negative: ER-, PR-, and HER2-) [19].
Statistical analyses
The patients' survival rates were estimated by the Kaplan-Meier method. To estimate the overall survival (OS), patients were followed-up from the date of surgical excision of primary breast cancer until the date of death. Similarly, to estimate the recurrence-free survival rate, patients were followed-up from the date of surgical excision of primary breast cancer tumor until the date of the first recurrence. Patients who were lost during follow-up or died from causes other than breast cancer were excluded from the analysis. The survival curves of 2 or more groups were compared with one another by the log rank test and the Cox proportional hazard model. The correlation between IGF-1R expression and categorical variables (age, grade, stage, lymph node [LN] status, hormone receptor status, and molecular subtype) was analyzed using Pearson chi-square test or Fisher exact test. Only p-values of less than 0.05 were considered significant. All statistical analyses were performed using the SPSS statistical software version 18.0 (SPSS Inc., Chicago, USA).
RESULTS
Baseline clinical characteristics
Of the 1,198 invasive breast cancer samples collected, 1,114 samples were included in the final analysis. The remaining samples were excluded due to noninformative cores or loss of cores while performing the IGF-1R immunostaining. The 1,114 breast cancer cases included in this study comprised 961 cases of invasive ductal carcinoma, 23 cases of mucinous carcinoma, 29 cases of tubular carcinoma, and 101 cases of carcinomas of other histological types. The median patient age was 46 years (range, 20-89 years) and the median follow-up period was 77 months. In total, 212 patients experienced relapse or death (19.0%) and 144 patients (12.9%) died. The median time between the date of diagnosis and the date of death was 41.2 months (range, 41 days-92 months). Complete IHC and SISH data were available for 968 cases, which could be classified into four molecular subtypes: luminal A (544 cases, 56.2%), luminal B (79 cases, 8.2%), HER2 (113 cases, 11.7%), and triple-negative (232 cases, 24.0%).
IGF-1R expression and its association with clinical/histological features
Positive IGF-1R expression was observed in 728 (65.4%) of the 1,114 samples. Of the 968 samples that could be classified, positive IGF-1R expression was observed in 651 samples (67.3%). This subset included samples from all four subtypes. IGF-1R was frequently expressed in the luminal A and luminal B subtypes (84.4% and 75.9%, respectively). However, only 21.2% of the HER2 subtypes were IGF-1R positive (p<0.001). In the triple-negative subtype, 46.6% of the cases were IGF-1R positive (Table 1).
Table 1.
Insulin-like growth factor 1 receptor expression in molecular subtypes
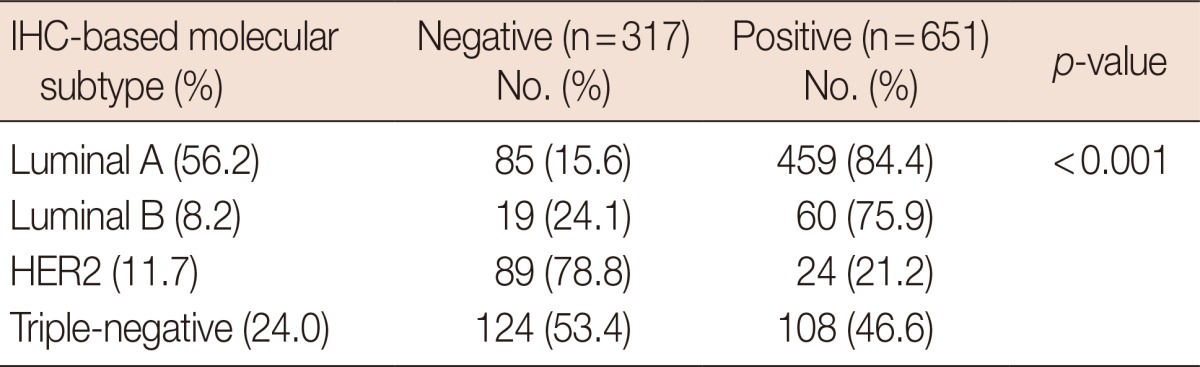
IHC=immunohistochemistry; HER2=human epidermal growth factor receptor 2.
Positive IGF-1R expression was associated with a positive hormone receptor status (for both ER and PR) and the absence of HER2 gene amplification (p<0.001). In addition, IGF-1R positivity was associated with low histological grades (p<0.001). There was no correlation between IGF-1R expression and age, T stage (tumor size), and the presence or absence of lymph node metastasis (LN status) (Table 2).
Table 2.
Correlation between insulin-like growth factor 1 receptor expression and clinical/histological features and hormonal receptor status
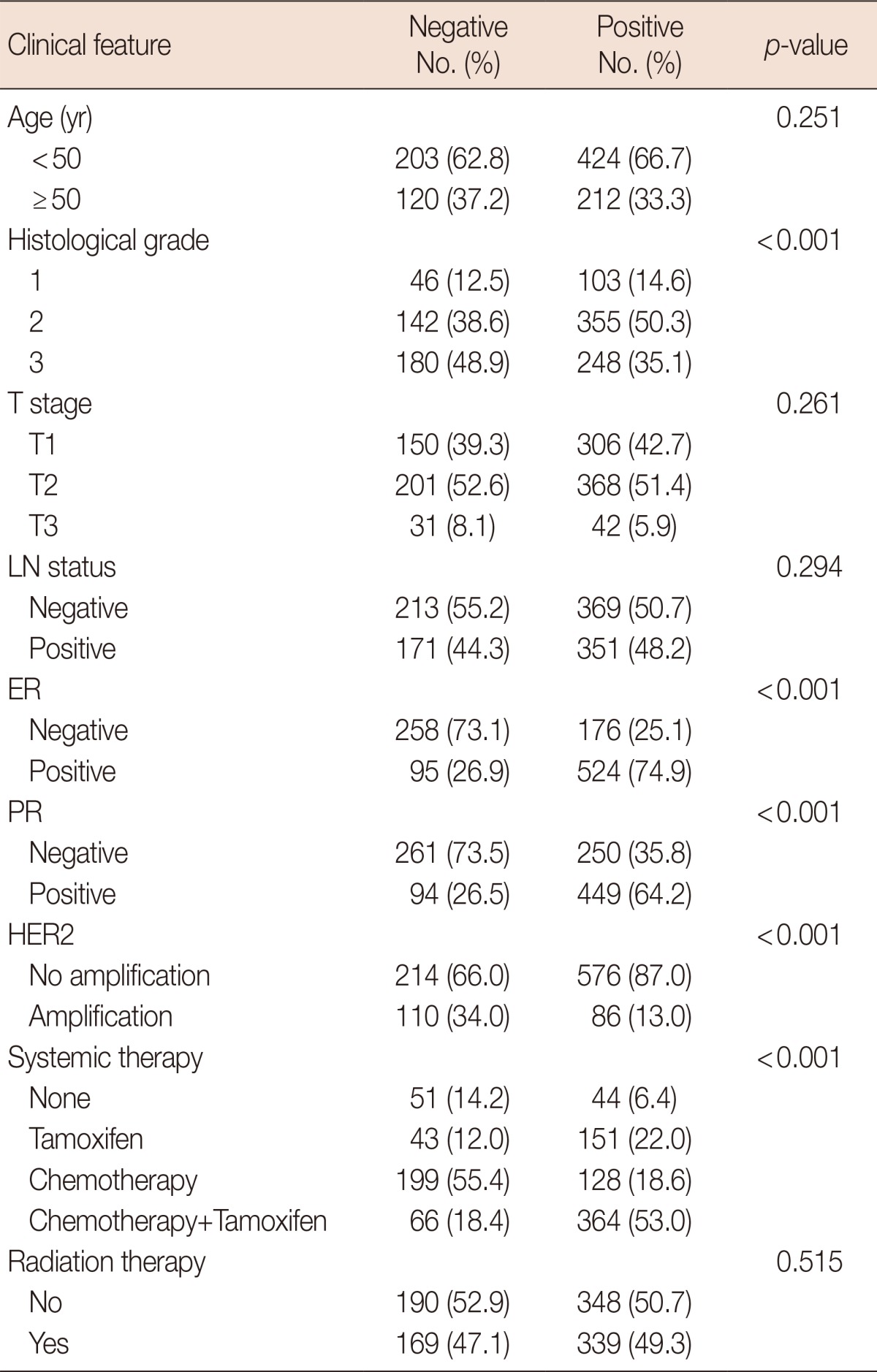
LN=lymph node; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
IGF-1R expression and survival
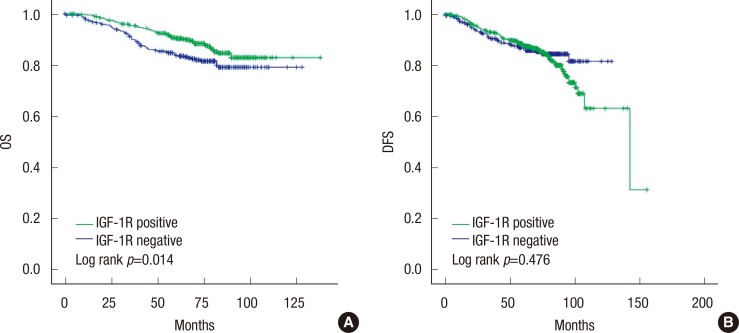
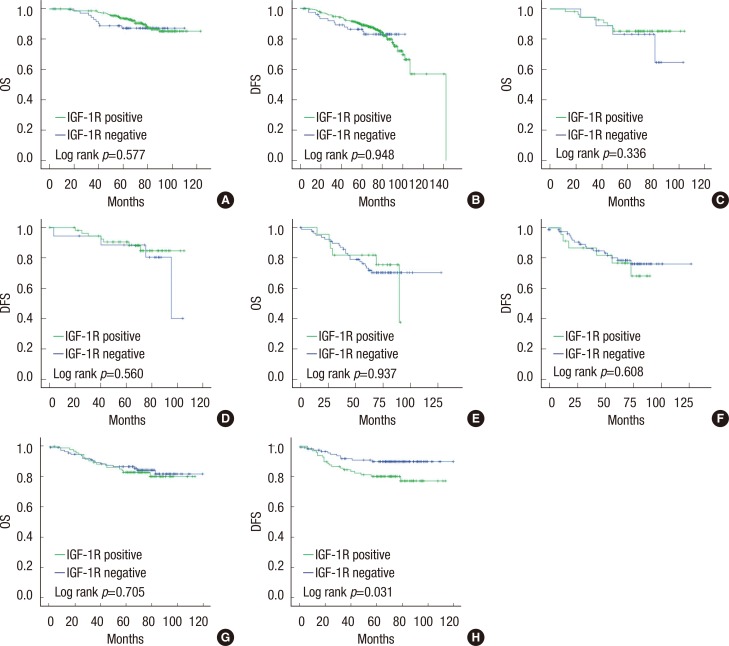
OS was significantly longer in breast cancer cases with positive IGF-1R expression than in those with negative IGF-1R expression (p=0.014) (Figure 2A). However, IGF-1R expression did not correlate with disease-free survival (DFS) (Figure 2B). Univariate analyses revealed that other factors might be associated with a poor OS, including a high histological grade (p=0.001), a high T stage (p<0.001), positive LN metastasis (p=0.001), and the HER2 (p<0.001) or triple-negative (p=0.018) subtypes (Table 3). In the multivariate analysis, a high T stage, positive LN status, and the HER2 subtype were statistically significant poor prognostic factors, while negative IGF-1R expression lost statistical significance (Table 4). We also assessed the prognostic impact of IGF-1R expression in each molecular subtype. In luminal A, luminal B, and HER2 subtypes, OS and DFS were not associated with IGF-1R expression. In the triple-negative subtype, DFS was significantly shorter in cases with positive IGF-1R expression (p=0.031), but OS did not appear to be associated with IGF-1R expression (Figure 3).
Figure 2.
Kaplan-Meier analysis of (A) overall survival (OS) and (B) disease-free survival (DFS) in breast cancer.
IGF-1R=insulin-like growth factor 1 receptor.
Table 3.
Univariate analyses of overall survival in all invasive breast cancer
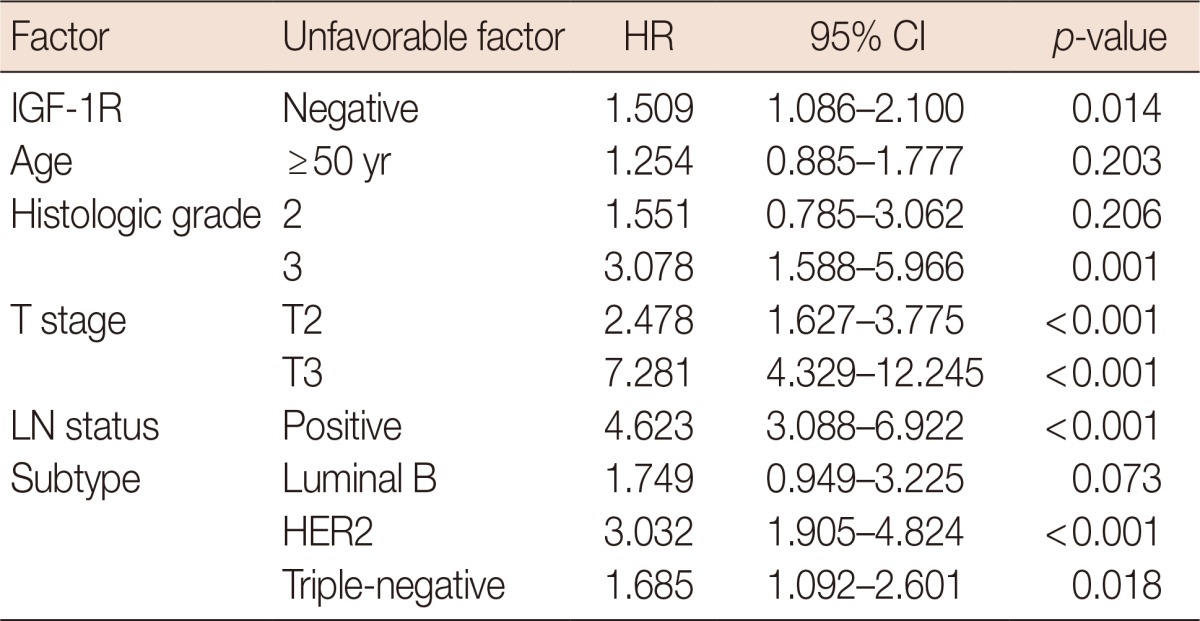
HR=hazard ratio; CI=confidence interval; IGF-1R=insulin-like growth factor 1 receptor; LN=lymph node; HER2=human epidermal growth factor receptor 2.
Table 4.
Multivariate analyses of overall survival in all invasive breast cancer
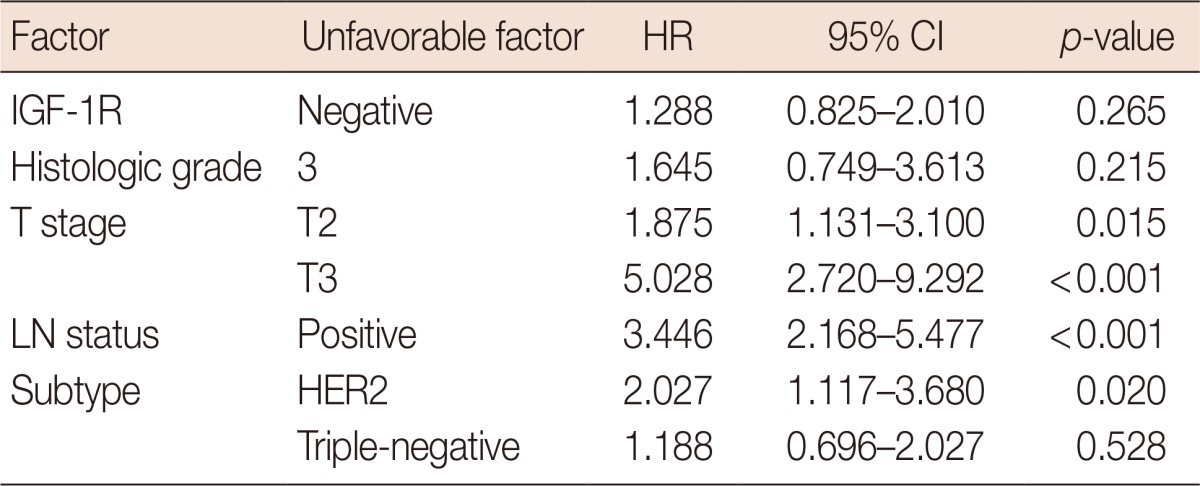
HR=hazard ratio; CI=confidence interval; IGF-1R=insulin-like growth factor 1 receptor; LN=lymph node; HER2=human epidermal growth factor receptor 2.
Figure 3.
Kaplan-Meier analysis of overall survival (OS) and disease-free survival (DFS) in breast cancer subtypes: luminal A (A and B), luminal B (C and D), HER2 (E and F), and triple-negative subtypes (G and H).
IGF-1R=insulin-like growth factor 1 receptor.
DISCUSSION
This multicenter study included a large number of patients with diverse subtypes of carcinoma. The results revealed that positive IGF-1R expression is associated with a favorable prognosis and a positive hormone receptor status. Our findings are in agreement with previous studies that showed that a high IGF-1R level is a favorable prognostic indicator in breast cancer [20]. Papa et al. [21] showed that increased IGF-1R expression is a favorable prognostic indicator and positively correlates with ER expression. Shin et al. [22] quantified the mRNA levels of the IGF-1 and IGF-1R and demonstrated that patients with high expression levels of IGF-1 in cancer tissues tended to have better OS and DFS. In a recent study, increased IGF-1R expression, as measured by IHC, was associated with improved outcomes in luminal B subtype cancers and a poor outcome in HER2 subtype cancers [14]. However, other studies suggest that IGF-1R expression is associated with a poor prognosis and unfavorable clinical markers. Phosphorylated IGF-1R was detected in all breast cancer subtypes and was associated with a poor prognosis [13]. In early stage metastatic HER2-positive breast cancers that had been treated with trastuzumab, IGF-1R positivity was significantly associated with the presence of high-grade tumors, a high mitotic index, and vascular invasion [23]. However, these studies either examined the expression patterns of phosphorylated genes or were carried out in patients treated with HER2-targeted therapy. The patients included in our study were not treated with trastuzumab. This might explain the contradiction between the results of the previous studies and those of the present study.
In our study, we showed that IGF-1R expression was correlated with OS rates, and that IGF-1R positivity was associated with a number of favorable prognostic factors such as a positive hormone receptor status (ER and PR), absence of HER2 amplification, and a low histological tumor grade. These results might explain the loss of statistical significance in the multivariate analysis of OS.
We also investigated the correlation between IGF-1R expression and outcomes in different breast cancer subtypes. No correlation could be detected between IGF-1R expression and outcome in luminal A/B and HER2 subtypes. However, in the triple-negative subtype, positive IGF-1R expression was associated with a shorter DFS. The triple-negative breast cancer subtype is a highly diverse group. Recently, Lehmann et al. [24] suggested that triple-negative breast cancer subtyping is necessary to enhance the design and effectiveness of molecular-based therapies. Lehmann et al. [24] identified six triple-negative subtypes by cluster analysis, including the basal-like 2 and mesenchymal-like types, which express IGF-1R. However, the authors did not investigate the association between the six triple-negative subtypes and cancer outcome. It is possible that each subtype has a different outcome. Therefore, additional studies need to be conducted to evaluate the relationship between the six triple-negative subtypes, IGF-1R expression, and outcome of cancer.
In this study, we observed positive IGF-1R expression in all breast cancer subtypes. Therefore, IGF-1R targeting might represent an attractive strategy for the treatment of breast cancer. Drugs that specifically target IGF-R are now being developed. IGF-1R-targeted therapy might be beneficial to patients who do not respond to other targeted therapies (such as trastuzumab and tamoxifen) and to patients with the triple-negative subtype of cancer. At present, therapeutic strategies that co-target IGF-1R and HER2 are being studied and tested in a HER2-overexpressing breast cancer model. Studies on the interaction and crosstalk between IGF-1R and HER2 in BT474 cells showed that IGF-1R inhibition leads to a reduction in HER2 phosphorylation. Combining IGF-1R inhibitors with trastuzumab or HER2 kinase inhibitors resulted in a synergistic growth inhibition and increased apoptosis [25]. Furthermore, IGF-1R inhibition by genetic or pharmacologic approaches was associated with antitumor activity in cell lines and xenograft models derived from trastuzumab-naive and trastuzumab-resistant HER2-positive breast cancers [8]. Therefore, IGF-1R immunostaining may be used to screen and select patients who might benefit from IGF-1R-targeted therapy.
We defined IGF-1R expression according to the HER2 expression scoring system described in the Hercep Test™ manual because the membrane staining pattern of IGF-1R could be easily evaluated using this system. Although, this method is different from that used by Yerushalmi et al. [14], the results correspond well with those of previous studies.
In conclusion, we found that positive IGF-1R expression was observed in all breast cancer subtypes and its expression was correlated with a favorable prognosis. IGH-1R might be a useful immunohistochemical marker for the prediction of cancer outcome and for the selection of patients for IGF-1R targeted therapy.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, et al. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res. 1990;50:48–53. [PubMed] [Google Scholar]
- 2.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen TO, Andrews HN, Cheang M, Kucab JE, Hsu FD, Ragaz J, et al. Expression of the insulin-like growth factor I receptor and urokinase plasminogen activator in breast cancer is associated with poor survival: potential for intervention with 17-allylamino geldanamycin. Cancer Res. 2004;64:286–291. doi: 10.1158/0008-5472.can-03-1242. [DOI] [PubMed] [Google Scholar]
- 4.Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:485–498. doi: 10.1007/s10911-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 5.Pegram MD, Pietras R, Bajamonde A, Klein P, Fyfe G. Targeted therapy: wave of the future. J Clin Oncol. 2005;23:1776–1781. doi: 10.1200/JCO.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Yee D. The therapeutic potential of agents targeting the type I insulin-like growth factor receptor. Expert Opin Investig Drugs. 2004;13:1569–1577. doi: 10.1517/13543784.13.12.1569. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 8.Nahta R. Deciphering the role of insulin-like growth factor-I receptor in trastuzumab resistance. Chemother Res Pract. 2012;2012:648965. doi: 10.1155/2012/648965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 10.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 11.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 13.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 14.Yerushalmi R, Gelmon KA, Leung S, Gao D, Cheang M, Pollak M, et al. Insulin-like growth factor receptor (IGF-1R) in breast cancer subtypes. Breast Cancer Res Treat. 2012;132:131–142. doi: 10.1007/s10549-011-1529-8. [DOI] [PubMed] [Google Scholar]
- 15.Robbins P, Pinder S, de Klerk N, Dawkins H, Harvey J, Sterrett G, et al. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol. 1995;26:873–879. doi: 10.1016/0046-8177(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 16.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991; 19; 403-410. Histopathology. 2002;41(3A):151–152. [PubMed] [Google Scholar]
- 17.Bae YK, Gong G, Kang J, Lee A, Cho EY, Lee JS, et al. HER2 status by standardized immunohistochemistry and silver-enhanced in situ hybridization in Korean breast cancer. J Breast Cancer. 2012;15:381–387. doi: 10.4048/jbc.2012.15.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 19.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 20.Bonneterre J, Peyrat JP, Beuscart R, Demaille A. Prognostic significance of insulin-like growth factor 1 receptors in human breast cancer. Cancer Res. 1990;50:6931–6935. [PubMed] [Google Scholar]
- 21.Papa V, Gliozzo B, Clark GM, McGuire WL, Moore D, Fujita-Yamaguchi Y, et al. Insulin-like growth factor-I receptors are overexpressed and predict a low risk in human breast cancer. Cancer Res. 1993;53:3736–3740. [PubMed] [Google Scholar]
- 22.Shin A, Ren Z, Shu XO, Cai Q, Gao YT, Zheng W. Expression patterns of insulin-like growth factor 1 (IGF-I) and its receptor in mammary tissues and their associations with breast cancer survival. Breast Cancer Res Treat. 2007;105:55–61. doi: 10.1007/s10549-006-9427-1. [DOI] [PubMed] [Google Scholar]
- 23.Gallardo A, Lerma E, Escuin D, Tibau A, Muñoz J, Ojeda B, et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106:1367–1373. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakraborty AK, Liang K, DiGiovanna MP. Co-targeting insulin-like growth factor I receptor and HER2: dramatic effects of HER2 inhibitors on nonoverexpressing breast cancer. Cancer Res. 2008;68:1538–1545. doi: 10.1158/0008-5472.CAN-07-5935. [DOI] [PubMed] [Google Scholar]