Abstract
Background
The safety of extracorporeal photopheresis (ECP) in steroid-refractory chronic graft-versus-host disease (SR-cGVHD) has been explored in multiple studies but reported response rates (RR) vary significantly across studies.
Methods
We conducted a meta-analysis to assess the efficacy of ECP for SR-cGVHD. A search of electronic databases for studies published between 1984 and 2012 was conducted. End points included RR: complete response (CR), overall response rates (ORR), and organ-specific RR. The initial search generated 312 studies, of which 18 met the selection criteria (N=595). A random effects model was used for pooled rates.
Results
Pooled CR rates and ORR were 29% (confidence interval [CI], 19-42%) and 64% (CI, 65-82%), respectively. One-year overall survival was available for 4 studies only and was 49% (CI, 29-70%). The pooled RR for skin, liver, ocular, oral, lung, gastrointestinal and musculoskeletal SR-cGVHD was 74%, 68%, 60%, 72%, 48%, 53%, and 64%, respectively. There was a significant heterogeneity among studies due to differences in ECP schedules and duration. No significant differences in responses to ECP for pediatric and adult populations were found. Sensitivity analysis could not be undertaken due to a limited number of prospective studies.
Conclusion
ECP is an effective therapy for oral, skin, and liver SR-cGVHD, with modest activity in lung and gastrointestinal SR-cGVHD.
Keywords: Graft-versus-host disease, Extracorporeal photopheresis, Meta-analysis
INTRODUCTION
The incidence of chronic graft-versus-host disease (cGVHD) continues to rise as the number of allogeneic stem cell transplants (allo-SCT) has increased to >25,000 annually worldwide. The utilization of peripheral blood as a preferred stem cell source for allografts may have led to an increased incidence of cGVHD [1]. Aside from being the leading cause of treatment-related mortality among long-term survivors of allo-SCT, it also has a significant impact on quality of life [2]. Corticosteroids remain the backbone for initial cGVHD treatment [3], but overall prognosis remains poor despite a half the patients responding to this therapy. A strict uniform criterion for steroid-refractory disease does not exist but may include progression on prednisone at 1 mg/kg/d for 2 weeks, stable disease at >0.5 mg/kg/d, and inability to taper prednisone below 0.5 mg/kg/d [4].
There is no consensus regarding the optimal treatment for patients with steroid-refractory cGVHD (SR-cGVHD). Both pharmacologic and non-pharmacologic therapies have been evaluated with limited success. Drugs that have shown some activity include calcineurin inhibitors, tyrosine kinase inhibitors, purine analogs, mammalian target of rapamycin (mTOR) inhibitors, and monoclonal antibodies. Kharfan-Dabaja et al. [5] conducted a systematic review to evaluate the efficacy of rituximab in SR-cGVHD and reported a pooled proportion overall response rate (ORR) of 0.66 (95% confidence interval [CI], 0.57-0.74). Among the non-pharmacologic modalities, extracorporeal photopheresis (ECP) has acceptable response rates (RR) in both cutaneous and systematic manifestations of cGVHD [6, 7, 8, 9, 10, 11, 12]. In 2010, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) launched the largest intervention study (BMT CTN #0801) in cGVHD; a phase II/III trial to compare various treatments including ECP. The ECP arm of the parallel phase II study closed due to slow accrual. Since prospective data on the safety and overall efficacy of ECP with regard to standardized clinical RR and overall survival (OS) consists only of small phase I and phase II trials, we conducted a systematic review and meta-analysis to provide estimates of the overall impact of ECP on SR-cGVHD RR.
MATERIALS AND METHODS
Study selection
We included prospective and retrospective studies examining ECP as a treatment for SR-cGVHD. Studies where ECP was given for a minimum of 4 treatments, and which included both acute and cGVHD, were included; however, only the number of patients with cGVHD were included in the analysis. Studies that utilized the addition of calcineurin inhibitors (tacrolimus or cyclosporin) to ECP were included. Case reports and review articles were excluded. Studies with <5 cGVHD patients were excluded (Fig. 1).
Fig. 1.
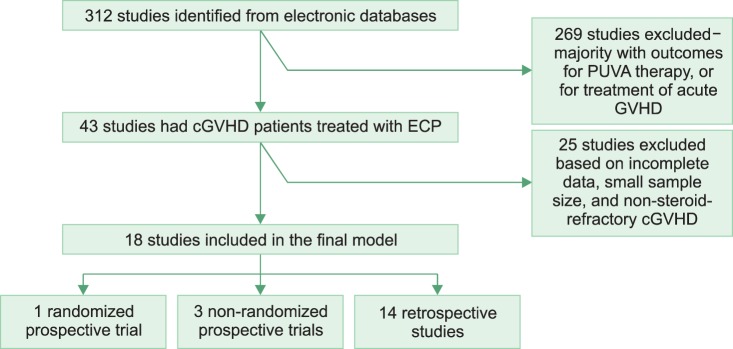
Identification and selection of studies for steroid-refractory chronic graft-versus-host disease (SR-cGVHD). Default criteria for cGVHD diagnosis were based on the NIH consensus criteria as below: Diagnosis of cGVHD requires the presence of at least 1 diagnostic clinical sign of cGVHD or the presence of at least 1 distinctive manifestation confirmed by pertinent biopsy or other relevant tests in the same or another organ [13].
Outcomes
The outcomes measured were complete RR (CRR) and clinical ORR following ECP treatment, including both classical RR and the National Institutes of Health (NIH) cGVHD score [13, 14].
Data sources and search strategies
A comprehensive search of several databases for studies published in any language from 1984 to August 2012 was conducted. The databases searched were: Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, and Scopus. Specific vocabulary supplemented with keywords was used to search for ECP therapy for GVHD, and the results were limited to controlled, prospective, and retrospective studies.
Publication bias
We searched for unpublished studies using electronic databases from annual meetings or conference abstracts of the American Society of Blood and Marrow Transplantation (ASBMT) from 1990 to 2012, European Group for Blood and Marrow Transplantation (EBMT) from 2000 to 2012, International Society for Pediatric Oncology (SIOP) from 2007 to 2012, and the American Society of Hematology (ASH) from 2001 to 2012. For ongoing trials, the http://clinicaltrials.gov/ and http://controlled-trials.com/ databases were searched. We were unable to statistically test for publication bias via funnel plot asymmetry tests due to the small number of studies included and also heterogeneity.
Quality assessment and data extraction
Two reviewers (SH and MM) critically appraised the studies from the search results, and also extracted the outcome data independently. Disagreements were resolved by discussion and consensus. The only randomized trial [15] comparing standard therapy to standard therapy and ECP was evaluated using the Cochrane Collaboration's tool for risk of assessment bias.
Statistical methods
For each study, we estimated the event rate (i.e., cumulative incidence of the outcome at the end of the study follow-up period) and the associated 95% CI. Event rates were pooled across studies using the random effects model. Statistical heterogeneity was assessed using the I2 test [16].
RESULTS
Identification of studies
The initial electronic search of multiple electronic databases based on the selection criteria generated 312 studies. Of these, 269 were excluded because of: (a) small sample size of <5 patients; (b) psoralen plus ultraviolet A (PUVA) treatment was utilized; (c) ECP was used for acute GHVD only. Forty-three studies had cGVHD patients who were treated with ECP, while 25 were excluded because they either did not meet the strict selection criteria or very limited information was available on the RR of the cGVHD patients. Out of 18 studies for analysis, only 4 were prospective trials (Table 1). Outcomes from a final sample size of 595 patients were analyzed.
Table 1.
Quality of the selected studies.
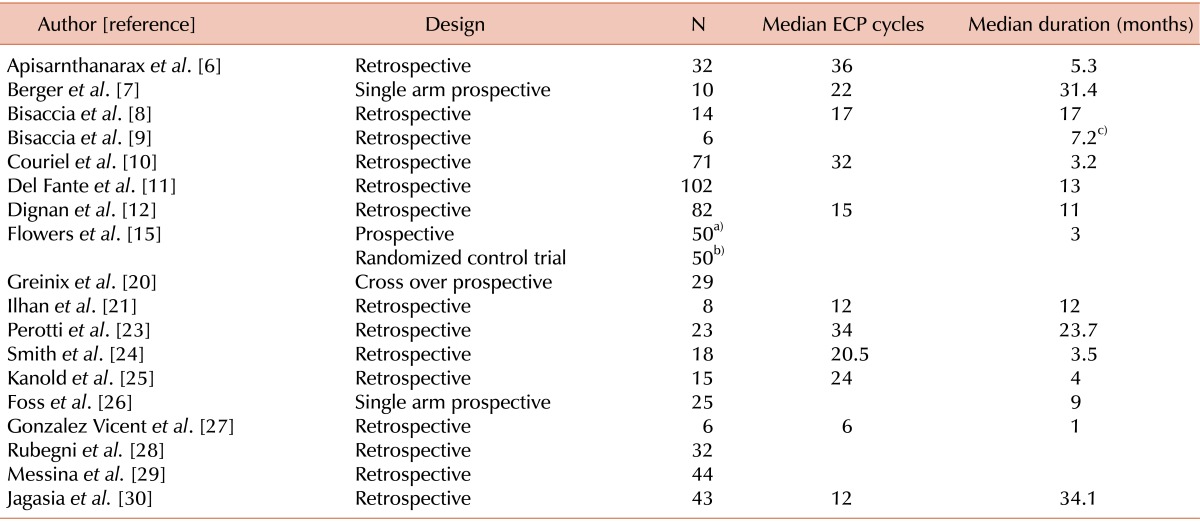
a)ECP+ Standard therapy, b)Standard therapy, c)mean value.
Abbreviation: ECP, extracorporeal photopheresis.
Only 1 randomized controlled trial by Flowers et al. [15] was identified, which compared ECP and conventional therapy to conventional therapy alone in SR-cGVHD. The quality appraisal for this clinical trial using the Cochrane Collaboration's tool indicated a low risk of bias considering adequate methodology described for sequence generation, allocation concealment, and for selected outcome reporting.
These findings highlight the problems mentioned by Martin et al. [17], who after a comprehensive review of published reports, also found numerous qualitative deficiencies in studies of secondary treatments for cGVHD. In this report, fewer than 10% published studies tried to minimize selection bias, and investigators in most studies did not use consistent treatment regimens. Similarly in our analysis, different ECP regimen cycles and duration were used, hence we can only report a median number of ECP cycles and median duration of ECP.
Response rates
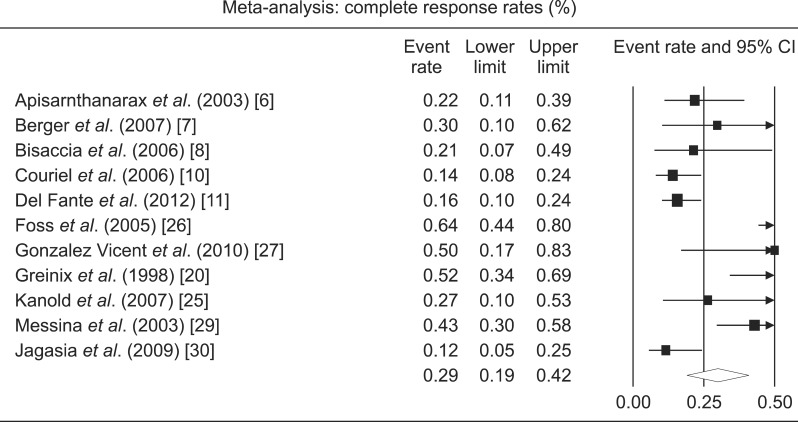
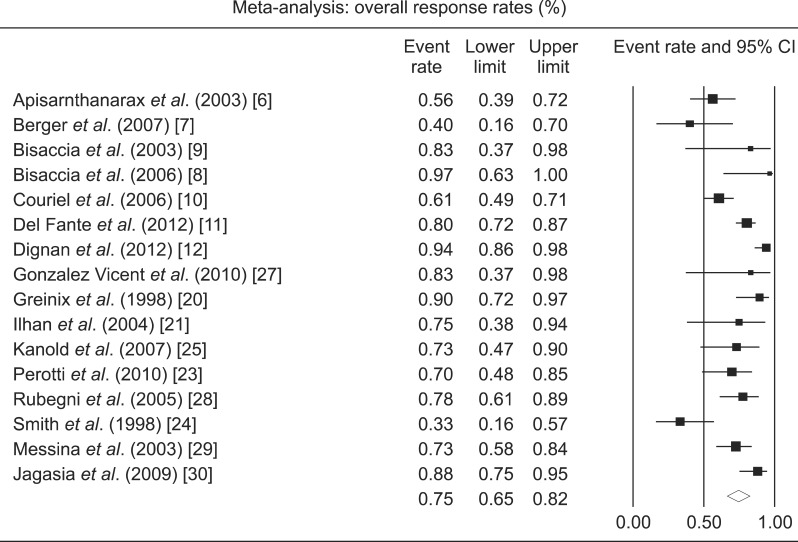
CR rates were available from 11 studies with a total of 372 patients (Table 2; Fig. 2). Only 3 were prospective studies (N=64). The pooled CR rate for ECP was 29% (CI, 19-42%). There was a significant heterogeneity among the studies (I2=79%). ORR were available from 16 studies with a total of 554 patients (Fig. 3). Seven were prospective studies with a total of 64 patients. The pooled ORR of both retrospective and prospective studies was 64% (CI, 65-82%). The 1-year OS rate was available for 4 studies and was 49% (CI, 29-70%). There was a significant heterogeneity among the studies (I2=72%).
Table 2.
Response rates for steroid-refractory chronic graft-versus-host disease.
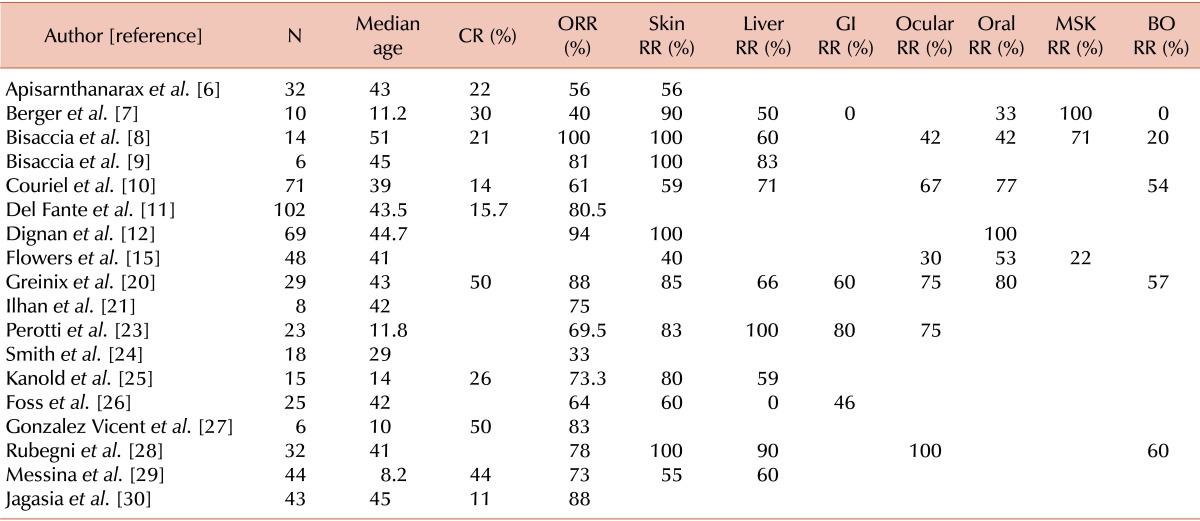
Abbreviations: CR, complete response rates; ORR, overall response rates; GI, gastrointestinal; MSK, musculoskeletal; BO, bronchiolitis obliterans.
Fig. 2.
Forest plot of the complete response rates following extracorporeal photopheresis for chronic graft-versus-host disease.
Fig. 3.
Forest plot of the overall response rates following extracorporeal photopheresis for chronic graft-versus-host disease.
Efficacy of ECP for skin SR-cGVHD
Skin was the most commonly involved organ in the selected studies. RR were available for 13 studies with a total of 399 patients and the pooled RR was 74% (CI, 60-85%; I2=79%).
Efficacy of ECP for liver SR-cGVHD
RR were available for 10 studies with a total of 269 patients. The pooled RR was 68% (CI, 57-77%; I2=57%).
Efficacy of ECP for ocular SR-cGVHD
RR were available from 6 studies with a total of 217 patients. The pooled RR was 60% (CI, 40-78%; I2=83%).
Efficacy of ECP for lung SR-cGVHD
Bronchiolitis obliterans (BO) was the most common presentation of lung cGVHD. RR were available from 5 studies with a total of 156 patients. The pooled RR was 48% (CI, 33-63%; I2=62%).
Efficacy of ECP for oral SR-cGVHD
RR were available from 6 studies with a total of 274 patients. The pooled RR was 72% (CI, 51-86%; I2=81%).
Efficacy of ECP for gastrointestinal (GI) tract SR-cGVHD
RR were available from 4 studies with a total of 87 patients. The pooled RR was 53% (CI, 21-83%; I2=77%).
Efficacy of ECP for musculoskeletal system SR-cGVHD
RR were available from 3 studies with a total of 72 patients. The pooled RR was 64% (CI, 18-94%; I2=88%).
Evaluation of the age effect
We compared the efficacy of ECP in adult and pediatric populations. The pooled CR rate for adults was obtained from 7 studies and was 26% (CI, 14-42%). ORR in adults was available from 11 studies and was 78% (CI, 66-86%). The pooled CR rate for the pediatric population was available from 4 studies and was 39% (CI, 29-51%; P=0.17). ORR in pediatric patients was available from 5 studies and was 69% (CI, 58-78%; P=0.24). The difference between adults and pediatric CR and ORR was not statistically significant.
DISCUSSION
Although a number of therapeutic modalities have demonstrated responses in SR-cGVHD, most of these treatments do not affect each organ system uniformly. Cellular, pharmacologic, and ECP-based therapies have been evaluated in a cGVHD paradigm and have shown differential results. Mesenchymal stromal cell therapies for GVHD have recently generated considerable interest, but direct clinical experience for SR-cGVHD is limited [18], in contrast to its well-established efficacy in the treatment of acute GVHD [19]. Since ECP has a remarkable safety record in cGVHD treatment and pharmacologic therapies may be associated with significant adverse effects, the efficacy of ECP on organs affected by cGVHD was evaluated systematically to assess all the current evidence for each organ system.
In the final analysis, 18 studies were included, of which 4 were prospective trials. The CR rate following ECP was found to be 29%, whereas the ORR was 64%. A poor 1-year OS of 49% was found in SR-cGVHD patients. We report superior RR following ECP for oral cavity, skin, and liver SR-cGVHD, and a modest RR for SR-cGVHD of the musculoskeletal system, GI tract, eyes, and lungs.
Historically, skin has been reported as the organ most responsive to ECP in SR-cGVHD as shown in Table 2, with a RR ranging from 40-100%. Our results indicate that more than two-thirds of patients responded well to ECP, which is consistent with previous studies. Such high RR in skin have been consistently reported only with rituximab [5].
ECP has been reported to have a variable effect on visceral organs involved by cGVHD. For liver cGVHD, Greinix et al. [20] reported a 66% RR following ECP with complete normalization of bilirubin and the transaminases, while Couriel et al. [10] reported a RR of 71% in 15 liver cGVHD patients. Our pooled RR of 68% was consistent with these studies, indicating the efficacy of ECP for liver SR-cGVHD.
BO signifies an extremely poor prognosis in cGVHD patients. Retrospective studies have indicated that ECP primarily stabilizes BO [21, 22] and is insufficient to render CR in these patients. Recently Lucid et al. [22] reported the outcome following ECP use and focused specifically on BO after allo-SCT, which yielded a RR of 67% in 9 patients. Our final analysis did not include this study in the pooled results due to a strictly defined selection criterion in the electronic searches. Results from our study, which included 156 patients with BO, indicate that approximately a half of these patients responded to ECP; this is impressive given the futility of most other interventions in highly refractory BO.
The results of the response following ECP for GI tract SR-cGVHD are extremely variable. Perrotti et al. [23] reported that 6 of 8 patients with GI cGVHD responded to ECP, in contrast to Berger et al.'s report where all 3 patients showed a response [7]. Our meta-analysis indicates a modest response with a half of GI SR-cGVHD patients responding to ECP.
Several limitations of this analysis must be noted; the most important being the absence of uniform criteria for assessment of cGVHD as an endpoint in the studies included. The NIH consensus development project published uniform criteria for the reporting of cGVHD in 2005 [13]. Very few studies published since 2005 have reported outcomes based on these criteria.
The precision of pooled (meta-analytic) effect size is affected by the small sample size of the studies, therefore we performed a meta-analysis to increase power and precision. Only 1 randomized trial for SR-cGVHD and 3 non-randomized prospective studies were found during the literature search. Thus the totality of evidence indicating ECP efficacy remains small and clinicians should address the level of uncertainty about the RR in their discussions with patients.
Most investigators utilized a different schedule of ECP cycles e.g., some studies evaluated RR when ECP was undertaken at a twice weekly schedule, while other studies reported outcomes with initial biweekly therapy.
Despite the above-mentioned limitations, this is the first meta-analysis analyzing the RR following ECP for SR-cGVHD and the estimates provided, despite some uncertainty, are the best available. Shared decision-making strategies using our results may help patients to proceed with options most consistent with their values and preferences.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 4.Wolff D, Schleuning M, von Harsdorf S, et al. Consensus Conference on Clinical Practice in Chronic GVHD: Second-Line Treatment of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2011;17:1–17. doi: 10.1016/j.bbmt.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15:1005–1013. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Apisarnthanarax N, Donato M, Korbling M, et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003;31:459–465. doi: 10.1038/sj.bmt.1703871. [DOI] [PubMed] [Google Scholar]
- 7.Berger M, Pessolano R, Albiani R, et al. Extracorporeal photopheresis for steroid resistant graft versus host disease in pediatric patients: a pilot single institution report. J Pediatr Hematol Oncol. 2007;29:678–687. doi: 10.1097/MPH.0b013e31814d66f5. [DOI] [PubMed] [Google Scholar]
- 8.Bisaccia E, Palangio M, Gonzalez J, et al. Treatment of extensive chronic graft-versus-host disease with extracorporeal photochemotherapy. J Clin Apher. 2006;21:181–187. doi: 10.1002/jca.20084. [DOI] [PubMed] [Google Scholar]
- 9.Bisaccia E, Palangio M, Gonzalez J, Adler KR, Rowley SD, Goldberg SL. Treating refractory chronic graft-versus-host disease with extracorporeal photochemotherapy. Bone Marrow Transplant. 2003;31:291–294. doi: 10.1038/sj.bmt.1703830. [DOI] [PubMed] [Google Scholar]
- 10.Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 11.Del Fante C, Scudeller L, Viarengo G, Bernasconi P, Perotti C. Response and survival of patients with chronic graft-versus-host disease treated by extracorporeal photochemotherapy: a retrospective study according to classical and National Institutes of Health classifications. Transfusion. 2012;52:2007–2015. doi: 10.1111/j.1537-2995.2011.03542.x. [DOI] [PubMed] [Google Scholar]
- 12.Dignan FL, Greenblatt D, Cox M, et al. Efficacy of bimonthly extracorporeal photopheresis in refractory chronic mucocutaneous GVHD. Bone Marrow Transplant. 2012;47:824–830. doi: 10.1038/bmt.2011.186. [DOI] [PubMed] [Google Scholar]
- 13.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 15.Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Martin PJ, Inamoto Y, Carpenter PA, Lee SJ, Flowers ME. Treatment of chronic graft-versus-host disease: Past, present and future. Korean J Hematol. 2011;46:153–163. doi: 10.5045/kjh.2011.46.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng JY, Du X, Geng SX, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45:1732–1740. doi: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 20.Greinix HT, Volc-Platzer B, Rabitsch W, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–3104. [PubMed] [Google Scholar]
- 21.Ilhan O, Arat M, Arslan O, et al. Extracorporeal photoimmunotherapy for the treatment of steroid refractory progressive chronic graft-versus-host disease. Transfus Apher Sci. 2004;30:185–187. doi: 10.1016/j.transci.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant. 2011;46:426–429. doi: 10.1038/bmt.2010.152. [DOI] [PubMed] [Google Scholar]
- 23.Perotti C, Del Fante C, Tinelli C, et al. Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion. 2010;50:1359–1369. doi: 10.1111/j.1537-2995.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith EP, Sniecinski I, Dagis AC, et al. Extracorporeal photochemotherapy for treatment of drug-resistant graft-vs.-host disease. Biol Blood Marrow Transplant. 1998;4:27–37. doi: 10.1016/s1083-8791(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 25.Kanold J, Merlin E, Halle P, et al. Photopheresis in pediatric graft-versus-host disease after allogeneic marrow transplantation: clinical practice guidelines based on field experience and review of the literature. Transfusion. 2007;47:2276–2289. doi: 10.1111/j.1537-2995.2007.01469.x. [DOI] [PubMed] [Google Scholar]
- 26.Foss FM, DiVenuti GM, Chin K, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35:1187–1193. doi: 10.1038/sj.bmt.1704984. [DOI] [PubMed] [Google Scholar]
- 27.Gonzàlez Vicent M, Ramirez M, Sevilla J, Abad L, Diaz MA. Analysis of clinical outcome and survival in pediatric patients undergoing extracorporeal photopheresis for the treatment of steroid-refractory GVHD. J Pediatr Hematol Oncol. 2010;32:589–593. doi: 10.1097/MPH.0b013e3181e7942d. [DOI] [PubMed] [Google Scholar]
- 28.Rubegni P, Cuccia A, Sbano P, et al. Role of extracorporeal photochemotherapy in patients with refractory chronic graft-versus-host disease. Br J Haematol. 2005;130:271–275. doi: 10.1111/j.1365-2141.2005.05586.x. [DOI] [PubMed] [Google Scholar]
- 29.Messina C, Locatelli F, Lanino E, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol. 2003;122:118–127. doi: 10.1046/j.1365-2141.2003.04401.x. [DOI] [PubMed] [Google Scholar]
- 30.Jagasia MH, Savani BN, Stricklin G, et al. Classic and overlap chronic graft-versus-host disease (cGVHD) is associated with superior outcome after extracorporeal photopheresis (ECP) Biol Blood Marrow Transplant. 2009;15:1288–1295. doi: 10.1016/j.bbmt.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]