Abstract
Purpose
To test whether the risk of age-related macular degeneration (AMD) decreases with vigorous physical activity.
Methods
This was a prospective study of self-reported clinically diagnosed macular degeneration in male (n=29,532) and female (n=12,176) runners followed prospectively for 7.7 years. Survival analyses of incident AMD versus average running distance (kilometers per day), cardiorespiratory fitness (10-km footrace performance), body mass index (BMI), cigarette use, and diet at baseline.
Results
The 110 men and 42 women reporting incident AMD were older than those unaffected (mean ± SE: 54.81 ± 0.97 vs. 44.86 ± 0.06 years), and the men were significantly more likely to have once smoked cigarettes (50.6 vs. 41.2%, P = 0.04 when adjusted for age). Age-and sex-adjusted AMD risk was greater in the men and women who consumed more meat (3.17 ± 0.20 vs. 2.55 ± 0.02 servings/wk) and less fruit (9.41 ± 0.70 vs. 10.92 ± 0.05 pieces/wk). The men and women reporting incident AMD ran for exercise significantly less than those who remained unaffected, when adjustment was made for age and sex (4.57 ± 0.30 vs. 5.34 ± 0.02 km/d, P ≤ 0.01). When adjusted for age, sex, diet, and smoking history, the relative risk for AMD decreased 10% per km/d increment in running distance. Moreover, compared with the men and women who averaged less than 2 km/d, those averaging 2 to 4 km/d had 19% lower adjusted risk, and those averaging ≥4 km/d had 42% to 54% lower adjusted AMD risk.
Conclusions
Higher doses of vigorous exercise (running) are associated with lower incident AMD risk independent of weight, cardiorespiratory fitness, and cigarette use. Limitations of the analyses include the select nature of the sample and reliance on self-report of both running history and clinically diagnosed AMD.
Age-related macular degeneration (AMD) is the leading cause of irreversible visual loss in older white Americans [1], affecting approximately 2% of Americans 52 to 64 years of age and 28% of those ≥75 years of age [2]. The wet (exudative or neovascular) form of macular degeneration, which represents approximately 90% of AMD vision loss, occurs when bleeding or leaking of blood from vessels from the choroids penetrate the retina [3]. Dry (nonexudative) AMD is more common but progresses more slowly and represents approximately 10% of severe vision loss [3] Early AMD is characterized by changes in macular retinal pigment epithelium or the presence of drusen. Given the limited potential for restoring vision loss, attention has focused on early recognition and treatment to prevent progression.
Exercise is not currently advocated for AMD prevention [4], although at least three studies suggest its possible role in lowering AMD risk [5-7]. Physical activity lowers cardiovascular disease [8], which is possibly predictive of AMD [9 –12]. In addition, physical activity improves various cardiovascular disease risk factors that have also been associated with AMD risk (albeit not always consistently so): adiposity [13-16], and blood pressure [17-20].
This report examines the relationship of incident self-reported, clinically diagnosed AMD during 7.7 years of follow-up of the National Runners’ Health Study. This cohort is unique among prospective epidemiologic studies in being specifically recruited to assess the health benefits and burdens of physical activity. Population- or occupationally-based cohorts tend to include few vigorously active individuals, which limits the ability to draw inferences on vigorous exercise and health. Although runners may not be representative of the population in general, the physiological processes affecting macular degeneration in runners are likely to be the same as in nonrunners. Moreover, the health benefits of greater doses of more vigorous exercise are relevant to the one-half of all adult Americans who already meet or exceed current physical activity guideline levels [21].
METHODS
The design and methods of the National Runners’ Health Study are described elsewhere [22-10]. Briefly, this cohort of runners, 18 years old and older, was recruited between 1991 and 1993 by distributing a two-page questionnaire nationally to runners identified through subscription lists to running magazines and among participants of foot race events. Approximately 15% returned baseline questionnaires among the total number contacted (the exact number is not known because of uncertainty of the number actually distributed and the proportion of subjects who receive duplicate questionnaires). The questionnaire solicited information on demographics, running history, weight history, smoking habits, prior history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, and diabetes. Eighty percent of the original cohort provided follow-up surveys to us 7 years later or were known dead. The study protocol was approved by the University of California Berkeley Committee for the Protection of Human Subjects, and all participants signed committee-approved informed consents, in accordance with the Declaration of Helsinki.
Participants reported whether they had received a clinical diagnosis of macular degeneration since their baseline questionnaire and provided the year of diagnosis. Subjects reporting being diagnosed in the same year as their baseline survey or before were excluded from the analyses. Running distances were reported in usual miles run per week at baseline. There were strong correlations between repeated questionnaires for self-reported running distance (r = 0.89), and self-reported running distance has been shown to be significantly associated with a number of biomarkers traditionally associated with physical activity, including HDL-cholesterol, triglycerides, and LDL cholesterol concentrations, systolic and diastolic blood pressures, fasting plasma glucose concentrations, BMI, and body circumferences [28]. Although other leisure-time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was added to the survey) show that running represents (mean ± SD) 91.5% ± 19.1% and 85.2% ± 24.0% of all vigorously intense activity in men and women, respectively, and 73.5% ± 23.7% and 69.4% ± 25.7% of total leisure-time physical activity, respectively.
BMI was calculated as self-reported weight in kilograms divided by the square of self-reported height in meters. Self-reported waist circumferences were elicited by the question, “Please provide, to the best of your ability, your body circumference in inches.” without further instruction. There are strong correlations between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) [29], and for self-reported running distances versus self-reported BMIs and waist circumferences in cross-sectional analyses [28,30]. Self-reported waist circumferences are somewhat less precise, as indicated by their correlations with reported circumferences on a second questionnaire (r = 0.84) and with their clinical measurements (r = 0.68) [29]. Intakes of meat, fish, and fruit were based on the questions: “During an average week, how many servings of beef, lamb, or pork do you eat,” “...servings of fish do you eat,” and “...pieces of fruit do you eat?” Alcohol intake was estimated from the corresponding questions for 4-oz. (112 mL) glasses of wine, 12-oz. (336 mL) bottles of beer, and mixed drinks and liqueurs. Alcohol was computed as 10.8 g per 4-oz glass of wine, 13.2 g per 12 oz. bottle of beer, and 15.1 g per mixed drink. Correlations between these responses and values obtained from 4-day diet records in 110 men were r = 0.65 for alcohol intake, r = 0.46 for red meat, r = 0.38 for fruit, and r = 0.19 for fish. These values agree favorably with published correlations between food records and more extensive food frequency questionnaires for red meat (r =0.50), wine (r = 0.66), beer (r = 0.70), and mixed drinks (r = 0.72), somewhat less favorably for fruit intake (r = 0.50), and less favorably for fish intake (r = 0.51) [31]. For this report, baseline cardiorespiratory fitness was defined as speed in meters per second of the participant’s best time in a 10-km race during the previous 5 years (reported as finishing time in minutes). Published data support the use of running performance to approximate maximum oxygen consumption (VO2max) [32–34].
Statistical Analyses
The Cox proportional hazards model (JMP software version 5.0; SAS Institute, Cary, NC) was used to estimate the dose–response relationships of incident AMD to baseline running distance, cardiorespiratory fitness, body weight, and circumferences. Reported weekly intakes of alcohol, meat, fish, and fruit, age, and BMI were used as covariates when analyzing physical activity. Subjects who were diabetic at baseline were excluded from all analyses.
RESULTS
There were 110 men and 42 women who reported receiving a clinical diagnosis of AMD during 7.7 years of follow-up (Table 1). Those reporting AMD were older than those unaffected (Table 1), and the men were significantly more likely to have once smoked cigarettes (i.e., percentage of current and past smokers in diagnosed versus nondiagnosed: 50.6% vs. 41.2%, P = 0.04 when adjusted for age, analyses not displayed). The statistically greater use of hypertensive medications and slower 10-km race performance times for the men and women who developed AMD (Table 1) appeared to be due to their older age, since the significance of these effects were lost when adjusted for age in addition to sex. Incident AMD was significantly associated with age-and sex-adjusted greater meat intake and lower fruit intake. AMD risk was unrelated to baseline dose of vitamin C (P = 0.32) and vitamin E intake (P = 0.89, analyses not displayed). Table 1 shows that the men and women who developed AMD ran on average 1 km per day less than those who remain undiagnosed without age adjustment (P ≤ 0.0001), and about three quarters of a kilometer per day less after adjustment for age and sex (P = 0.01).
TABLE 1.
Baseline characteristics of 29,532 men and 12,176 women by incident AMD status
| Unadjusted | Age-and Sex-Adjusted | |||
|---|---|---|---|---|
| Incident AMD | Present | Absent | Present | Absent |
| Sample (N) | 152 | 41,556 | 152 | 41,556 |
| Female (%) | 27.63 | 29.20 | ||
| Age (y) | 54.22 ± 0.92 | 43.09 ± 0.05* | ||
| Follow-up duration (y) | 8.02 ± 0.14 | 7.66 ± 0.01† | 8.31 ± 0.16 | 7.68 ± 0.01* |
| Education (y) | 16.23 ± 0.20 | 16.34 ± 0.01 | 16.48 ± 0.20 | 16.57 ± 0.01 |
| Running distance (km/day) | 4.34 ± 0.23 | 5.33 ± 0.02* | 4.60 ± 0.26 | 5.28 ± 0.02‡ |
| Years of running | 13.77 ± 0.61 | 12.25 ± 0.04† | 11.50 ± 0.59 | 12.32 ± 0.04 |
| 10-km performance (m/s) | 3.51 ± 0.05 | 3.82 ± 0.00* | 3.76 ± 0.04 | 3.82 ± 0.00 |
| BMI (kg/m2) | 23.23 ± 0.24 | 23.11 ± 0.01 | 23.38 ± 0.20 | 23.34 ± 0.01 |
| Waist circumference (cm) | 82.19 ± 0.83 | 79.95 ± 0.05‡ | 81.16 ± 0.51 | 80.16 ± 0.04† |
| Ever smoked (%) | 53.95 | 36.38* | 45.23 | 39.12 |
| Meat (servings/wk) | 2.94 ± 0.21 | 2.52 ± 0.01† | 3.17 ± 0.20 | 2.55 ± 0.02‡ |
| Fish (servings/wk) | 1.67 ± 0.12 | 1.46 ± 0.01 | 1.48 ± 0.11 | 1.47 ± 0.01 |
| Fruit (pieces/wk) | 10.69 ± 0.68 | 11.17 ± 0.04 | 9.41 ± 0.70 | 10.92 ± 0.05† |
| Alcohol (g/day) | 11.07 ± 1.39 | 8.89 ± 0.06† | 1.02 ± 0.10 | 0.91 ± 0.01 |
| Hypertensive medications (%) | 9.21 | 3.34* | 4.49 | 2.55 |
| Lipid-lowering medication (%) | 3.95 | 1.92 | 1.65 | 1.61 |
Data are the mean ± SE. Significance of difference by analysis of covariance:
P ≤ 0.0001;
P ≤ 0.05;
P ≤ 0.01.
Table 2 presents the survival analyses of running distance versus incident AMD adjusted for age, prior smoking, hypertension, and intakes of meat, fish, fruit, and alcohol. Each kilometer per day increment in running distance was associated with a 10% reduction in risk with or without adjustment for BMI (P = 0.01). Comparable results were obtained when the analyses were repeated in the subset of men and women who had provided their best 10-km performance. Age adjusted running distance and 10-km performance correlated moderately in both men (r = 0.44) and women (r = 0.41). Adjustment for 10-km performance (a measure of cardiorespiratory fitness) had no effect on the relative risk for AMD per km/d run, and AMD risk was unrelated to cardiorespiratory fitness with (P = 0.42, Table 1) or without adjustment for km/d run (P = 0.91, not displayed).
TABLE 2.
Relative risk per kilometer/day run (95% Confidence Interval) from Cox proportional hazards analyses of AMD in 41,708 men and women
| All Runners | Runners Reporting Their 10-km Performance Times* | ||
|---|---|---|---|
| Relative Risk for Physical Activity (km/day) | Relative Risk for Physical Activity (km/day) | Relative Risk for Cardiorespiratory Fitness (m/s) | |
| Without BMI adjustment | 0.90 | 0.90 | 0.96 |
| (0.84-0.96) | (0.84-0.97) | (0.63-1.43) | |
| P = 0.002 | P = 0.006 | P = 0.83 | |
| BMI adjusted | 0.90 | 0.90 | 0.92 |
| (0.83-0.97) | (0.83-0.97) | (0.60-1.39) | |
| P = 0.003 | P = 0.005 | P = 0.69 | |
Adjusted for age, sex, smoker status, and intakes of meat, fish, fruit, and alcohol.
34,035 men and women provided 10-km performance times (i.e., cardiorespiratory fitness).
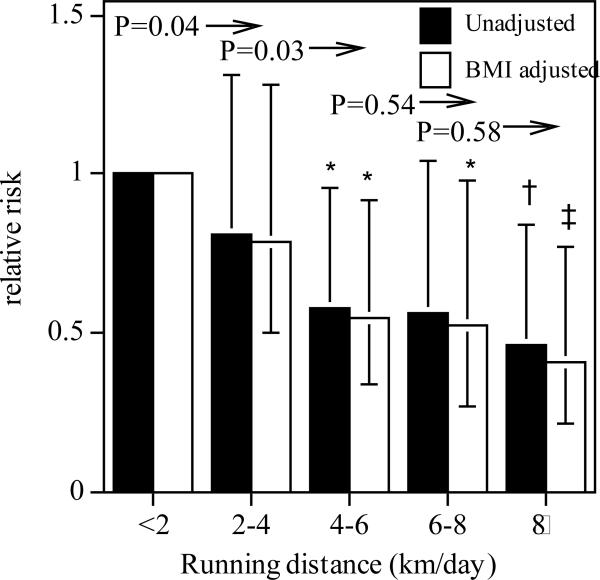
Figure 1 presents the relative risk for macular degeneration by average running distance. Compared to running under 2 km/d, those who exceeded 4 km/d were generally at significantly lower risk for macular degeneration, even when adjusted for BMI. The relationship appears convex, with the risk reduction largely occurring for progressive increments in running distance through 4 km/d. Specifically, the risk was significantly lower in the men and women who ran ≥2 km/d than in those who ran less (P = 0.04) and was significantly lower in those who ran ≥4 km/d than in those who ran 2 to 4 km/d (P = 0.03). The men and women who ran an average of 8 km/d or more had 54% less risk of macular degeneration compared to those averaging <2 km per day (59% lower risk when adjusted for BMI).
FIGURE 1.
Relative risk of AMD by average distance run per day in 41,708 nondiabetic, nonsmoking men and women. All results are adjusted for age (age and age2), sex, and weekly intakes of meat, fish, fruit, and alcohol, with additional adjustment for BMI where indicated. Significant risk reductions relative to the least active men: *P ≤ 0.05, †P ≤ 0.01, and ‡P ≤ 0.001. Significance levels to the left of arrows are for all greater distances run vs. the distance run under the significance level.
Additional analyses were performed excluding younger individuals for whom the risk for macular degeneration was low and in males and females separately. Excluding men and women < 40 years of age yielded essentially the same results as the entire sample. There were 136 reported diagnoses of macular degeneration in the 25,335 men and women who were 40 years old or older at baseline. The relative risk for macular degeneration per kilometer/day in this older subset was 0.94 (P = 0.04), with a 95% confidence interval of (0.88 to 0.97). The relative risk per kilometer/day run was not significantly different (P = 0.63) between the men (relative risk = 0.91, P = 0.01 when analyzed separately) and the women (relative risk = 0.94; [0.84, 1.05], P = 0.29 when analyzed separately). The effect of distance run on AMD risk was not affected by age (i.e., significance of interaction, P = 0.12), alcohol intake (P = 0.58), smoking, (P = 0.63), or the intakes of meat (P = 0.18), fish (P = 0.62), or fruit (P = 0.17).
DISCUSSION
These analyses demonstrate in a large cohort of men and women that greater doses of vigorous physical activity were associated with lower age-adjusted AMD risk. Running an average of 2 or more kilometer/day significantly reduced the risk by 36%, and running >8 km/d reduced the risk by 54%, compared to running <2 km/d. These age-adjusted risk reductions could not be explained by BMI, intakes of meat, fish, fruit, or alcohol, or smoking history.
Three other studies have reported on the association between physical activity and AMD. Patients with exudative AMD were reported to be less physically active in the Eye Disease Case Control Study but the difference failed to achieve statistical significance [7]. In the Beaver Dam Eye Study [6], physical activity was assessed from city blocks walked per day, flights of stairs climbed per day, and sweat-inducing bouts of exercise per week. Stair climbing was associated with lower age-related risk but not when adjusted for covariates. City blocks walked, and three or more bouts per week of sweat-inducing exercise reduced the risk for exudative AMD by 30% and 70%, respectively. Neither early AMD nor geographic atrophy was related to physical activity in the Beaver Dam Eye Study. Seddon et al. [5] reported that when compared to no vigorous physical activity, three sessions per week of vigorous activity was associated with a 25% reduction in the progression to geographic atrophy and neovascular disease during 4.6 years of follow-up in 261 patients with early or intermediate AMD.
The mechanisms relating physical activity to macular degeneration are not known. A key feature separating running's effect on AMD from running's other health effects are: (1) the risk reduction was independent of adiposity as measured by both BMI or waist circumference, and (2) the risk reduction was independent of cardiorespiratory fitness as measured by 10-km performance. Others have reported BMI or waist circumference–related [13–16] or unrelated [12,16] to AMD risk. The current results are not necessarily inconsistent with those reporting a relationship, given that there may be a J-shaped relationship between BMI and AMD risk [14.35], and the runners are considerably leaner than other studied populations [27]. Earlier papers from the National Runners’ Health Study have reported that significant portions of the reductions in hypertension, diabetes, or hypercholesterolemia risk per kilometers/ week run were attributable to the runners’ leanness [24]. Moreover, reductions in incident hypertension, diabetes, and hypercholesterolemia were more strongly related to baseline cardiorespiratory fitness than to distance run [24]. Thus, the physiological mechanisms responsible for lower AMD risk may be different from those that reduce hypertension, cholesterol, and diabetes risk in runners.
Several studies report that higher plasma concentrations of high-density lipoprotein (HDL) concentrations predict AMD [36 –38] risk and the risk for soft drusen [39], which would not explain the reduction in AMD risk with physical activity reported herein, or the AMD risk reduction associated with adiposity reported by others. It has been shown in these studies that men's plasma HDL-cholesterol concentrations increase significantly in relation to the distance run through at least 64 km/wk [28]. Exercise-induced increases in HDL-cholesterol have also been demonstrated in training studies [40]. Moreover, innate high HDL-cholesterol may predispose individuals to run longer distances [41]. However, the effects of exercise on HDL-cholesterol concentrations are mediated to a large extent by low body fat or weight loss [42], whereas AMD showed no relation to body weight in the present study. Others report that HDL-cholesterol concentrations show no relationship to AMD [43].
The pathogenesis of AMD may also involve C-reactive protein (CRP), a marker of nonspecific inflammation. The evidence relating AMD to CRP is mixed, with some studies showing an association [44–46] and others not [47– 49]. Although endurance trained individuals have lower CRP levels than do sedentary individuals [50], CRP shows a stronger inverse relationship to cardiorespiratory fitness than physical activity [51,52], whereas AMD was unrelated to 10-km performance (Table 2).
With respect to other variables, there was no relationship between macular degeneration and cholesterol-lowering medication use, which is consistent with prevailing opinion from other studies [53]. There was also no relationship between vitamin C or E supplements and incident macular degeneration, which agrees with the recent meta-analysis of nine prospective cohort studies and three randomized controlled clinical trials showing no evidence that dietary antioxidants prevented AMD [53]. The significant relationship between cigarette use and AMD is consistent with other accounts [9]. Others have shown that current smokers are at a higher risk for AMD than are nonsmokers or past smokers [54 –56]. However, the analyses of Table 1 grouped current smokers with ex-smokers because the 608 current smokers (1.5% of the sample, of whom only two developed AMD) provided insufficient statistical power for assessing their separate relationship to AMD. Diets characterized by greater intake of meat and less fruit consumption may reflect in part various nutrients others have associated with AMD risk, including total [57,58] and saturated [59] fat intake and antioxidant carotenoids (lutein and zeaxanthin) found in dark green or yellow vegetables [60]. An absence of heavy drinkers may explain the failure to find an association between AMD and alcohol intake [61].
The primary limitation of these analyses is that they are based on self-report. The proportion of subjects who received eye examination to properly assess macular degeneration is not known. In addition to being less reliable than clinically verified diagnoses, the self-report provides no information on whether the macular degeneration was exudative (wet) or nonexudative (dry), or early or late stage, which could affect their etiology. A sedentary control group was also not included for comparison with the runners. There were 14,225 men and 3,118 women aged 43 years of age or older whose incident rate for macular degeneration was 0.81 and 1.26 per 1000 person-years of follow-up, respectively. This incidence for AMD is less than reported for the Beaver Dam Study, which may be due to the better eye health or the less-complete ADM ascertainment of the runners’ sample. Based on data reported by others, it seems unlikely that the observed differences in self-reported clinically diagnosed macular degenerations were due to less frequent clinical visits among leaner, higher mileage runners. Schaumberg et al. [62] report that leaner women tend to have more frequent eye examinations than do heavier women. The Health Professionals Study reported that their more vigorously active participants had more routine medical check-ups than did less active men [63], and there was no difference in routine medical check-up by activity level in the Nurses’ Health Study [64]. Although the prospective design specifically excluded individuals with preexisting macular degeneration from being counted as incident events, visual impairment associated with undiagnosed AMD may affect running distance, although there is no direct evidence of this. Only 15% of the targeted population of runners provided baseline surveys; however, the goal was not to obtain a purely representative sample of runners but rather to obtain a sufficient number of participants to assess prospectively the associations between running and incident disease.
In summary, these data suggest that higher doses of vigorous physical activity were related to a lower risk of self-reported, clinically diagnosed AMD. The average distance run in the least active group of runners (<2 km/d) is the energy equivalent of 30 minutes of walking briskly 5 days per week. This is the minimum physical activity level currently recommended by the Centers for Disease Control, the American Heart Association, and the American College of Sports Medicine [21,65]. Figure 1 shows a 19% risk reduction in men who exercised at twice the recommended minimum guideline levels, and a 42% reduction in those whose weekly exercise was threefold higher. Although there are important health benefits in meeting the guideline levels compared with being sedentary, these data suggest that lower AMD risk is yet another benefit to exercising substantially more than the minimum recommended (i.e., in addition to lower risks for incident cataracts [23], diabetes [24], hypertension [24], hypercholesterolemia [24], benign prostate hyperplasia [22], gout [25], gallbladder disease [26], and stroke [66].
References
- 1.Seddon JM, Chen CA. The epidemiology of age-related macular degeneration. Int Ophthalmol Clin. 2004;44:17–39. doi: 10.1097/00004397-200404440-00004. [DOI] [PubMed] [Google Scholar]
- 2.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glucoma, diabetic retinopathy, macular degeneration, and visual acuity in general population of 2631 adults. 1973–1975. Surv Ophthalmol. 1980;24(suppl):335–610. [PubMed] [Google Scholar]
- 3.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Guymer RH, Chong EW. Modifiable risk factors for age-related macular degeneration. Med J Aust. 2006;184:455–458. doi: 10.5694/j.1326-5377.2006.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 5.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785–792. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 6.Knudtson MD, Klein R, Klein BEK. Physical activity and the 15-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Br J Ophthalmol. 2006;90:1461–1463. doi: 10.1136/bjo.2006.103796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Eye Disease Case-Control Study Group Risk factors for neovascular age related macular degeneration. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1992;110:1701–1708. doi: 10.1001/archopht.1992.01080240041025. [DOI] [PubMed] [Google Scholar]
- 8.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan JS, Mitchell P, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2007;114:1143–1150. doi: 10.1016/j.ophtha.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Deng Y, Klein BE, et al. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women's Health Initiative Sight Exam ancillary study. Am J Ophthalmol. 2007;143:473–483. doi: 10.1016/j.ajo.2006.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaine G, Hullo A, Sahel J, et al. Case-control study of the risk factors for age related macular degeneration. France-DMLA Study Group. Br J Ophthalmol. 1998;82:996–1002. doi: 10.1136/bjo.82.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vingerling JR, Dielemans I, Bots ML, et al. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol. 1995;142:404–409. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 13.Smith W, Mitchell P, Leeder SR, et al. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:583–587. doi: 10.1001/archopht.116.5.583. [DOI] [PubMed] [Google Scholar]
- 14.Schaumberg DA, Christen WG, Hankinson SE, et al. Body mass index and the incidence of visually significant age-related maculopathy in men. Arch Ophthalmol. 2001;119:1259–1265. doi: 10.1001/archopht.119.9.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein BE, Klein R, Lee KE, et al. Measures of obesity and age-related eye diseases. Ophthalmic Epidemiol. 2001;8:251–262. doi: 10.1076/opep.8.4.251.1612. [DOI] [PubMed] [Google Scholar]
- 16.Moeini HA, Masoudpour H, Ghanbari H. A study of the relation between body mass index and the incidence of age related macular degeneration. Br J Ophthalmol. 2005;89:964–966. doi: 10.1136/bjo.2005.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg J, Flowerdew G, Smith E, et al. Factors associated with age-related macular degeneration: an analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol. 1988;128:700–710. doi: 10.1093/oxfordjournals.aje.a115023. [DOI] [PubMed] [Google Scholar]
- 18.Vidaurri JS, Pe'er J, Halfon ST, et al. Association between drusen and some of the risk factors for coronary artery disease. Ophthalmologica. 1984;188:243–247. doi: 10.1159/000309370. [DOI] [PubMed] [Google Scholar]
- 19.Hyman L, Schachat AP, He Q, et al. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 20.Fraser-Bell S, Wu J, Klein R, et al. Cardiovascular risk factors and age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;145:308–316. doi: 10.1016/j.ajo.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 22.Williams PT. Effects of running distance and performance on incident benign prostatic hyperplasia. Med Sci Sports Exer. 2008;40:1733–1739. doi: 10.1249/MSS.0b013e31817b8eba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams PT. Prospective epidemiological cohort study of reduced risk of incident cataract with vigorous physical activity and cardiorespiratory fitness during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2008;49:95–100. doi: 10.1167/iovs.08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams PT. Vigorous exercise, fitness and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.William PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr. 2008;87:1480–1487. doi: 10.1093/ajcn/87.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams PT. Independent effects of cardiorespiratory fitness, vigorous physical activity, and body mass index on clinical gallbladder disease risk. Am J Gastroenterol. 2008;103:2239–2247. doi: 10.1111/j.1572-0241.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams PT, Hoffman K, La I. Weight-related increases in hypertension, hypercholesterolemia, and diabetes risk in normal weight male and female runners. Arterioscler Thromb Vasc Biol. 2007;27:1811–1819. doi: 10.1161/ATVBAHA.107.141853. [DOI] [PubMed] [Google Scholar]
- 28.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners: The National Runners’ Health Study. Arch Intern Med. 1997;157:191–198. [PMC free article] [PubMed] [Google Scholar]
- 29.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obes Relat Metab Disord. 2004;28:120–128. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 30.Williams PT, Pate RR. Cross-sectional relationships of exercise and age to adiposity in 60,617 male runners. Med Sci Sports Exerc. 2005;37:1329–1337. doi: 10.1249/01.mss.0000174894.05236.45. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 32.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:875–888. [PubMed] [Google Scholar]
- 33.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann NY Acad Sci. 1977;301:484–494. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 34.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–204. [PubMed] [Google Scholar]
- 35.Smith W, Mitchell P, Leeder SR, Wang JJ. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:583–587. doi: 10.1001/archopht.116.5.583. [DOI] [PubMed] [Google Scholar]
- 36.Klein R, Klein BE, Knudtson MD, et al. Subclinical atherosclerotic cardiovascular disease and early age-related macular degeneration in a multiracial cohort: the Multiethnic Study of Atherosclerosis. Arch Ophthalmol. 2007;125:534–543. doi: 10.1001/archopht.125.4.534. [DOI] [PubMed] [Google Scholar]
- 37.van Leeuwen R, Klaver CC, Vingerling JR, et al. Cholesterol and age-related macular degeneration: is there a link? Am J Ophthalmol. 2004;137:750–752. doi: 10.1016/j.ajo.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 39.Delcourt C, Michel F, Colvez A, Lacroux A, Delage M, Vernet MH, POLA Study Group Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8:237–249. doi: 10.1076/opep.8.4.237.1613. [DOI] [PubMed] [Google Scholar]
- 40.Wood PD, Stefanick ML, Dreon DM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 41.Williams PT, Stefanick ML, Vranizan KM, Wood PD. The effects of weight loss by exercise or by dieting on plasma high-density lipoprotein (HDL) levels in men with low, intermediate, and normal-to-high HDL at baseline. Metabolism. 1994;43:917–924. doi: 10.1016/0026-0495(94)90277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams PT. Weight set-point theory and the high-density lipoprotein concentrations of long-distance runners. Metabolism. 1990;39:460–467. doi: 10.1016/0026-0495(90)90003-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abalain JH, Carre JL, Leglise D, et al. Is age-related macular degeneration associated with serum lipoprotein and lipoparticle levels? Clin Chim Acta. 2002;326:97–104. doi: 10.1016/s0009-8981(02)00288-7. [DOI] [PubMed] [Google Scholar]
- 44.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291:704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 45.Vine AK, Stader J, Branham K, Musch DC, Swaroop A. Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology. 2005;112:2076–2080. doi: 10.1016/j.ophtha.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 47.Klein R, Klein BE, Knudtson MD, Wong TY, Shankar A, Tsai MY. Systemic markers of inflammation, endothelial dysfunction, and age-related maculopathy. Am J Ophthalmol. 2005;140:35–44. doi: 10.1016/j.ajo.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 48.Klein R, Klein BE, Marino EK, et al. Early age-related maculopathy in the cardiovascular health study. Ophthalmology. 2003;110:25–33. doi: 10.1016/s0161-6420(02)01565-8. [DOI] [PubMed] [Google Scholar]
- 49.McGwin G, Hall TA, Xie A, Owsley C. The relation between C reactive protein and age related macular degeneration in the Cardiovascular Health Study. Br J Ophthalmol. 2005;89:1166–1170. doi: 10.1136/bjo.2005.067397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomaszewski M, Charchar FJ, Przybycin M, et al. Strikingly low circulating CRP concentrations in ultramarathon runners independent of markers of adiposity: how low can you go? Arterioscler Thromb Vasc Biol. 2003;23:1640–1644. doi: 10.1161/01.ATV.0000087036.75849.0B. [DOI] [PubMed] [Google Scholar]
- 51.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol. 2007;102:1374–1379. doi: 10.1152/japplphysiol.01028.2006. [DOI] [PubMed] [Google Scholar]
- 52.Verdaet D, Dendale P, De Bacquer D, Delanghe J, Block P, De Backer G. Association between leisure time physical activity and markers of chronic inflammation related to coronary heart disease. Atherosclerosis. 2004;176:303–310. doi: 10.1016/j.atherosclerosis.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Chuo JY, Wiens M, Etminan M, Maberley DA. Use of lipid-lowering agents for the prevention of age-related macular degeneration: a meta-analysis of observational studies. Ophthalmic Epidemiol. 2007;14:367–374. doi: 10.1080/09286580701421684. [DOI] [PubMed] [Google Scholar]
- 54.Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137:190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- 55.Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Christen WG, Glynn RJ, Manson JE, Ajani UA, Buring JE. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA. 1996;276:1147–1151. [PubMed] [Google Scholar]
- 57.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho E, Hung S, Willett WC, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr. 2001;73:209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- 59.Mares-Perlman JA, Brady WE, Klein R, et al. Dietary fat and age-related maculopathy. Arch Ophthalmol. 1995;113:743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- 60.Mozaffarieh M, Sacu S, Wedrich A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: a review based on controversial evidence. Nutr J. 2003;2:20. doi: 10.1186/1475-2891-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Alcohol consumption and the risk of age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2008;145(4):707–715. doi: 10.1016/j.ajo.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Schaumberg DA, Glynn RJ, Christen WG, Hankinson SE, Hennekens CH. Relations of body fat distribution and height with cataract in men. Am J Clin Nutr. 2000;72:1495–1502. doi: 10.1093/ajcn/72.6.1495. [DOI] [PubMed] [Google Scholar]
- 63.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–425. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 64.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–784. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 65.U.S. Department of Health and Human Services . Physical Activity and Health: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- 66.Williams PT. Reduction in incident stroke risk with vigorous physcial activity during 7.7-year follow-up of the National Runners’ Health Study. Stroke. doi: 10.1161/STROKEAHA.108.535427. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]