Abstract
Purpose
Mutations in BRCA genes are the main cause of hereditary breast cancer in Korea. The aim of this study was to investigate the characteristics of breast cancers involving BRCA1 (BRCA1 group) and BRCA2 (BRCA2 group) mutations.
Methods
We retrospectively reviewed the medical records of patients with BRCA1 (BRCA1 group) or BRCA2 (BRCA2 group) mutation positive breast cancer from multiple centers and compared the data to that of the Korean Breast Cancer Society registry (registry group).
Results
The patients of the BRCA1 group were diagnosed at a younger age (median age, 37 years) and had tumors of higher histological (61.3% with histological grade 3) and nuclear (37.5% with nuclear grade 3) grade than those of the registry group. In addition, the frequency of ductal carcinoma in situ in the BRCA1 group was lower (3.7%) than in the registry group, and the BRCA1 group were more likely to be triple-negative breast cancer (61.3%). Patients in the BRCA2 group were also younger at diagnosis (mean age, 41 years) and were more likely to have involvement of the axillary node than the registry group (45.5% vs. 33.5%, p=0.002). The BRCA1 and BRCA2 groups did not show a correlation between tumor size and axillary node involvement.
Conclusion
We report the characteristics of BRCA mutation positive breast cancer patients in the Korean population through multicenter data and nation-wide breast cancer registry study. However, BRCA-mutated breast cancers appear highly complex, and further research on their molecular basis is needed in Korea.
Keywords: BRCA1 genes, BRCA2 genes, Breast neoplasms, Korea
INTRODUCTION
Globally, breast cancer is a common cancer in women, with approximately 7% of breast cancers being caused by a hereditary predisposition [1]. Hereditary breast cancer is generally caused by mutations in the BRCA1 or BRCA2 gene [2,3], with a high penetrance of BRCA1 or BRCA2 genetic mutations. In fact, it is estimated that women with either a BRCA1 or a BRCA2 genetic mutation have a 60% to 80% risk of developing breast cancer in their lifetime [4]. In a previous study, the estimated cumulative risk of breast cancer until the age of 70 years was 72.1% and 66.3% in BRCA1 and BRCA2 mutation carriers, respectively, in Korea [5].
Breast cancer patients can present with various clinical, pathological and molecular characteristics, some of which have prognostic significance. Several studies have shown that breast cancers involving BRCA1 or BRCA2 mutation are different from sporadic breast cancer cases. Several studies have demonstrated that BRCA1 mutation carriers often do not express the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) (termed triple-negative breast cancer) [6,7,8,9]. In contrast, breast cancer cases with a BRCA2 mutation share similar pathologic features to sporadic cancers [9,10,11].
Breast cancer phenotypes resulting from a patients' genetic background are also likely to be racially influenced. Therefore, conducting race and country-specific investigations is important to understand the potential effects of race on breast cancer phenotypes. However, few such studies have been performed in Korea, and these studies were single center-based investigations with small cohort sizes [12].
To the best of our knowledge, this is the first study to use multicenter and registry data in Korea. The purpose of this study was to determine the clinical and pathological characteristics of breast cancer in Korean patients with positive for BRCA1 or BRCA2 mutations, compared to sporadic breast cancer from the Korean breast cancer registry.
METHODS
Study population and data collection
A total of 181 women were confirmed to be breast cancer patients with either BRCA1 or BRCA2 genetic mutations (termed the BRCA1 group or the BRCA2 group, respectively) by BRCA genetic testing. Until December 2010, patients were enrolled from eight hospitals (Asan Medical Center, Seoul National University Bundang Hospital, Seoul National University Hospital, Soonchunhyang University Hospital, Korea Cancer Center Hospital, Hallym University Sacred Heart Hospital, Samsung Medical Center, and Ajou University Hospital). All patients were previously enrolled in the Korean Hereditary Breast Cancer Study (KOHBRA) [13]. Patients undergoing neoadjuvant treatment were excluded. Clinical and pathological details of all breast cancer patients were obtained from the registry of the Korean Breast Cancer Society, which is representative of the general population of breast cancer patients in Korea (termed the registry group). This registry is a prospectively maintained database of the Korean Breast Cancer Society [14], and is estimated to include >50% of all newly diagnosed breast cancer patients in Korea every year [15]. In the registry group, patients undergoing neoadjuvant treatment were also excluded. Up to December 2010, there were 55,387 breast cancer patients from the general population with available information in the registry database. The electronic medical records of patients in the BRCA1 and BRCA2 groups were reviewed and reconfirmed by each of the eight hospitals. Medical records of patients in the registry group (from the Korean breast cancer registry) were reviewed to obtain clinical and pathological information, including age at diagnosis, tumor size, axillary lymph node metastasis involvement, distant metastasis, Nottingham histological grade (modification of the Scarff-Bloom-Richardson grading system), modified Blacks nuclear grade, nodal status, as well as the expression status of the estrogen receptor, progesterone receptor, and HER2. In this study, hormone receptor and HER2 status were reviewed retrospectively by pathology reports at each hospital. Negative HER2 status was defined as "negative" or "1+" on immunohistochemistry in the medical records of each hospital and in the Korean breast cancer registry.
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB number, 2010-0476) and the registry committee of the Korean Breast Cancer Society.
Statistical analysis
An independent t-test was used to compare the mean age and mean tumor size across all groups. In addition, a chi-square test was used to determine differences between groups in the following characteristics: T stage, axillary lymph node metastasis, distant metastasis, histological grade, nuclear grade, nodal status, and the expression status of the estrogen receptor, progesterone receptor, and HER2. In all analyses, p-values less than 0.05 were considered statistically significant. All of the statistical analyses were performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, USA).
RESULTS
Characteristics of patients in the BRCA1 and BRCA2 groups compared to those in the registry group
The median age at the time of diagnosis of patients in the BRCA1 group was 37 years (range, 19-62 years), which was younger than that of patients in the registry group (median age, 48 years; range, 12-96 years; p<0.001). However, there was no difference in tumor size between the BRCA1 group and the registry group. Furthermore, patients in the BRCA1 group had tumors of higher histological (61.3% with histological grade 3, p>0.001) and nuclear (37.5% with nuclear grade 3, p=0.004) grade than did general breast cancer patients of the registry group. The frequency of ductal carcinoma in situ in the BRCA1 group (3.7%) was lower than that in the registry group (10.3%). However, there was no significant difference in the proportion of patients with axillary node metastasis between the BRCA1 group and the registry group.
The BRCA1 group included more hormone receptor-negative cases and had a lower proportion of cases with HER2 overexpression, compared to the registry group. Triple-negative breast cancer cases were more prevalent than general breast cancer cases in the registry group (61.3% vs. 12.4%, p<0.001).
Similar to the BRCA1 group, the BRCA2 group had a younger median age at diagnosis than the registry group (41 years vs. 48 years, p<0.001). Despite no significant differences in tumor size, tumors of the BRCA2 group involved axillary nodes more often than did those of the registry group (45.5% vs. 33.5%, p=0.002). As observed in the BRCA1 group, there was also less HER2 overexpression in the BRCA2 group than in the registry group; however the distribution of HER2 expression grade differed between the BRCA2 group and the registry group. There were no other significant differences between the BRCA2 and the registry groups.
Triple-negative breast cancer presented more often in the BRCA1 group than in the BRCA2 and registry groups (BRCA1 group, 61.3%; BRCA2 group, 12.9%; and registry group, 12.4%) (Table 1).
Table 1.
Characteristics of the BRCA1, BRCA2, and registry groups
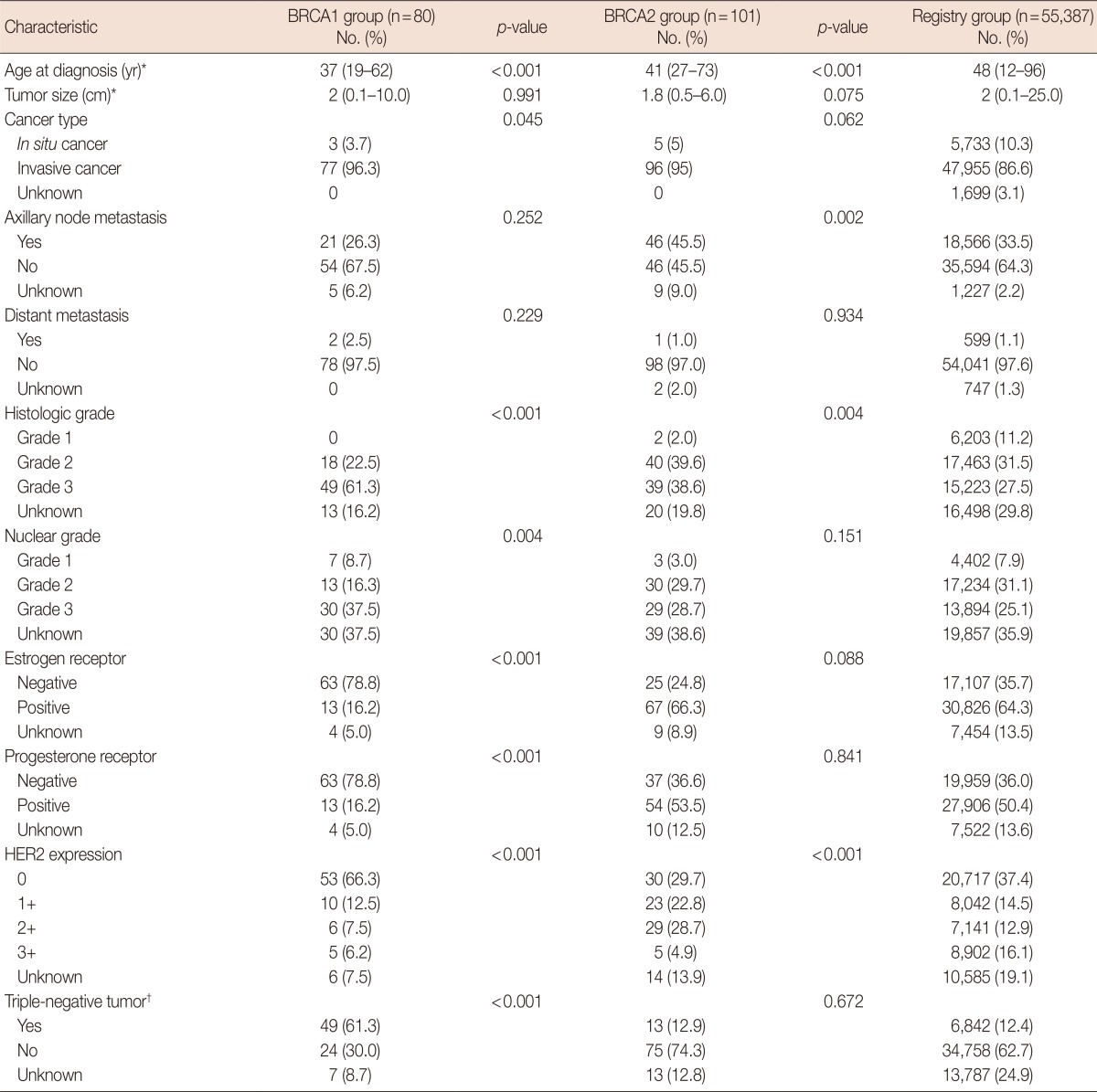
HER2=human epidermal growth factor receptor 2.
*Median (range); †Estrogen receptor (-) & progesterone receptor (-) & HER2 expression: 0 or 1+.
Nodal status according to tumor size in the BRCA1, BRCA2, and registry groups
The frequency of axillary node metastasis in breast cancer increases with increasing tumor size. However, unlike the registry group, there was no significant odds ratio of axillary lymph node involvement and increasing tumor size cancer in the BRCA1 and BRCA2 groups. In addition, BRCA1 group was even more likely to involve axillary lymph nodes in smaller-sized tumors (Table 2). A significant positive correlation was noted between tumor size and axillary nodal involvement in triple-negative and non-triple-negative patients of registry group, while no such correlation was seen in breast cancer cases of BRCA mutation carriers (Table 3).
Table 2.
Odds ratio of axillary node involvement according to tumor size in the BRCA1, BRCA2, and registry groups
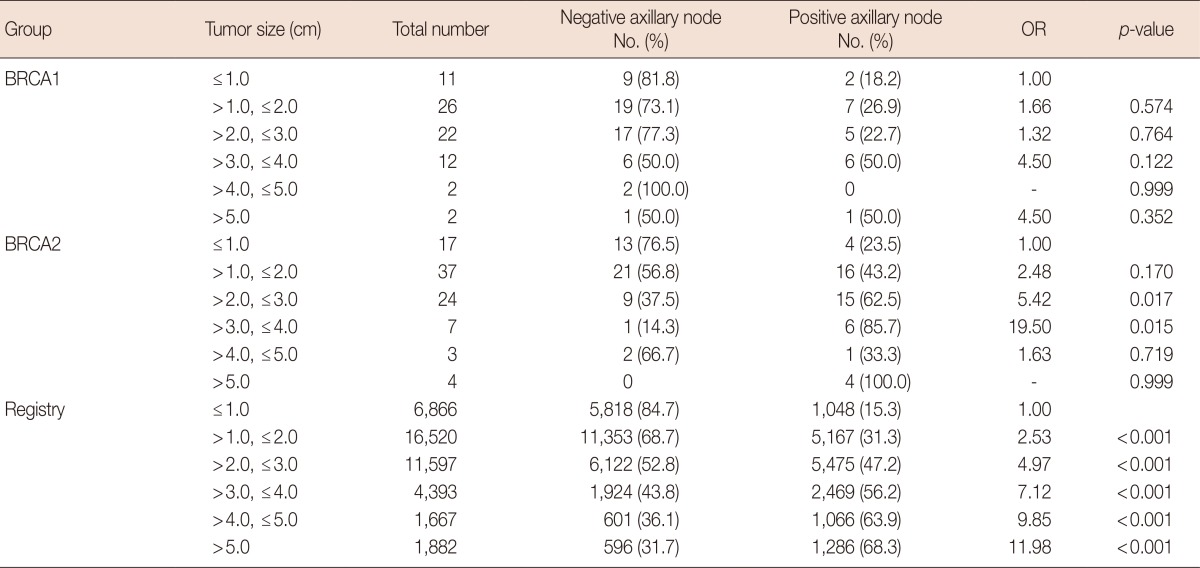
OR=odds ratio of positivity of axillary lymph node involvement.
Table 3.
Tumor size and axillary lymph node involvement in the BRCA1 and BRCA2 groups compared to triple-negative and non-triple-negative breast cancer in the registry group
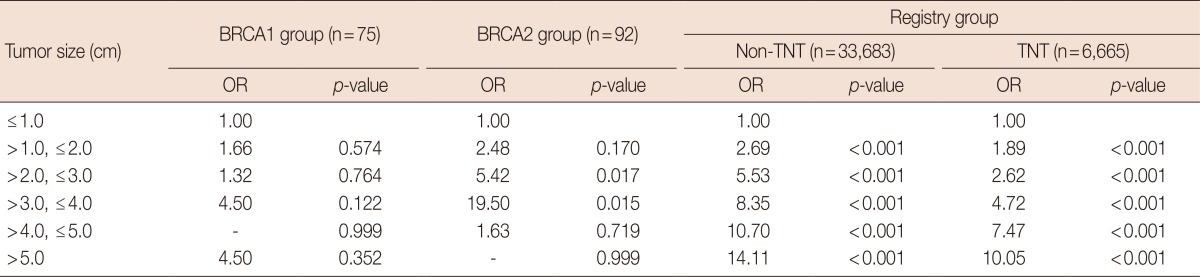
OR=odds ratio of positivity of axillary lymph node involvement; Registry=patients of registry data; Non-TNT=non-triple-negative tumor; TNT=triple-negative tumor.
DISCUSSION
To the best of our knowledge, this is the first to utilize multicenter data and Korean breast cancer registry data to describe the clinical and pathological characteristics of BRCA1 and BRCA2 mutation-positive breast cancer cases in Korea. The features of BRCA1- and BRCA2-positive breast cancers were compared to those of the registry group. While cases of the registry group were not specifically identified as sporadic breast cancers, the registry group can be assumed to represent sporadic breast cancers considering the low BRCA mutation prevalence in Korea [3,7].
Consistent with the findings of previous studies in other countries, this study showed differences in the characteristics of the BRCA1 and BRCA2 groups compared to the registry group, which represents sporadic breast cancer cases in Korea [9,11,16,17].
Patients in the BRCA1 and BRCA2 groups were diagnosed with breast cancer at a younger age than those in the registry group, which confirms similar data from previous studies. However, BRCA mutation testing tends to be more easily performed in younger than in older breast cancer patients, which may present a selection bias in the present study as well as in previous studies in other countries [9]. The BRCA1 group presented with tumors that were higher histological and nuclear grades compared to the registry group. In contrast to the BRCA1 group, which had a low proportion of ductal carcinoma in situ, the T stage distribution of the BRCA2 group was not significantly different from that of the registry group. However, there was more frequent involvement of the axillary lymph node in patients of the BRCA2 group than in those of the registry group.
In breast cancer, the status of estrogen receptor, progesterone receptor, and HER2 expression has important clinical implications for disease treatment and prognosis [18]. Most studies showed a higher frequency of the triple-negative subtype of breast cancer in patients with a BRCA1 mutation [9,16,17]. In this study, there was a significantly higher proportion of triple-negative breast cancer cases (no expression of the estrogen receptor, progesterone receptor, and no overexpression of HER2) in the BRCA1 group. In contrast, there was a similar proportion of triple-negative breast cancer cases in the BRCA2 group compared to the registry group. Interestingly, in a previous study, breast cancers involving BRCA1 mutations were shown to cluster with the estrogen receptor-negative basal-like subgroup [19]. The underlying molecular basis of this phenotype specificity observed in patients with BRCA1 mutations remains unknown. However, several investigators have attempted to identify the mechanism underlying estrogen receptor-negativity in breast cancers involving BRCA1 mutations and have suggested that BRCA1 may transcriptionally induce estrogen receptor α (ERα) mRNA expression [20]. Therefore, BRCA1 mutations and the subsequent loss of heterozygosity in breast and ovary cancer patients may result in the inhibition of ERα expression [21]. However, the molecular basis of breast cancers involving BRCA mutations remains highly complex and dependent on potentially unknown functions of BRCA, such as possible effects of BRCA on the expression of progesterone receptor and HER2.
In general, the risk of axillary lymph node metastasis increases with increasing tumor size. However, a significant correlation between tumor size and axillary nodal involvement was not seen in patients of the BRCA1 and BRCA2 groups in this study. Considering the high proportion of triple-negative breast cancer cases in the BRCA1 group, we hypothesized that triple-negative breast cancers may be less susceptible to a correlation between tumor size and axillary node metastasis [22]. Therefore, we investigated the effect of triple-negative breast cancer status on the characteristics of tumors of the BRCA1 group. We first combined cases from all three groups (BRCA1, BRCA2, and registry groups) and then divided the cases into 2 groups (triple-negative breast cancers and non-triple-negative breast cancers). Consequently, in contrast to the findings from the BRCA1 and BRCA2 groups, in both triple-negative and non-triple-negative breast cancer groups, the odds ratio of axillary lymph node involvement increased according to tumor size. In particular, the BRCA1 group showed lower odds ratio of axillary lymph node involvement than did the registry group, suggesting that BRCA1-mutated breast cancers have other mechanisms for axillary node metastasis. Previous studies by other groups suggested that BRCA1-mutated breast cancers metastasize by early hematogenous dissemination regardless of tumor size, rather than by lymphatic spread [23].
In the present study, the BRCA1 group included a small proportion of patients with ductal carcinoma in situ tumors and high grade and hormone receptor-negative tumors. In addition, BRCA2 group tumors were more likely to involve the axillary lymph nodes. Therefore, breast cancers involving BRCA mutations showed several features associated with poor patient prognosis. However, previous studies did not show poor prognosis in BRCA mutation carriers. In an unselected cohort of triple-negative breast cancer cases, BRCA mutation carriers had a significantly better prognosis than patients without BRCA mutations [24,25]. These results are not consistent with the poor prognostic features of the patients of the BRCA1 and BRCA2 groups in this study. Therefore, further research is required to confirm the prognostic significance of BRCA mutations in breast cancer.
In conclusion, BRCA1 mutation-positive breast cancer cases have unique clinical and pathological features such as a younger patient age at diagnosis, lower proportion of ductal carcinoma in situ, higher tumor grade, and higher proportion of triple-negative breast cancer status. Breast cancers involving BRCA2 mutations were also diagnosed at a younger age and more frequently involved axillary lymph nodes compared to the breast cancers from the registry group. Further studies are required to investigate the impact of BRCA mutations on the unique clinical and pathologic characteristics of breast cancer patients. In addition, the present results suggest that a varied approach tailored to individual patients, from screening and diagnosis to treatment and monitoring, is required to manage breast cancer patients with BRCA mutations in Korea.
ACKNOWLEDGMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare, and Family Affairs, Korea (number, 1020350), and by a grant from Asan Institute for Life Sciences, Seoul, Korea (number, 2011-510).
We thank all participants and investigators of the KOHBRA study: Beom Seok Kwak, Byeong-Woo Park, Byung Ho Son, Byung-In Moon, Cha Kyong Yom, Chan Heun Park, Chan Seok Yoon, Chang Hyun Lee, Dae Sung Yoon, Dong-Young Noh, Doo Ho Choi, Eundeok Chang, Eun-Kyu Kim, Eunyoung Kang, Hae Kyung Lee, Hai-Lin Park, Hyde Lee, Hyeong-Gon Moon, Hyun-Ah Kim, Il-Kyun Lee, Jeong Eon Lee, Jong Won Lee, Jong-Han Yu, Joon Jeong, Jung-Hyun Yang, Keumhee Kwak, Ki-Tae Hwang, Ku Sang Kim, Lee Su Kim, Min Hee Hur, Min Hyuk Lee, Myung Chul Chang, Nam Sun Paik, Sang Ah Han, Sang Seol Jung, Sang Uk Woo, Se Jeong Oh, Sehwan Han, Sei Joong Kim, Sei-Hyun Ahn, Seok-Jin Nam, Seung Sang Ko, Sue Kyoung Park, Sung Hoo Jung, Sung Soo Kang, Sung Yong Kim, Sung-Won Kim, Tae Hyun Kim, Tae Woo Kang, Wonshik Han, Woo-Chul Noh, Yong Lai Park, Yongsik Jung, Young Jin Suh, Young Tae Bae, Young Up Cho, Young-Ik Hong, Sue K. Park, Yoon Joo Jung, Su Yun Choi, Young Bum Yoo, Soo-Jung Lee.
The Korean Breast Cancer Society thanks the following members who participated in Korean Breast Cancer Registry: Kang SY, Kang SH, Kang YJ, Kang EY, Kang HS, Kang HJ, Go BG, Koh JH, Ko CD, Kwak BS, Kwak JH, Kwak HN, Kim KS, Kim KC, Kim DI, Kim SY, Kim SW, Kim SY, Kim SW, Kim SJ, Kim SK, Kim YH, Kim WW, Kim JY, Kim JI, Kim LS, Kim JS, Kim JR, Kim TH, Kim HK, Kim HS, Kim HJ, Nam SJ, Nam SY, Nam YH, Noh DY, Noh WC, Ryu WS, Moon BI, Moon HG, Min JW, Park KS, Park MH, Park SH, Park ST, Park YL, Park WC, Park JP, Park HL, Park HK, Park HB, Park HY, Bae YT, Bae JW, Paik NS, Baek JM, Bong JG, Suh YJ, Sun WY, Son KY, Son GS, Son BH, Song BJ, Song YJ, Song JY, Shin SH, Shin JH, Shin HJ, Shin HC, An MH, Ahn SK, Yang GS, Yang JH, Yom CK, Oh SJ, Yoo YB, Yoon DS, Yun MY, Yoon JH, Lee KS, Lee KP, Lee DS, Lee MR, Lee MH, Lee SJ, Lee SY, Lee SH, Lee AB, Lee YL, Lee WH, Lee EJ, Lee YO, Lee IK, Lee JA, Lee JE, Lee JW, Lee JY, Lee JJ, Lee JS, Lee JH, Lee CH, Lee HD, Lee HY, Lim YA, Lim WS, Lim JY, Lim CW, Chang MC, Chang ES, Chang JN, Jun SY, Jeon YW, Jeon YS, Jeon CW, Chung MS, Jung PJ, Jung BH, Jung SS, Jung SY, Jung YS, Jung YJ, Jung EJ, Chung IY, Jeong J, Jung JH, Jeong HY, Jo BH, Cho SH, Cho YU, Cho JY, Cho JS, Chae BJ, Choi MS, Choi SY, Choi SH, Choi YJ, Choi JH, Han JW, Han SA, Han SW, Han AR, Han WS, Hwang KT, Hwang SH.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77:2318–2324. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2318::AID-CNCR21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Robson ME, Boyd J, Borgen PI, Cody HS., 3rd Hereditary breast cancer. Curr Probl Surg. 2001;38:387–480. [PubMed] [Google Scholar]
- 3.Seo JH, Cho DY, Ahn SH, Yoon KS, Kang CS, Cho HM, et al. BRCA1 and BRCA2 germline mutations in Korean patients with sporadic breast cancer. Hum Mutat. 2004;24:350. doi: 10.1002/humu.9275. [DOI] [PubMed] [Google Scholar]
- 4.King MC, Marks JH, Mandell JB New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 5.Han SA, Park SK, Ahn SH, Son BH, Lee MH, Choi DH, et al. The breast and ovarian cancer risks in Korea due to inherited mutations in BRCA1 and BRCA2: a preliminary report. J Breast Cancer. 2009;12:92–99. [Google Scholar]
- 6.Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat. 2008;108:289–296. doi: 10.1007/s10549-007-9600-1. [DOI] [PubMed] [Google Scholar]
- 7.Li WF, Hu Z, Rao NY, Song CG, Zhang B, Cao MZ, et al. The prevalence of BRCA1 and BRCA2 germline mutations in high-risk breast cancer patients of Chinese Han nationality: two recurrent mutations were identified. Breast Cancer Res Treat. 2008;110:99–109. doi: 10.1007/s10549-007-9708-3. [DOI] [PubMed] [Google Scholar]
- 8.Musolino A, Bella MA, Bortesi B, Michiara M, Naldi N, Zanelli P, et al. BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study. Breast. 2007;16:280–292. doi: 10.1016/j.breast.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loman N, Johannsson O, Bendahl PO, Borg A, Fernö M, Olsson H. Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer. 1998;83:310–319. [PubMed] [Google Scholar]
- 11.Noguchi S, Kasugai T, Miki Y, Fukutomi T, Emi M, Nomizu T. Clinicopathologic analysis of BRCA1- or BRCA2-associated hereditary breast carcinoma in Japanese women. Cancer. 1999;85:2200–2205. [PubMed] [Google Scholar]
- 12.Son BH, Kwak BS, Kim JK, Kim HJ, Hong SJ, Lee JS, et al. Changing patterns in the clinical characteristics of Korean patients with breast cancer during the last 15 years. Arch Surg. 2006;141:155–160. doi: 10.1001/archsurg.141.2.155. [DOI] [PubMed] [Google Scholar]
- 13.Kim EK, Kim KS, Park SK, Ahn SH, Lee MH, Kim SW, et al. The Korean hereditary breast cancer (KOHBRA) study: protocol review. J Breast Cancer. 2007;10:241–247. [Google Scholar]
- 14.Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–2368. doi: 10.1200/JCO.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- 15.Jung YS, Na KY, Kim KS, Ahn SH, Lee SJ, Park HK, et al. Nation-wide Korean breast cancer data from 2008 using the breast cancer registration program. J Breast Cancer. 2011;14:229–236. doi: 10.4048/jbc.2011.14.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagos-Jaramillo VI, Press MF, Ricker CN, Dubeau L, Mai PL, Weitzel JN. Pathological characteristics of BRCA-associated breast cancers in Hispanics. Breast Cancer Res Treat. 2011;130:281–289. doi: 10.1007/s10549-011-1570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082–1089. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayraktar S, Glück S. Molecularly targeted therapies for metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2013;138:21–35. doi: 10.1007/s10549-013-2421-5. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosey AM, Gorski JJ, Murray MM, Quinn JE, Chung WY, Stewart GE, et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst. 2007;99:1683–1694. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorski JJ, Kennedy RD, Hosey AM, Harkin DP. The complex relationship between BRCA1 and ERalpha in hereditary breast cancer. Clin Cancer Res. 2009;15:1514–1518. doi: 10.1158/1078-0432.CCR-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 23.Foulkes WD, Metcalfe K, Hanna W, Lynch HT, Ghadirian P, Tung N, et al. Disruption of the expected positive correlation between breast tumor size and lymph node status in BRCA1-related breast carcinoma. Cancer. 2003;98:1569–1577. doi: 10.1002/cncr.11688. [DOI] [PubMed] [Google Scholar]
- 24.Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010;119:13–24. doi: 10.1007/s10549-009-0566-z. [DOI] [PubMed] [Google Scholar]
- 25.Cortesi L, Masini C, Cirilli C, Medici V, Marchi I, Cavazzini G, et al. Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer. 2010;10:90. doi: 10.1186/1471-2407-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]


