Abstract
Purpose
Stearoyl-CoA desaturase 1 (SCD1) is a novel therapeutic target in various malignancies, including breast cancer. The present study was designed to investigate the effect of the pharmacologic inhibition of SCD1 on fatty acid composition in tissue explant cultures of human breast cancer and to compare these effects with those in adjacent nonneoplastic breast tissue.
Methods
Paired samples of tumor and adjacent noncancerous tissue were isolated from 12 patients with infiltrating ductal breast cancer. Samples were explant cultured in vitro, exposed to the highly selective SCD1 inhibitor CAY10566, and examined for fatty acid composition by gas liquid chromatography. The cytotoxic and antigrowth effects were evaluated by quantification of lactate dehydrogenase release and by sulforhodamine B (SRB) measurement, respectively.
Results
Breast cancer tissue samples were found to have higher levels of monounsaturated fatty acids (MUFA) (p<0.001) and arachidonic acid (20:4n-6, p<0.001) and a lower level of linoleic acid (18:2n-6, p=0.02) than the normal-appearing breast tissues. While exhibiting no evident cytotoxicity, treatment with the SCD1 inhibitor, CAY10566 (0.1-1 µM), for 48 hours significantly increased 18:2n-6 levels in both the tumor and adjacent normal-appearing tissue (approximately 1.2 fold, p<0.05). However, the breast cancer tissue samples showed significant increases in the levels of MUFA and 20:4n-6 compared to the normal-appearing breast tissues (p<0.05). The SRB growth assay revealed a higher rate of inhibition with the SCD1 inhibitor in breast cancer tissues than in normal-appearing tissues (p<0.01, 41% vs. 29%). The SCD1 inhibitor also elevated saturated fatty acid (1.46-fold, p=0.001) levels only in the tumor tissue explant.
Conclusion
The fatty acid composition and response to SCD1 inhibition differed between the explant cultures from breast cancer and the adjacent normal-appearing tissue. Altered fatty acid composition induced by SCD1 inhibition may also, in addition to Δ9 desaturation, modulate other reactions in de novo fatty acid synthesis and lipogenesis, and subsequently affect the overall survival and progression of breast cancer.
Keywords: Breast neoplasms, Fatty acid desaturases, Tissue culture techniques
INTRODUCTION
Breast cancer is one of the most common malignancies among women worldwide; however, the pathology of this disease has not yet been elucidated [1]. Multiple biochemical and molecular factors have been reported to be involved in the pathogenesis and progression of breast cancer. Cancer cells require high levels of biomolecules to maintain rapid growth and division. This is mainly achieved by modifying metabolic pathways in de novo synthesis [2]. In general, cells are able to synthesize fatty acids de novo. However, healthy cells preferentially take up exogenous fatty acids. In contrast, breast tumor cells, as well as other tumor cells, obtain the majority of their fatty acid requirement by de novo synthesis. This phenomenon depends on the increased expression of fatty acid biosynthetic enzymes that produce the required fatty acids in large quantities [3].
Enhanced fatty acid desaturation is an important lipid modification process in cancer cells, in which the cellular content of monounsaturated fatty acids (MUFA) is increased [4]. Stearoyl-CoA desaturase 1 (SCD1) is an important regulatory enzyme in cellular de novo fatty acid synthesis. The activity of this enzyme provides essential precursors for structural cell components and bioactive metabolites. Upregulated levels of SCD1 activity have been reported in various malignant cells [5]. Enhanced activity of SCD1 has also been associated with the pathogenesis of certain aspects of tumor behavior, including tumor cell survival and growth [6]. It has also been shown that knockdown of SCD1 gene expression in A549 human lung adenocarcinoma cells decreases the ratio of MUFA/saturated fatty acids (SFA) in total lipids, significantly delays the formation of tumors, and reduces the growth rate of tumors formed [7].
Despite the aforementioned research on the antitumor effects of SCD1 inhibition, there has been no study to date specifically evaluating the metabolic effect of SCD1 inhibition in breast cancer. Therefore, the present study was designed to examine the effect of pharmacologic SCD1 inhibition on the fatty acid composition of breast tissue in explant cultures from patients with infiltrating ductal carcinoma (IDC).
METHODS
Materials
Cell culture materials, media, fetal bovine serum (FBS) and standard fatty acid methyl esters were obtained from Sigma Chemicals Company (St. Louis, USA). CAY10566 was purchased from Cayman Chemicals (Ann Arbor, USA). All other chemicals used were of analytical grade and obtained from Sigma Chemicals Company.
Primary cell culture
Human breast tissue samples were obtained from 12 women aged 43 to 65 years with recently diagnosed IDC, classified as grade II or III according to the Nottingham Grading System [8]. All patients were scheduled to undergo breast carcinoma surgery at the University Hospital. No patient had previously undergone radiation, surgery, or cytotoxic chemotherapy. Patients over 65 years of age and those with a smoking history, those receiving nutritional supplementation, and those with hypercholesterolemia or diabetes were excluded from the study. The study was approved by the ethics committee of Tabriz University of Medical Sciences (Institutional Review Board permission number, 9182), and all patients provided written informed consent.
Small samples of both the breast carcinoma and the adjacent normal-appearing tissue (1-1.5 cm beyond the tumor and the resection borders) were obtained simultaneously during surgery. All specimens were histologically assessed by a single pathologist to confirm the histopathologic status, homogeneity, and integrity of the tissue.
Each sample was immersed into serum-free Iscove's Modified Dulbecco's Medium with glutamine and transported to the laboratory within 30 minutes after surgery. The glandular tissue was dissected from the fat and fibrous tissue, minced with scissors into 7 to 10 mg pieces, and plated onto 24-well plates in triplicate. Explants were cultured in Dulbecco's Modified Eagle Medium F12 supplemented with 10% FBS, 100 IU/mL penicillin, and 100 µg/mL streptomycin. Cultures were maintained at 37℃ in 5% CO2 in a humidified incubator. They were serum starved for 12 hours and then treated with the indicated concentrations of the highly selective SCD1 inhibitor, CAY10566 (0-10 µM).
Fatty acid analysis
Tissue lipids were extracted by using the Bligh-Dyer method and were esterified with methanol during catalysis with acetyl chloride [9]. Fatty acid methyl esters were extracted and analyzed for fatty acid composition as described previously [10]. Briefly, fatty acid methyl ester derivatives formed by isolated tissue lipids were separated on a 60×0.25-mm Teknokroma TR CN100 column using a Buck Scientific model 610 gas chromatograph (SRI Instruments, Torrance, USA) equipped with a split injector and a flame ionization detector. Helium was used as the carrier gas. The oven temperature was increased from 170℃ to 210℃ at the rate of 1℃/min and then maintained stable for 45 minutes. Tridecanoic acid (13:0) was used as the internal standard. Peak retention times were identified by injecting known standards.
Viability and growth assays
Lactate dehydrogenase (LDH) release into the medium was assessed as an indicator of cytotoxicity. The LDH activity was measured in the supernatant of the explant culture using a standard NADH-linked enzymatic assay (Pars Azmoon, Tehran, Iran). Cytotoxicity was expressed as the relative LDH release compared to the control culture medium. The effect of the SCD1 inhibitor on explant growth was evaluated by measuring cellular protein content using the sulforhodamine-B (SRB) colorimetric assay [11]. The color intensity of solubilized SRB was read by an Immunoscan model 310 microplate reader (Labsystems, Helsinki, Finland) at 570 nm.
Statistical analysis
Analysis of variance was used to compare the group means. The significance of differences between groups was determined using paired-sample t-tests, and a p-value of <0.05 was considered statistically significant. All analyses were performed using SPSS for windows version 11.0 (SPSS Inc., Chicago, USA).
RESULTS
The clinicopathologic details of the subjects in this study are presented in Table 1. Table 2 shows the level of fatty acids measured by gas liquid chromatography in each breast carcinoma and the matched adjacent normal-appearing tissue. SFA were the major fatty acids in both tissues, followed by MUFA and n-6 polyunsaturated fatty acids (PUFA). The level of the major MUFA, oleic acid (18:1n-9), was higher in the cancer tissue samples than in the normal-appearing breast tissue. Among the PUFA assayed, there was a statistically significant increase in the arachidonic acid (20:4n-6) level in the breast cancer tissue (2.2 fold) relative to the mean value in the adjacent normal-appearing breast tissue. Overall, there were no significant differences in the total SFA and PUFA levels between normal-appearing breast and breast cancer tissues. However, the breast cancer tissues showed a significantly higher ratio of MUFA/SFA (p<0.001) (Table 2).
Table 1.
Clinicopathological and molecular characteristics in the 12 women with infiltrating ductal carcinoma
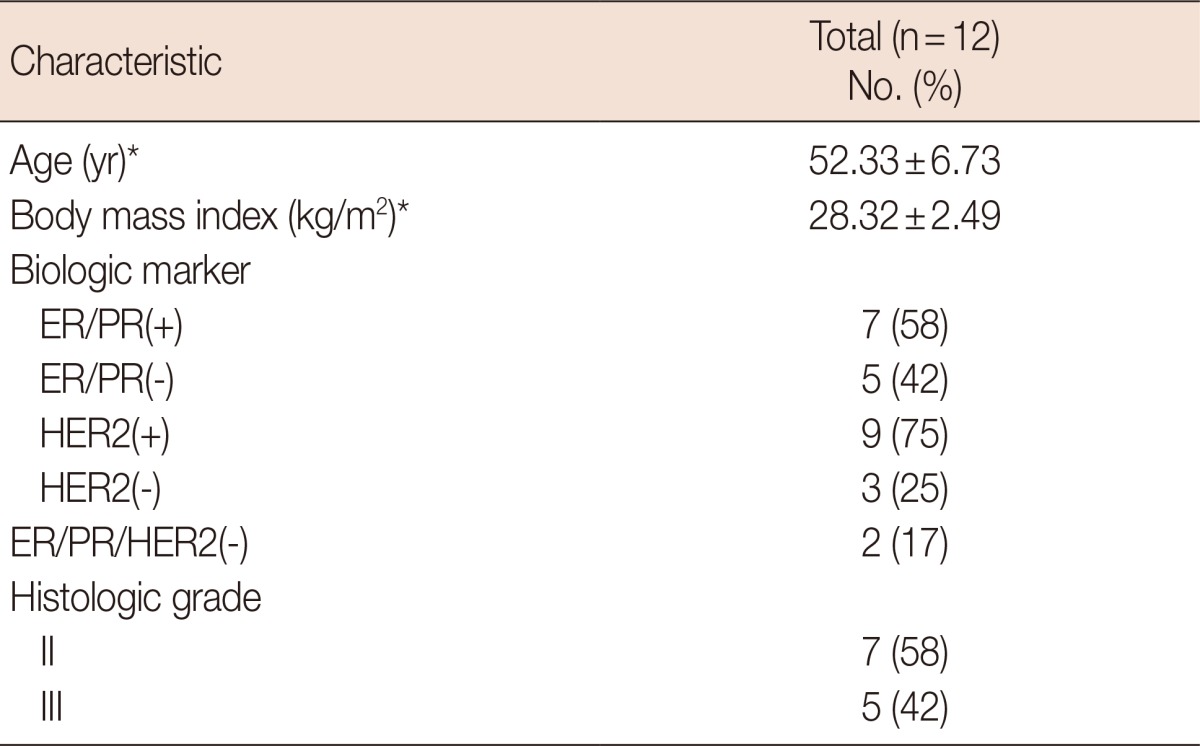
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Mean±SD.
Table 2.
Fatty acid composition of normal-appearing and infiltrating ductal carcinoma tissue
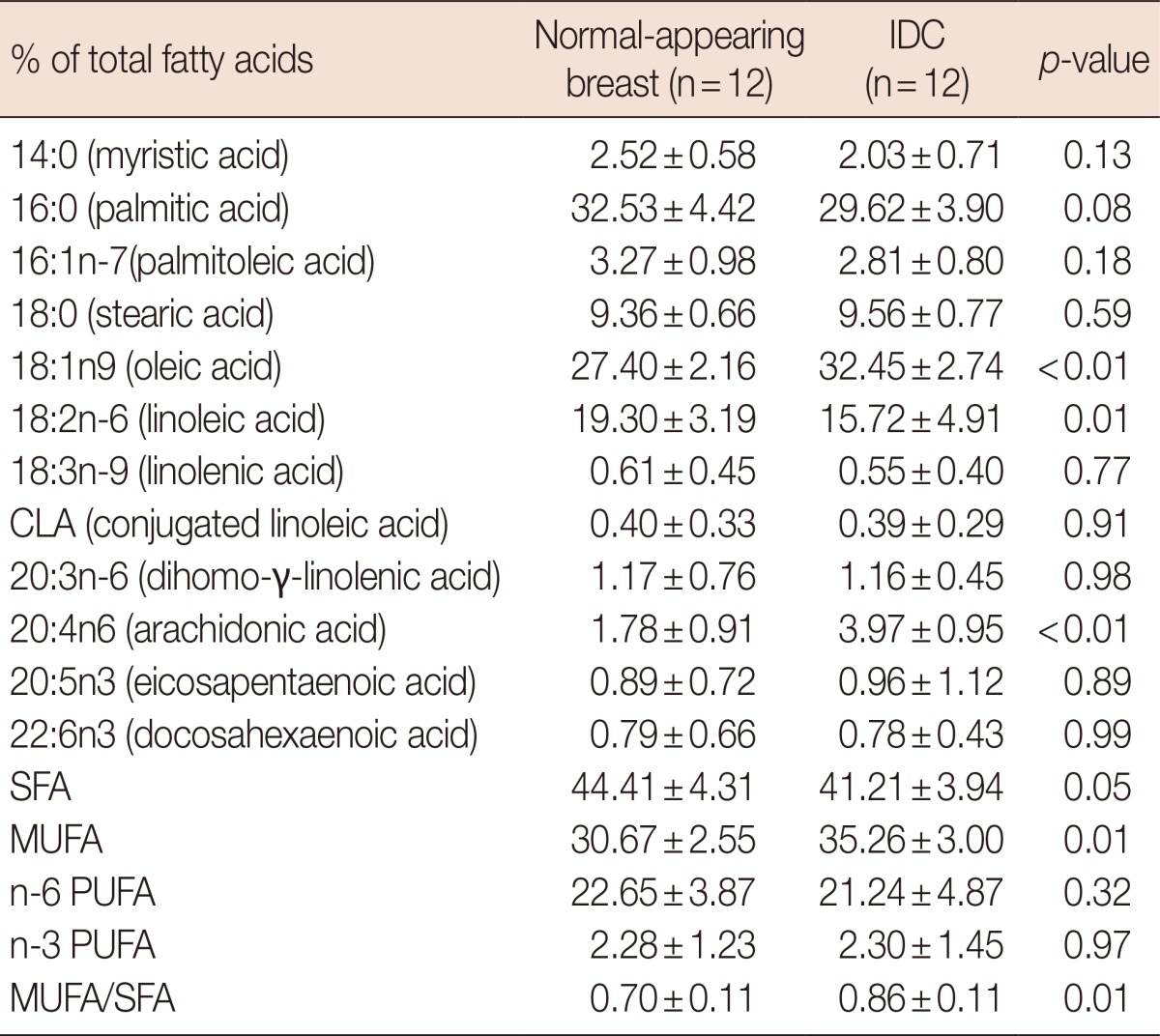
Explants were cultured for 48 hours. Lipid extracts were prepared and analyzed by gas liquid chromatography for a comprehensive fatty acid profile. Values are expressed as means±SD of three independent determinations.
IDC=infiltrating ductal carcinoma; SFA=saturated fatty acids; MUFA=monounsaturated fatty acids; PUFA=polyunsaturated fatty acids.
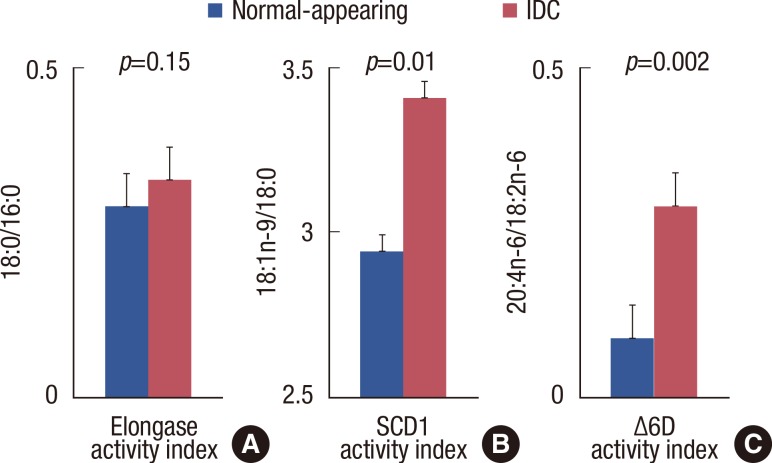
The observed values of the ratio of specific classes of fatty acids, which are regarded as indices of desaturation/elongation, in the cancerous and nonneoplastic breast tissues were compared, as shown in Figure 1. The ratio of 18:0/16:0 was calculated as an index for elongase activity. The 18:1n-9/18:0 and 20:4n-6/18:2n-6 ratios were calculated as indices of SCD1 and Δ6 fatty acid desaturase activity, respectively. The 18:1n-9/18:0 (p=0.01) and 20:4n-6/18:2n-6 (p=0.002) ratios were higher in the cancerous tissue (Figure 1). The latter effect was a result of a greater increase in the percentage of 20:4n-6 than in the percentage of 18:2n-6.
Figure 1.
Derived fatty acid indices of normal-appearing breast and infiltrating ductal carcinoma (IDC) tissue in the subject population. Explants were cultured for 48 hours. Lipid extracts were prepared and analyzed by gas liquid chromatography for a comprehensive fatty acid profile. (A) Elongase activity index (18:0/16:0). (B) Stearoyl-CoA desaturase 1 (SCD1) activity index (18:1n-9/18:0). (C) Δ6D activity index (20:4n-6/18:2n-6). Data are means±SD of three independent determinations, n=12. p, paired t-test.
Δ6D=Δ6 fatty acid desaturase; 16:0=palmitic acid; 18:0=stearic acid; 18:1n-9=oleic acid; 18:2n-6=linoleic acid; 20:4n-6=arachidonic acid.
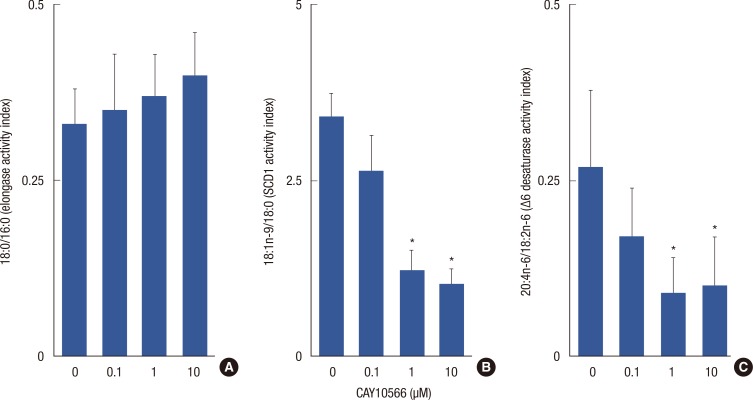
A 48-hour incubation of breast cancer tissue explants in vitro with different doses of CAY10566 resulted in significant alterations in fatty acid composition (data not shown). Figure 2 shows the effects of three doses of CAY10566 on the ratio of specific classes of fatty acids in the cancer tissue explants. While no significant changes were observed in the 18:0/16:0 ratio, the 18:1n-9/18:0 and 20:4n-6/18:2n-6 ratios decreased in explant tissues exposed to a dose of 1.0 or 10 µM (p<0.05) (Figure 2B and C).
Figure 2.
Effects of different doses of CAY10566 on derived fatty acid indices of infiltrating ductal carcinoma tissue. Explants were cultured for 48 hours. Lipid extracts were prepared and analyzed by gas liquid chromatography for a comprehensive fatty acid profile. (A) Elongase activity index (18:0/16:0). (B) Stearoyl-CoA desaturase 1 (SCD1) activity index (18:1n-9/18:0). (C) Δ6D activity index (20:4n-6/18:2n-6). Data are means±SD of three independent determinations, n=12.
Δ6D=Δ6 fatty acid desaturase; 16:0=palmitic acid; 18:0=stearic acid; 18:1n-9=oleic acid; 18:2n-6=linoleic acid; 20:4n-6=arachidonic acid.
*p<0.05 (CAY10566 treated vs. untreated explant) (Tukey's test, α=0.05).
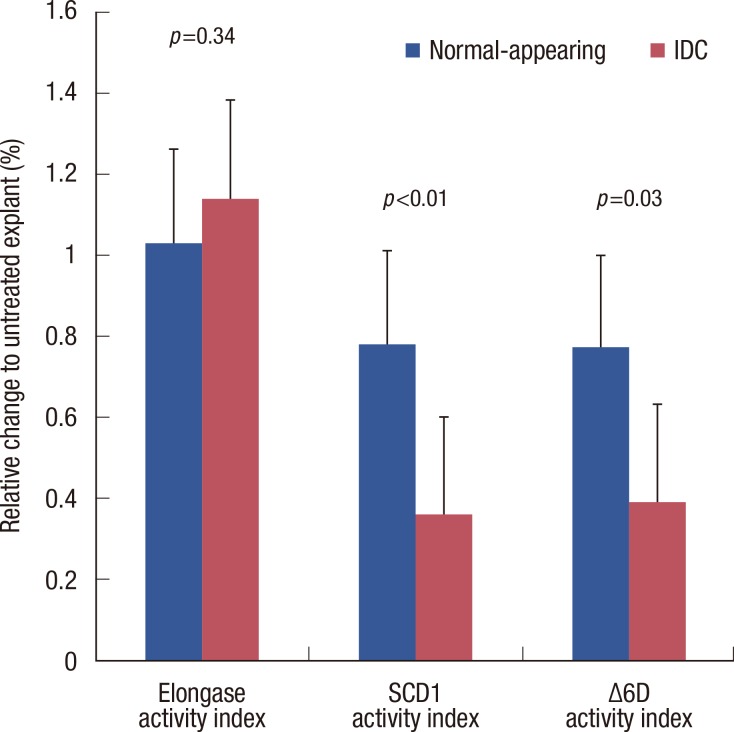
In the next set of experiments, we evaluated the effective dose of CAY10566 by comparing fatty acid variation between normal-appearing tissue explants and cancer tissue explants (Table 3). The MUFA/SFA ratio was significantly downregulated in breast cancer than in normal-appearing breast tissue (p<0.001). Specifically, the 18:1n-9/18:0 ratio showed a larger fold change in the breast cancer tissues than in the normal-appearing breast tissues (0.36 vs. 0.78, p<0.001). Moreover, the effective dose of CAY10566 produced a greater decrease in the 20:4n-6/18:2n-6 ratio in the breast cancer tissues (0.39 fold) as compared to the nonneoplastic breast tissues (0.77 fold, p=0.01) (Figure 3). In contrast, incubation with CAY10566 resulted in a significant upregulation of 18:2n-6 (approximately 1.2 fold) in normal-appearing breast and breast cancer tissues. However, no such change was observed for total PUFA in CAY10566-treated explants (Table 3).
Table 3.
Fatty acid changes in normal-appearing and infiltrating ductal carcinoma explants induced by the stearoyl CoA desaturase 1 inhibitor CAY10566
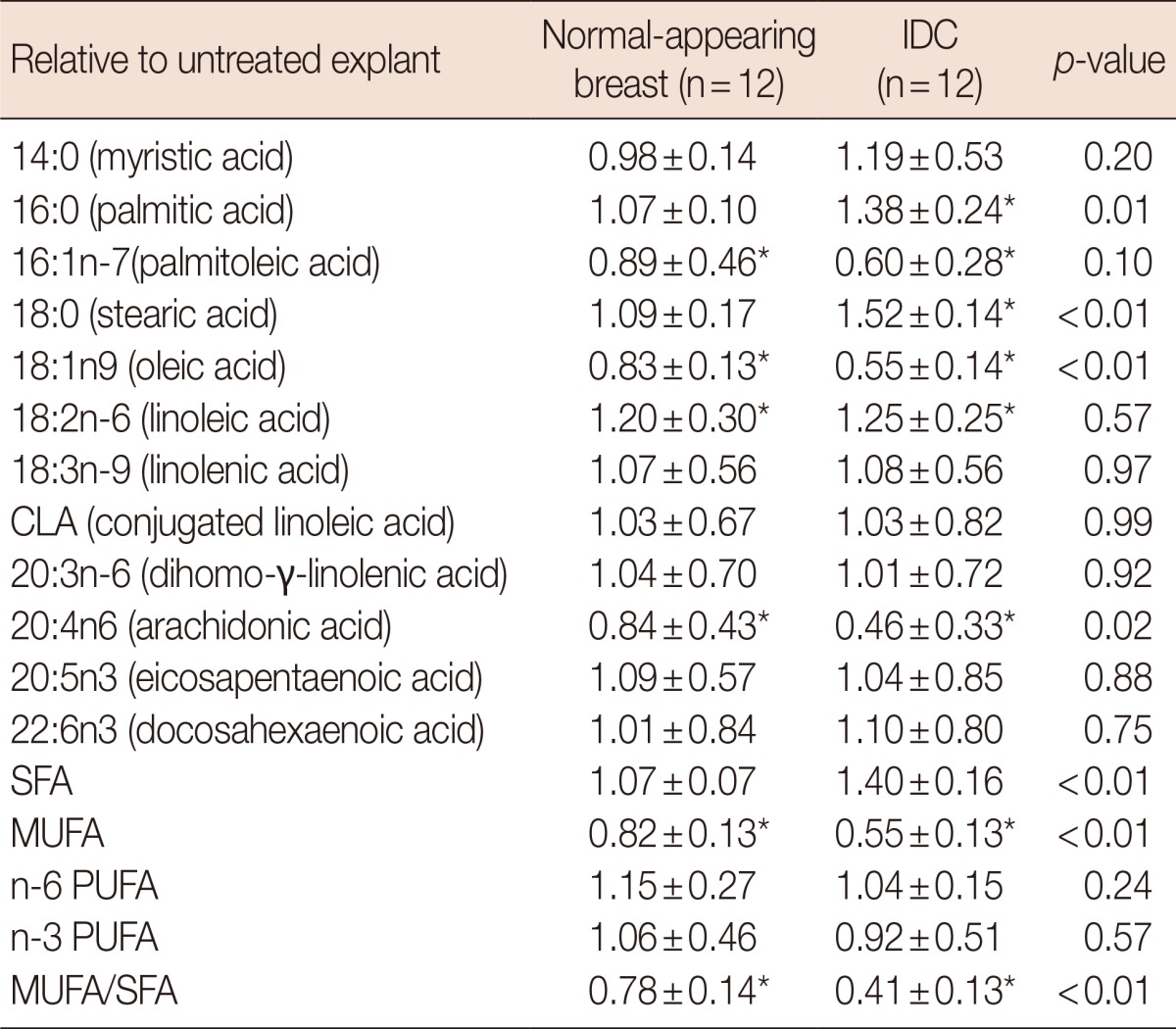
Explants were incubated with CAY10566 (1.0 µM) for 48 hours. Lipid extracts were prepared and analyzed by gas liquid chromatography for a comprehensive fatty acid profile. Values are expressed as means±SD of three independent determinations.
IDC=infiltrating ductal carcinoma; SFA=saturated fatty acids; MUFA=monounsaturated fatty acids; PUFA=polyunsaturated fatty acids.
*p<0.05 (CAY10566 treated vs. untreated explant).
Figure 3.
Percent of changes in derived fatty acid indices between normal-appearing and infiltrating ductal carcinoma (IDC) explants induced by CAY10566 (1.0 µM). Explants were incubated with CAY10566 (1.0 µM) for 48 hours. Lipid extracts were prepared and analyzed by gas liquid chromatography for a comprehensive fatty acid profile. Elongase activity index, 18:0/16:0; stearoyl-CoA desaturase 1 (SCD1) activity index, 18:1n-9/18:0; Δ6D activity index, 20:4n 6/18:2n 6. Data are means±SD of three independent determinations, n=12. p, paired t-test.
Δ6D=Δ6 fatty acid desaturase; 16:0=palmitic acid; 18:0=stearic acid; 18:1n-9=oleic acid; 18:2n-6=linoleic acid; 20:4n-6=arachidonic acid.
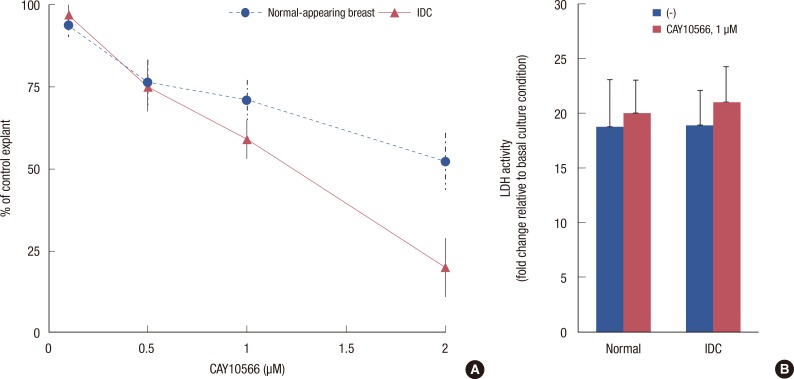
Cytotoxic assays showed a relatively high basal LDH activity, likely as a consequence of mechanical damage at the cut surface. However, concentrations of the SCD1 inhibitor at or below 1.0 µM did not cause a statistically significant increase in cytotoxicity compared to the untreated control (data not shown). The SRB growth assay revealed that the pharmacologic inhibition of SCD1 repressed the growth and proliferation of both explants in a dose-dependent fashion (Figure 4A). In our experiments, a concentration of 1.0 µM of CAY10566 elicited a higher inhibition rate in the breast cancer tissues than in the normal-appearing breast tissues (p<0.01, 41% vs. 29%). However, as shown in Figure 4B, the cytotoxic effects at the same dose (1.0 µM) did not differ between tumor explant cultures and normal-appearing breast explant cultures.
Figure 4.
Effect of stearoyl-CoA desaturase 1 inhibition on cell viability and growth between normal-appearing and infiltrating ductal carcinoma (IDC) explants. (A) Sulforhodamine B growth assay following treatment with the indicated doses (0-2.0 µM) of CAY10566 for 48 hours. (B) Lactate dehydrogenase (LDH) release induced by 1.0 µM CAY10566. Data are presented as fold induction (means±SD of three independent determinations, n=12) relative to the untreated explant.
DISCUSSION
SCD1 has been shown to be a critical factor in multiple biological functions, including the cell cycle and proliferation [12], and MUFA such as 16:1n-7 and 18:1n-9 are the immediate products of this enzyme. Previous studies reported that SCD1 inhibition causes cancer cell death by depleting 18:1n-9 in different types of cells [6]. These effects may constitute an underlying mechanism of the anti-growth effect of SCD1 inhibition in cancer cells [6,7,13]. In support of this hypothesis, SCD1 expression and activity is upregulated in several spontaneous and chemically induced tumors [5,14,15]. Our results demonstrated a considerably higher proportion of MUFA and 20:4n-6 in the human breast cancer tissues than in the normal appearing breast tissues. We also found that cellular fatty acid response to the pharmacologic inhibition of SCD1 is more prominent in breast cancer tissues than in normal-appearing breast tissue explants.
The response to SCD1 inhibition, as measured by the fold change in explant tissue fatty acids, is greater in the breast tumors, which are more sensitive to such inhibition, than in the normal-appearing breast tissues. This result did not appear to be caused by any nonspecific cytotoxic effects as indicated by the unchanged LDH leakage at the examined concentrations. Our findings confirm previous studies [16] showing that cancer cells are more likely to be dependent on endogenous fatty acid metabolism than nontransformed cells.
The increased conversion of SFA to MUFA, associated with increased SCD1 activity, is a common feature of tumors [12]. Minville-Walz et al. [16] have shown that the inhibition of SCD1 leads to the blocking of proliferation in both cancer and noncancer cells, but induces cell death only in cancer cells. It has been proposed that noncancer cells synthesize cellular membrane lipids mostly by uptake of exogenous fatty acids whereas cancer cells proliferate at a higher rate and need de novo fatty acid synthesis. SCD1 activity provides a constant MUFA supply, as well as precursors for de novo fatty acid modification, including chain elongation and desaturation that are essential for cell growth and proliferation.
In support of our findings, it has been reported that, while the levels of 18:2n 6 are significantly lower in breast cancer tissue, the levels of 20:4n 6 are significantly higher in breast cancer tissue than in grossly normal or interface tissues [17]. In the tumor tissue explants obtained from patients with IDC in our study, SCD1 inhibition induced a more pronounced effect on the tissue level of 20:4n-6. Furthermore, SCD1 inhibitor treatment remarkably downregulated the ratio of 20:4n 6/18:2n 6 in breast cancer tissue explants, as compared with normal-appearing tissue explants. Correspondingly, it has been shown that antisense oligonucleotide-mediated knockdown of SCD1 results in SFA and 18:2n-6 enrichment of macrophages in transgenic mice [18]. In a recent study, Pender-Cudlip et al. [19] showed that Δ6 fatty acid desaturase activity and prostaglandin E2, a pro-inflammatory 20:4n 6-derived eicosanoid, levels are higher in breast cancer tissue than in adjacent noncancerous tissue. These results imply that the apparent suppressive effect of SCD1 inhibition on 20:4n 6 in breast cancer tissue explants may be attributable to a positive regulation of the Δ6 desaturase pathway by augmented activity of SCD1 or its MUFA products in the tumor tissues of IDC patients. Consistent with this, SCD1 inhibition has recently been shown to elicit antisteatotic and anti-inflammatory effects similar to Δ6 desaturase inhibition in mice hepatocytes.
Although previous research has shown the beneficial effect of SCD1 inhibition in breast cancer, this is the first study to examine the effect of a highly selective SCD1 inhibitor on the fatty acid composition of a physiologically relevant human tissue explant system. In our study, we performed the LDH assay in conjunction with the SRB assay to assess cytotoxicity and growth, given their relatively high sensitivity and ease of application. To expand on the results of this study, apoptotic and proliferation pathways in relation to SCD1 expression levels and fatty acid status may be evaluated using primary breast explant cultures. Our results imply a key role for SCD1 in the modification of breast cancer fatty acid metabolism, by comparing the selective inhibition of desaturation in human IDC explant tissue to that in the corresponding normal-appearing breast tissue. Our findings along with the published mechanistic studies on SCD1 point to a central role for SCD1 in the development of breast tumors.
In conclusion, our study showed that the fatty acid composition and the response to SCD1 inhibition differed between the explant cultures from breast cancer tissues and the adjacent normal-appearing tissues. We speculate that the altered fatty acid composition induced by SCD1 inhibition may also, in addition to Δ9 desaturation, modulate other reactions in de novo fatty acid synthesis and lipogenesis, and subsequently affect breast cancer survival and progression.
ACKNOWLEDGMENTS
This study was conducted as part of a Master's thesis project no. 91/2-4/6 at the Tabriz University of Medical Sciences for the Fatemeh Mohammadzadeh under the supervision of Masoud Darabi. The research was financially supported by a grant to Masoud Darabi (research project number, 9182) from the Biotechnology Research Center of Tabriz University of Medical Sciences. The authors acknowledge the Faculty of Advanced Medical Sciences at Tabriz University of Medical Sciences for providing tissue culture laboratory facilities.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 2.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 3.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henley BJ, et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res. 2011;9:1551–1561. doi: 10.1158/1541-7786.MCR-11-0126. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Ding SF, Habib NA, Fermor BF, Wood CB, Gilmour RS. Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. Int J Cancer. 1994;57:348–352. doi: 10.1002/ijc.2910570310. [DOI] [PubMed] [Google Scholar]
- 6.Mason P, Liang B, Li L, Fremgen T, Murphy E, Quinn A, et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS One. 2012;7:e33823. doi: 10.1371/journal.pone.0033823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaglia N, Igal RA. Inhibition of stearoyl-CoA desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol. 2008;33:839–850. [PubMed] [Google Scholar]
- 8.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 9.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 10.Noori M, Darabi M, Rahimipour A, Rahbani M, Abadi NA, Darabi M, et al. Fatty acid composition of HDL phospholipids and coronary artery disease. J Clin Lipidol. 2009;3:39–44. doi: 10.1016/j.jacl.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 12.Igal RA. Roles of stearoylCoA desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers (Basel) 2011;3:2462–2477. doi: 10.3390/cancers3022462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess D, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS One. 2010;5:e11394. doi: 10.1371/journal.pone.0011394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verschoor ML, Verschoor CP, Singh G. Ets-1 global gene expression profile reveals associations with metabolism and oxidative stress in ovarian and breast cancers. Cancer Metab. 2013;1:17. doi: 10.1186/2049-3002-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thai SF, Allen JW, DeAngelo AB, George MH, Fuscoe JC. Detection of early gene expression changes by differential display in the livers of mice exposed to dichloroacetic acid. Carcinogenesis. 2001;22:1317–1322. doi: 10.1093/carcin/22.8.1317. [DOI] [PubMed] [Google Scholar]
- 16.Minville-Walz M, Pierre AS, Pichon L, Bellenger S, Fèvre C, Bellenger J, et al. Inhibition of stearoyl-CoA desaturase 1 expression induces CHOP-dependent cell death in human cancer cells. PLoS One. 2010;5:e14363. doi: 10.1371/journal.pone.0014363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azordegan N, Fraser V, Le K, Hillyer LM, Ma DW, Fischer G, et al. Carcinogenesis alters fatty acid profile in breast tissue. Mol Cell Biochem. 2013;374:223–232. doi: 10.1007/s11010-012-1523-4. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pender-Cudlip MC, Krag KJ, Martini D, Yu J, Guidi A, Skinner SS, et al. Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue. Cancer Sci. 2013;104:760–764. doi: 10.1111/cas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]