Abstract
To test prospectively in hypertensives whether moderate and vigorous exercise produce equivalent reductions in mortality, Cox-proportional hazard analyses were applied to energy expenditure (metabolic equivalents hours/day, METh/d) in 6,973 walkers and 3,907 runners who used hypertensive medications at baseline. 1121 died during 10.2-year follow-up: 695 cardiovascular disease (CVD, ICD10 I00-99, 465 underlying cause, 230 contributing cause), 124 cerebrovascular disease, 353 ischemic heart disease (ICD10 I20-25, 257 underlying, 96 contributing), 122 heart failure (ICD10 I50, 24 underlying, 98 contributing), and 260 dysrhythmias (ICD10 I46-49, 24 underlying, 236 contributing). Relative to <1.07 METh/d, running or walking 1.8-3.6 METh/d produced significantly lower all-cause (29% reduction, 95%CI: 17% to 39%, P=0.0001), CVD (34% reduction, 95%CI: 20% to 46%, P=0.0001), cerebrovascular disease (55% reduction, 95%CI: 27% to 73%, P=0.001), dysrhythmia (47% reduction, 95%CI: 27% to 62%, P=0.0001), and heart failure mortality (51% reduction, 95%CI: 21% to 70%, P=0.003), as did ≥3.6 METh/d with all-cause (22% reduction, 95%CI: 6% to 35%, P=0.005), CVD (36% reduction, 95%CI: 19% to 50%, P=0.0002), cerebrovascular disease (47% reduction, 95%CI: 6% to 71%, P=0.03), and dysrhythmia mortality (43% reduction, 95%CI: 16% to 62%, P=0.004). Diabetes and chronic kidney disease mortality also decreased significantly with METh/d. All results remained significant when BMI adjusted. Merely meeting guideline levels (1.07 to 1.8 METh/d) did not significantly reduced mortality. The dose-response was significantly nonlinear for all endpoints except diabetes, and cerebrovascular and chronic kidney disease. Results did not differ between running and walking. Thus, walking and running produce similar reductions in mortality in hypertensives.
Keywords: Hypertension, Exercise, Cardiovascular diseases, Diabetes Mellitus, Type 2, Renal Insufficiency, chronic
Hypertension increases the risk for stroke, ischemic heart disease, and all-cause mortality [1]. Physical activity reduces the risk for these conditions, which may be due, in part, to reductions in blood pressure [2]. A recent systematic review [3] of six prospective cohort studies of hypertensives [4-9] concluded that physical activity significantly decreases both all-cause and cardiovascular disease mortality. In fact, several of these studies suggested that the risk reductions could be even greater for hypertensives than normotensives [4,5].
Although hypertensives clearly benefit from physical activity, the particulars of the benefit are poorly understood, including the dose-response, the effect of intensity, and the specific diseases affected. The six hypertensive cohorts previously reported on were all general purpose [4-9], i.e., designed to relate a variety of variables to disease. As such, their most physically active groups were often not really very active, nor were their activities well-quantified (e.g., ≥30 minutes of moderate to vigorous exercise > once a week [6,8], regular vigorous exercise [4] or sports [9], or vigorous exercise >3 times/wk [7]), and therefore the cohorts provide little insight into the optimal exercise dose. None of these studies compared the benefits of moderate vs. vigorous exercise. Their endpoints included all-cardiovascular disease (CVD) [4,5,7,8], ischemic heart disease (IHD) [4,8], myocardial infarction [6] and cerebrovascular disease [6,8], while ignoring heart failure, cardiac dysrhythmia, and hypertensive heart disease. Hypertensives are also at increased risk for diabetes and renal failure [1], and the effects of exercise dose and intensity on these conditions also warrant investigation.
The National Runners’ and Walkers’ Health Studies [10-18] are the only large prospective cohorts designed specifically to assess the health benefits of exercise. Their advantages over cohorts of a more general purpose include: 1) greater statistical power due to the large sample size and broad activity range, 2) knowledgeable subjects committed to regular exercise regimens, 3) focus on specific exercises that are largely identical except for intensity, and 4) use of distance to calculate exercise energy expenditure, which has been shown to be superior to time-based calculations [13-15]. The ten-thousand plus hypertensive medication users from these cohorts were used to test whether the dose-response relationship between exercise and mortality: 1) is nonlinear; 2) differs significantly between running and walking, and 3) affects specific CVD diagnoses, diabetes, and chronic kidney disease.
Materials and Methods
Detailed descriptions of the methods and materials are presented in the online supplement. The National Death Index provided mortality surveillance through 2008 for the National Runners’ and Walkers’ Health Studies [10-18]. The runners reported the usual miles run per week, and the walkers reported usual miles walked per week and usual pace (minutes per mile). These values were used to estimate energy expenditure in metabolic equivalents (MET) [13-15], where one MET is the energy expended while sitting at rest (3.5 ml O2·kg−1·min−1) [19]. The study protocol was reviewed and approved by the Human Subjects Committee at Lawrence Berkeley National Laboratory for the protection of human subjects, and all subjects provided a signed statement of informed consent.
Statistics
Cox proportional hazard analyses (STATA version 11.1, StataCorp, College Station, TX) were used to test whether all cause mortality, and mortality from major cardiovascular disease (International Classification of Disease version 9 codes 390 to 459, version 10 code I00-99 [20]), ischemic heart disease (ICD9 410 –414, ICD10 I20-25), heart failure (ICD9 428, ICD10 I50), dysrhythmia (ICD9 427, ICD10 I46-49), cerebrovascular disease (ICD9 430 –438, ICD10 I60-69), hypertensive heart disease (ICD9 401-404, ICD10 I10-13), diabetes (ICD9 250, ICD10 E10-14), and chronic kidney disease (CKD, ICD9 585, ICD10 N18) were different between hypertensive medication users and nonusers, and within the users whether they were significantly related to METh/d run or walked when adjusted. Underlying and contributing (non-underlying entity axis diagnosis) causes of death were obtained from the National Death Index mortality surveillance. Results are presented as hazard ratios (HR), their fold increases in risk, and their percent reductions in the risk (calculated as 100*(HR-1)) for seven categories of walking or running energy expenditure: falling short of the current physical activity recommendations for health (<450 MET minutes per week =1.07 METh/d), meeting the recommendations (450 to 750 MET minutes per week =1.07 to 1.8 METh/d), exceeding the recommendations by 1- to 2-fold (1.8 to 3.6 METh/d), 2- to 3-fold (3.6-5.4 METh/d), 3 to 4-fold (5.4-7.2 METh/d), 4 to 5-fold (7.2-9.0 METh/d), and greater than or equal to 5-fold [19].
Results
Of the original 40,670 walkers and 111,080 runners surveyed at baseline with complete BMI and other data, there were 6,973 walkers (17.15%), and 3,907 runners (3.52%) who reported taking blood pressure medications on their baseline questionnaires (10,880 total subjects). As a group, the blood pressure medication users tended to be older (mean±SD: 58.58±12.04 vs. 43.61±12.11 years), include more African Americans (5.33% vs. 1.75%) and were more likely to walk than run (64.1% vs. 23.93%) than nonusers. Table S1 (supplement) presents the characteristics of the sample.
There were 1121 deaths during the hypertensives’ 10.2-year average follow-up: including 695 CVD-related (465 underlying cause, 230 contributing cause), 124 cerebrovascular disease-related (57 underlying, 67 contributing), 353 IHD-related (257 underlying, 96 contributing), 122 heart failure-related (24 underlying, 98 contributing), 260 dysrhythmia-related (24 underlying, 236 contributing), 99 diabetes-related (28 underlying, 71 contributing), and 28 chronic kidney disease-related deaths (6 underlying, 22 contributing). Compared to the 140,870 non-users of hypertensive medications in the total original cohort of runner and walkers, hypertensives were at significantly higher risk for mortality from all-causes (30.5% higher, 95%CI: 21.0% to 40.8%, P=10−10), and mortality related to CVD (47.5% higher, 95%CI: 33.5% to 62.8%, P=10−10), IHD (62.6% higher, 95%CI: 40.9% to 87.6%, P=0.009), cerebrovascular disease (48.3% higher, 95%CI: 18.2% to 86.1%, P=0.0008), cardiac dysrhythmia (44.6% higher, 95%CI: 23.4% to 69.3%, P<10−5), heart failure (48.2% higher, 95%CI: 17.0% to 87.7%, P=0.0006), hypertensive heart disease (188.4% higher, 95%CI: 131.9% to 258.5%, P<10−15), and diabetes (34.2% higher, 95%CI: 0.5% to 79.2%, P=0.05) when adjusted for age, sex, education, race, diet, aspirin use, prior heart attack, diabetes and cholesterol medication, smoking, and exercise.
All-cause mortality
Total mortality significantly increased in association with smoking (P=0.02), prior heart attack (P<10−7), greater alcohol intake (P=0.05), and diabetes medication use (P<10−5). Figure 1 shows a leveling off of the risk reduction above 1.8 to 3.6 METh/d (average 2.7 METh/d for the interval). Although the risk reductions were not significant for 7.2 to 9.0 METh/d and for ≥9.0 METh/d, these intervals each included <3% of the total sample. The shape of the graph, and the fact that for all CVD-related mortality there was no significant difference in risk among higher energy expenditures, led us to pool energy expenditure ≥3.6 METs for subsequent analyses. Table 1 shows that all-cause mortality decreased a nonsignificant 9% by meeting the current exercise guidelines (1.07 to 1.8 METh/d) compared to falling short of them (<1.07 METh/d), and decreased 29% by exceeding the guidelines by 1-to-2 fold (1.8-3.6 METh/d). Energy expenditure ≥3.6 METh/d did not appear to further reduce all-cause mortality vis-à-vis 1.8-3.6 METh/d.
Figure 1.
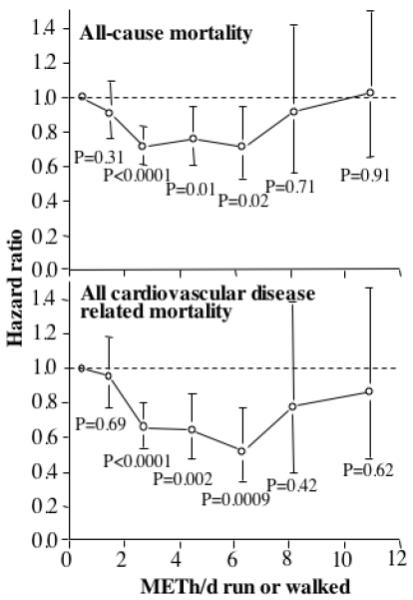
Relative risk (hazard ratio) relative to <1.07 METh/d of all cause and all cardiovascular disease related mortality vs. energy expenditure (METh/d) run or walked in 10,880 hypertensives followed prospectively for 10.2 years. Significance levels are relative to inadequate exercise (i.e., <1.07 METh/d). Brackets denote 95% confidence intervals. Adjusted for age (age, age2), sex, education, cohort, intakes of red meat, fruit, alcohol, prior heart attack, aspirin (tablets/day), diabetes and cholesterol medication use, and African American ethnicity.
Table 1.
Hazard ratios (95% confidence interval) relative to <1.07 METh/d for all cause, CVD-related, and non-CVD-related mortality in 10,880 runners and walkers on hypertensive medication.
| Dependent variable | 1.07 to 1.8 METh/d |
1.8 to 3.6 METh/d |
≥3.6 METh/d |
|---|---|---|---|
| All cause | |||
| No BMI adjustment | 0.91 (0.77, 1.09) P=0.31 |
0.71 (0.61, 0.83) P=0.0001 |
0.78 (0.65, 0.94) P=0.005 |
| BMI adjustment | 0.94 (0.79, 1.12) P=0.49 |
0.74 (0.63, 0.86) P=0.0001 |
0.82 (0.68, 0.99) P=0.04 |
| CVD-underlying | |||
| No BMI-adjustment | 0.89 (0.68, 1.16) P=0.41 |
0.61 (0.48, 0.78) P=0.0001 |
0.70 (0.53, 0.94) P=0.02 |
| BMI-adjustment | 0.93 (0.71, 1.22) P=0.60 |
0.64 (0.50, 0.82) P=0.0004 |
0.76 (0.57, 1.02) P=0.06 |
| All CVD-related | |||
| No BMI-adjustment | 0.96 (0.77, 1.19) P=0.70 |
0.66 (0.54, 0.80) P=0.0001 |
0.64 (0.50, 0.81) P=0.0002 |
| BMI-adjustment | 1.00 (0.80, 1.24) P=0.99 |
0.69 (0.57, 0.84) P=0.0003 |
0.68 (0.53, 0.88) P=0.003 |
| All diabetes-related | |||
| No BMI-adjustment | 1.22 (0.73, 2.00) P=0.43 |
0.56 (0.32, 0.95) P=0.03 |
0.59 (0.27, 1.20) P=0.15 |
| BMI-adjustment | 1.29 (0.77, 2.12) P=0.32 |
0.61 (0.34, 1.04) P=0.07 |
0.68 (0.30, 1.38) P=0.29 |
| Not CVD, diabetes, or CKD related | |||
| No BMI-adjustment | 0.84 (0.61, 1.14) P=0.26 |
0.83 (0.65, 1.08) P=0.17 |
1.06 (0.79, 1.43) P=0.70 |
Adjusted for age (age, age2), sex, education, cohort, intakes of red meat, fruit, alcohol, prior heart attack, aspirin (tablets/day), diabetes and cholesterol medication use, and African American ethnicity. Additional adjustment for BMI where indicated.
CVD-related mortality
Table 1 shows that the results were similar for CVD as an underlying cause, and total CVD-related deaths. In addition to age and sex, the risk factors for all CVD-related deaths were smoking (P<10−5), a prior heart attack (P<10−9), and diabetes medication use (P<10−7). There was a 34% decrease in total CVD-related mortality by exercising between 1.8 and 3.6 METh/d, which persisted for greater energy expenditures. Adjustment for BMI did not affect the results.
Table 2 present more-detailed analyses of CVD endpoints. Cerebrovascular disease-related mortality declined 19% for 1.07 to 1.8 METh/d, 55% for 1.8 to 3.6 METh/d, and remained relatively constant thereafter. Heart failure- and dysrhythmia-related mortality both showed near 50% reductions in risk for 1.8 to 3.6 and ≥3.6 METh/d. The reduction in IHD-related mortality per METh/d was not as great as for these other CVD endpoints. Hypertensive heart disease-related mortality was unrelated to exercise energy expenditure. Adjustment for BMI had little effect on the hazard ratios of Table 2. Figure 2 shows there was a 50% decrease in the risks by 1.8 METh/d for all cerebovascular disease, heart failure, dysrhythmia-related mortality, which continued through >3.6 METh/d. CVD-related deaths, exclusive of these endpoints, showed no significant relationship to METh/d. Nearly identical results were obtained when the analyses were restricted to the 115 deaths that listed cerebrovascular disease, heart failure, and dysrhythmia as their underlying cause.
Table 2.
Hazard ratios (95% confidence interval) relative to <1.07 METh/d for all CVD-related mortality in 10,880 runners and walkers on hypertensive medications.
| Dependent variable | 1.07 to 1.8 METh/d |
1.8 to 3.6 METh/d |
≥3.6 METh/d |
|---|---|---|---|
| Ischemic heart disease | |||
| No BMI adjustment | 0.82 (0.59, 1.13) P=0.23 |
0.71 (0.54, 0.93) P=0.01 |
0.83 (0.60, 1.15) P=0.27 |
| BMI adjustment | 0.87 (0.62, 1.19) P=0.38 |
0.75 (0.57, 0.99) P=0.04 |
0.91 (0.65, 1.27) P=0.59 |
| Cerebrovascular disease | |||
| No BMI-adjustment | 0.81 (0.48, 1.32) P=0.40 |
0.45 (0.27, 0.73) P=0.001 |
0.53 (0.29, 0.94) P=0.03 |
| BMI-adjustment | 0.83 (0.49, 1.36) P=0.46 |
0.46 (0.28, 0.76) P=0.002 |
0.56 (0.30, 0.99) P=0.05 |
| Dysrhythmia | |||
| No BMI-adjustment | 0.86 (0.59, 1.21) P=0.38 |
0.53 (0.38, 0.73) P=0.0001 |
0.57 (0.38, 0.84) P=0.004 |
| BMI-adjustment | 0.87 (0.61, 1.24) P=0.45 |
0.54 (0.39, 0.75) P=0.0003 |
0.59 (0.39, 0.88) P=0.009 |
| Heart Failure | |||
| No BMI-adjustment | 0.82 (0.49, 1.31) P=0.41 |
0.49 (0.30, 0.79) P=0.003 |
0.55 (0.27, 1.01) P=0.05 |
| BMI-adjustment | 0.92 (0.55, 1.48) P=0.73 |
0.57 (0.35, 0.91) P=0.02 |
0.68 (0.34, 1.26) P=0.23 |
| Hypertensive Heart Disease | |||
| No BMI-adjustment | 1.07 (0.70, 1.61) P=0.76 |
0.72 (0.49, 1.05) P=0.09 |
0.85 (0.53, 1.34) P=0.49 |
| BMI-adjustment | 1.12 (0.73, 1.69) P=0.59 |
0.76 (0.51, 1.12) P=0.17 |
0.92 (0.57, 1.47) P=0.73 |
Adjusted for age (age, age2), sex, education, cohort, intakes of red meat, fruit, alcohol, prior heart attack, aspirin (tablets/day), diabetes and cholesterol medication use, and African American ethnicity. Additional adjustment for BMI where indicated
Figure 2.
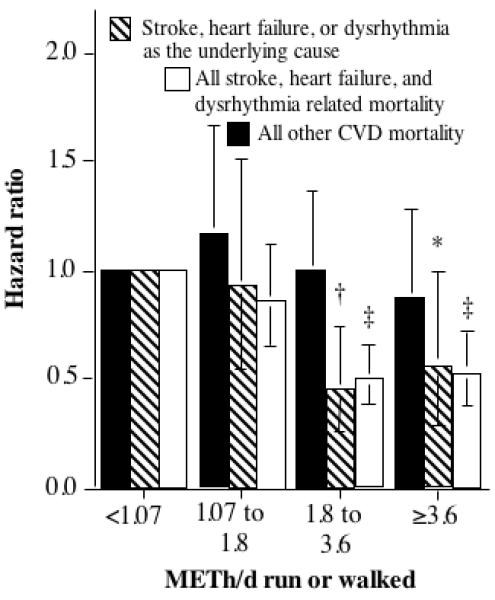
Relative risk (hazard ratio) of cardiovascular disease (CVD)-related mortality vs. energy expenditure (METh/d) run or walked in 10,880 hypertensives. Other CVD refers to all other CVD except for stroke, dysrhythmia, and heart failure related mortality. Significance levels, relative to inadequate exercise (i.e., <1.07 METh/d), are coded * P≤0.05, † P≤0.01; and ‡ P≤0.001. Brackets denote 95% confidence intervals. Adjusted for age (age, age2), sex, education, cohort, intakes of red meat, fruit, alcohol, prior heart attack, aspirin (tablets/day), diabetes and cholesterol medication use, and African American ethnicity.
Diabetes
On average, each METh/d increment in energy expenditure was associated with a 19.1% reduction in the risk for diabetes-related mortality (95%CI: 6.7% to 30.5%, P=0.003), which persisted when adjusted for BMI (16.4% reduction per METh/d, 95%CI: 3.6% to 28.6%, P=0.01).
Chronic kidney disease and other mortality
The risk for all CKD-related mortality decreased 25.4% per METh/d (95% CI: 4.8% to 44.2%, P=0.02 for a reduced model that included only significant covariates, P=0.06 for the full model). The significance of METh/d in the reduced model remained significant when adjusted for BMI (24.2% reduction per METh/d, 95%CI: 3.0% to 43.6%, P=0.03). Table 1 shows that the 409 deaths that did not include CVD, diabetes, or CKD as an underlying or contributing causes were not significantly related to exercise energy expenditure.
Nonlinear dose-response
A significant coefficient “β” in the regression model “α(METh/d) + β(METh/d)2 provided proof that dose-response relationship between mortality and exercise was nonlinear for total (P=0.005), CVD- (P=0.002), IHD- (P=0.02), dysrhythmia- (P=0.03), and heart failure-related mortality (P=0.05), but not for cerebrovascular disease (P=0.36), diabetes (P=0.59), or CKD (P=0.36). This represents nonlinearity in excess of that already implied by the proportional hazard model.
Running vs. Walking
Being a runner or a walker did not significantly affect the risk reduction for 1.8 to 3.6 METh/d, or ≥3.6 METh/d, for all CVD-related deaths (P=0.24 and P=0.37, respectively), CVD as the underlying cause (P=0.42 and P=0.24, respectively), all IHD-related deaths (P=0.48 and P=0.46, respectively), IHD as underlying cause (P=0.09 and P=0.07, respectively), and all deaths related to cerebrovascular disease (P=0.93 and P=0.58, respectively), heart failure (P=0.23 and P=0.51, respectively), and dysrhythmias (P=0.95 and P=0.62, respectively). Exercise mode also did not significantly affect the per METh/d declines in diabetes-related (P=0.94) or CDK-related deaths (P=0.46).
Discussion
Those in our study who took blood pressure medications remained at 48% greater risk for CVD mortality compared to cohort members not taking medications. We did not find that the current exercise guidelines were adequate to significantly reduce their CVD mortality. Most of the reduction in mortality was achieved by exercising slightly more, between 1.8 and 3.6 METh/d. At that level, the hypertensives’ CVD risks were reduced to those of the sedentary non-users of hypertensive medication in our cohort. We also found that the risk for two other fatal conditions that hypertensives are prone to developing, diabetes and CKD, declined with exercise. Running one kilometer per day expends about one METh/d [14,15], so 3.6 METh/d corresponds to running 15 miles per week. Walking and running appeared to produce comparable risk reductions provided the energy expenditure was the same, however, one must go about 50% further and take about twice as long to expend the same amount of energy by walking briskly as by running a 12-minute mile.
Our analyses suggest that exercise reduces the hypertensive’s risks for cerebrovascular disease, heart failure, and cardiac dysrhythmias. There were too few deaths for analyzing these conditions separately as underlying causes of death. Only 20% of the 122 heart failures, and 9% of the 260 cardiac arrhythmias, listed these conditions as the underlying cause. However, it does not appear from our analyses that the underlying cause of death provides greater specificity in defining the health benefits of exercise than all related mortality. Specifically, our results did not suggest any differences between using the 465 deaths that specifically identified CVD as the underlying cause and all 695 CVD-related causes of death. Moreover, Figure 2 shows that the risk reductions for the 115 underlying cerebrovascular disease, heart failure, and cardiac dysrhythmias deaths were entirely consistent with those of the 428 total deaths listing these conditions as either contributing or underlying causes.
The exercise-induced risk reductions we observed could be due, in part, to additional improvement in blood pressure control over medication alone. We have previously shown progressive, incremental reductions in both systolic and diastolic blood pressure with greater weekly running distance [10]. There is a doubling in both IHD- and cerebrovascular disease risk for every 20 mmHg increment in systolic blood pressure above 115 mmHg or for every 10 mmHg increment in diastolic blood pressure above 75 mmHg [1]. Physical activity also decreases other CVD-risk factors [2] that would otherwise compound the risk from hypertension [1]. For many endpoints reported here, the risk reductions were greater than generally reported by others. For example, meta-analyses of other population studies suggest that physical activity reduces stroke risk by about 19%, which is less than the approximately 50% risk reduction we observed [21]. The risk reduction may be greater for hypertensives, or the difference could reflect the technical advantages of using runners, walkers, and a distance-based metric for energy estimation.
Heart failure
Our data showed that exercise significantly reduced the risk of heart failure, consistent with reports from the Physician’s Health Study [22,23], Finnish men and women [24], and NHANES 1 [25]. The condition occurs when the heart is unable to provide adequate blood flow for tissue perfusion and metabolism. Heart failure is preceded by hypertension in about 90% of patients and often develops from antecedent coronary heart disease [26]. Therefore, in these hypertensives, exercise appeared to prevent or delay the cardiac remodeling that leads to heart failure.
Dysrhythmias
We also demonstrated a substantial decline in cardiac dysrhythmia with exercise that appeared to be equivalent for walkers and runners. This agrees with our recent report showing significant declines in incident physician-diagnosed cardiac arrhythmias by running and walking [11], while disagreeing with some others [27]. The Physicians Health Study reported a 53% greater risk in men who jogged five to seven days per week vis-à-vis non-joggers [27]. In contrast, the Cardiovascular Health Study reported a 48% lower risk in men and women who walked ≥ 60 blocks per week at > 3 mph as compared to those who walked ≤ 4 blocks per week at < 2 mph [28].
Diabetes
Hypertensives are 2.5-fold more likely to develop diabetes within 5 years than normotensives [29,30]. Hypertension may arise in part from insulin resistance [31,32]; indeed, treating insulin resistance often lowers blood pressure [33]. Insulin resistance is associated with endothelium-dependent vaso-contraction [34], hyperglycemia-induced glycation of endogenous protein, increased inflammatory responses leading to vasculature damage [35], endothelial dysfunction, reduced peripheral capillary density [36], and increased blood pressure. The significant reduction in diabetes-related mortality we observed is consistent with our previous finding of equivalent reductions in incident non-fatal type 2 diabetes in runners and walkers [16].
Chronic kidney disease
Hypertension is both a risk factor and a consequence of chronic renal disease [1]. Systemic elevated blood pressure leads to increased glomerular pressure, causing endothelial damage and fibrosis [37]. Chronic renal disease activates the renin-angiotensin system, which raises blood pressure [37]. Our analyses showed a 24.2% per METh/d reduction in chronic renal failure in hypertensives, independent of both diabetes and BMI.
Ischemic heart disease
As expected, IHD risk also declined with increasing exercise, although the effect appeared somewhat weaker than for other endpoints. Hypertension may increase IHD risk because coronary artery disease impedes myocardial oxygen supply [1]. Exercise may have compensated for the lack of oxygen via collateralization of coronary arteries [38]. In addition, exercise may attenuate plaque progression in coronary arteries and prevent infarction via myocardial preconditioning [38]. We found no association between METh/d run or walked and hypertensive heart disease, a condition that links arterial hypertension with left ventricular hypertrophy, myocardial fibrosis, and atherosclerotic coronary artery disease, and reduced exercise capacity.
Caveats
In these analyses, hypertensives were defined as self-identified users of hypertensive medications rather than being ascertained through the direct measurement of blood pressure, hence, some hypertensives not on medications will be incorrectly assigned to the non-hypertensive group. Although the design is prospective, and deaths occurring during the first year were excluded, we cannot rule out the possibility of reverse causality, i.e., that the extent of subclinical disease could affect MRTh/d run or walked. Compared with age-matched normotensive controls, hypertensive patients are reported to have up to 30% lower exercise capacity due to lower stroke volume, lower peak heart rate during exercise, and lower maximum heart rate [39]. However, we would expect this to have a greater effect on running than walking, which was not observed. We also note the risk of confounding by indication with respect to use of antihypertensive therapy. Our use of mortality endpoints precludes our being able to distinguish the effects of CVD-prevention from improved survival. In particular, exercise might ameliorate the hypertensives’ greater risk for death following a myocardial infarction [1]. We also caution that physician assignment of causes of death may be problematic [40-42], however, it is unclear how this could be influenced by METh/d of exercise. In addition, we note that despite their use of hypertensive medication, the cohort is likely to be more educated, and somewhat healthier as indicated by the low smoking rates, than the usual clinic population. In particular, only 3.5% of the running population reported using hypertensive medication compared to 17% of the walkers, which may be only partly due to the older age of the walkers. There may be a genetic predisposition to running, i.e., untrained rats selected over multiple generations for high-capacity running are able to run an 8.4-fold greater distance than rats selected for low-capacity running and have substantially better CVD-risk factor levels, including 13% lower 24-hour blood pressure [43]. However, it is not expected that the physiological effects of running and walking on CVD, diabetes, and chronic kidney disease would differ between the current sample and the general population. Consistent with this notion, the per METh/d reductions in disease risk were the same in runners and walkers, despite their difference in the prevalence of hypertension. Finally, we do not wish to imply that only those that run or walk ≥1.8 METh/d derive benefit from exercise. Others have shown that even as little as one hour per week of light-to-moderate intensity activity per day is associated with lower coronary heart disease risk in women over no regular exercise [44]
Conclusions
Our results provide strong justification for encouraging hypertensives to exercise. Asymptomatic hypertensive patients are known to have significantly greater carotid intima media thickness, an indicator of atherosclerosis associated with future cardiovascular events, than age matched normotensives [45]. Although exercise is clearly beneficial in normotensive patients (theoretically at least) the same benefits might not apply to exercise in hypertensives. During exercise, hypertensive patients show a greater rise in blood pressure and a blunted decline in systemic vascular resistance than normotensives [46,47]. Elevated systolic blood pressure during exercise is predictive of left ventricular hypertrophy, ≥ 1 vessel disease during coronary angiography, positive ischemic electrocardiographic findings, and cardiovascular disease mortality [39]. In our study, however, regular exercise significantly lowered the risk for a broad spectrum of diseases that hypertensives are at particular risk, including stroke, IHD, heart failure, diabetes, and CKD.
Perspectices
10,880 hypertensive medication users from the National Runners’ and Walkers’ Health Studies were used to test whether the dose-response relationship between exercise and mortality: 1) is nonlinear; 2) differs significantly between running and walking, and 3) affects specific cardiovascular disease (CVD) diagnoses, diabetes, and chronic kidney disease. Those in our study who took blood pressure medications remained at 60% greater risk for fatal CVD compared to those not taking medications. We did not find that the current exercise guidelines were adequate to significantly reduce their CVD mortality. Most of the reduction in mortality was achieved by exercising slightly more, between 1.8 and 3.6 METh/d. At that level, the hypertensives’ CVD risks were reduced to those of sedentary normotensives. Specifically, the risks of cerebrovascular disease, heart failure, and dysrhythmias were each reduced between 40% and 50%. IHD risk also declined with increasing exercise, although the effect appeared somewhat weaker than for these other endpoints. We also found that the risk for two other fatal conditions that hypertensives are prone to developing, diabetes and CKD, declined with exercise. Walking and running appeared to produce comparable risk reductions provided the energy expenditure was the same, however, one must go about 50% further and take about twice as long to expend the same amount of energy by walking briskly as by running a 12-minute mile. For many endpoints reported here, the risk reductions were greater than generally reported by others, which may reflect the technical advantages of using runners, walkers, and a distance-based metric for energy estimation.
Expanded Materials and Methods
The National Death Index provided mortality surveillance through 2008 for three cohorts: the first and second National Runners’ Health Study cohorts (NRHS-I and NRHS-II) and the National Walkers’ Health Study (NWHS). NRHS-I was recruited between 1991 and 1994 (primarily 1993), while NRHS-II and NWHS were recruited primarily between 1998 and 2001 [1-9]. The three cohorts may be more accurately characterized as a single cohort that targeted runners and walkers, since all three used the same questionnaire (modified slightly for the different activities), the same sampling domain (subscription lists to running and walking publications, running and walking events), the same survey staff, and were funded by the same grants. The National Death Index uses probabilistic matching based on available information on phonetic name, sex, social security number, birth date, and race to match cause of death to state-supplied date and causes of death from death certificates [10]. It has shown to identify over 90% of decedents and to show discrepancy from nosologist assigned causes of death in 4% of cases [11].
Participants completed baseline questionnaires on exercise, height, body weight, body circumferences, diet, cigarette use, and history of disease. The runners reported the usual miles run per week, and the walkers reported usual miles walked per week and usual pace (minutes per mile). These values were used to estimate energy expenditure in metabolic equivalents (MET), where one MET is the energy expended while sitting at rest (3.5 ml O2·kg−1·min−1) [12]. In walkers, METh/d walked was calculated by converting reported distances into durations (i.e., distance/mph), which were then multiplied by the MET value for the reported pace [4,6]. In runners, METh/d run was calculated as km run*1.02 METh/km [5,6].
Statistics
Two sample t-tests were used to compare the characteristics of the hypertensive medication users and non-users. Cox proportional hazard analyses (STATA version 11.1, StataCorp, College Station, TX) were used to test whether all cause mortality, and mortality from major cardiovascular disease (International Classification of Disease version 9 codes 390 to 459, version 10 code I00-99 [13]), ischemic heart disease (ICD9 410 –414, ICD10 I20-25), heart failure (ICD9 428, ICD10 I50), dysrhythmia (ICD9 427, ICD10 I46-49), cerebrovascular disease (ICD9 430 –438, ICD10 I60-69), hypertensive heart disease (ICD9 401-404, ICD10 I10-13), diabetes (ICD9 250, ICD10 E10-14), and chronic kidney disease (CKD, ICD9 585, ICD10 N18) were different between hypertensive medication users and non-users, and among users whether they were significantly related to METh/d run or walked when adjusted. Covariates included sex, baseline age (age and age2), education, African-American ethnicity, smoking, prior heart attack, diabetes and cholesterol medication use, aspirin (tablets/day), intakes of red meat, fruit, and alcohol, and cohort effects (NRHS-I, NRHS-II, NWHS). The covariates were chosen for their observed or accepted associations with disease outcomes, and to control for any differences between cohorts. Underlying and contributing (entity axis) causes of death were obtained from the National Death Index mortality surveillance [10]. Results are presented as hazard ratios (HR), their fold increases in risk, and their percent reductions in the risk (calculated as 100*(HR-1)) for seven categories of walking or running energy expenditure: falling short of the current physical activity recommendations for health (<450 MET minutes per week =1.07 METh/d), meeting the recommendations (450 to 750 MET minutes per week =1.07 to 1.8 METh/d), exceeding the recommendations by 1- to 2-fold (1.8 to 3.6 METh/d), 2- to 3-fold (3.6-5.4 METh/d), 3 to 4-fold (5.4-7.2 METh/d), 4 to 5-fold (7.2-9.0 METh/d), and exceeding the recommendations by greater than or equal to 5-fold [12]. A quadratic equation of METh/d run or walked was used to test for significant nonlinearity of each mortality-exercise relationship. Specifically, the significance of the coefficient “β” in the regression model “α(METh/d) + β(METh/d)2 in Cox proportional hazard analyses was used to formally prove that the dose-response relationship between mortality and exercise was nonlinear. Deaths occurring within one year of the baseline survey were excluded.
Supplementary Material
Novelty and Significance.
- What is new:
- In hypertensives, running or walking an average of 2.7 METh/d (equivalent to running 18.5 km or 11.5 mi per wk) was associated with significantly lower all-causes (29% lower), cardiovascular disease (39%), stroke (55%), ischemic heart disease (29%), heart failure (51%), and dysrhythmia mortality (47%).
- Each km run or walked per day was associated with a 19% reduction in diabetes and 25% reduction chronic kidney disease mortality.
- The benefits of walking and running in hypertensives were the same, provided that the total energy expended was the same, however, the patient would need to go 50% further and take twice as long to expend the same amount of energy by walking briskly as by running a 12-minute mile.
What Is Relevant?
Getting hypertensive patients to exercise may be one of the single most important things they can do for their health.
Summary
Running and walking substantially reduce mortality risk in hypertensives.
Acknowledgements
None
Sources of funding
This research was supported by grant HL094717 from the National Heart, Lung, and Blood Institute and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The author has declared that no competing interests exist.
Footnotes
Conflict of Interest
None to report
References
- 1.National High Blood Pressure Education Program . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute (US); Bethesda (MD): Aug, 2004. [PubMed] [Google Scholar]
- 2.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. U.S. Department of Health and Human Services; Washington, DC: 2008. pp. A1–H14. [Google Scholar]
- 3.Rossi A, Dikareva A, Bacon SL, Daskalopoulou SS. The impact of physical activity on mortality in patients with high blood pressure: a systematic review. J Hypertens. 2012;30:1277–1288. doi: 10.1097/HJH.0b013e3283544669. [DOI] [PubMed] [Google Scholar]
- 4.Engström G, Hedblad B, Janzon L. Hypertensive men who exercise regularly have lower rate of cardiovascular mortality. J Hypertens. 1999;17:737–742. doi: 10.1097/00004872-199917060-00003. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Wylie-Rosett J, Alderman MH. Exercise and cardiovascular outcomes by hypertensive status: NHANES I epidemiological followup study, 1971-1992. Am J Hypertens. 2005;18:751–758. doi: 10.1016/j.amjhyper.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Fossum E, Gleim GW, Kjeldsen SE, Kizer JR, Julius S, Devereux RB, Brady WE, Hille DA, Lyle PA, Dahlöf B. The effect of baseline physical activity on cardiovascular outcomes and new-onset diabetes in patients treated for hypertension and left ventricular hypertrophy: the LIFE study. J Intern Med. 2007;262:439–448. doi: 10.1111/j.1365-2796.2007.01808.x. [DOI] [PubMed] [Google Scholar]
- 7.Hu G, Jousilahti P, Antikainen R, Tuomilehto J. Occupational, commuting, and leisure-time physical activity in relation to cardiovascular mortality among Finnish subjects with hypertension. Am J Hypertens. 2007;20:1242–1250. doi: 10.1016/j.amjhyper.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Vatten LJ, Nilsen TI, Holmen J. Combined effect of blood pressure and physical activity on cardiovascular mortality. J Hypertens. 2006;24:1939–1946. doi: 10.1097/01.hjh.0000244941.49793.f9. [DOI] [PubMed] [Google Scholar]
- 9.Paffenbarger RS, Jung DL, Leung RW, Hyde RT. Physical activity and hypertension: an epidemiological view. Ann Med. 1991;23:319–327. doi: 10.3109/07853899109148067. [DOI] [PubMed] [Google Scholar]
- 10.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–198. [PMC free article] [PubMed] [Google Scholar]
- 11.Williams PT, Franklin B. Reduced incidence of cardiac arrhythmias in walkers and runners. PLoS ONE. 2013;8:e65302. doi: 10.1371/journal.pone.0065302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams PT. Vigorous exercise, fitness and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams PT. Advantage of distance- versus time-based estimates of walking in predicting adiposity. Med Sci Sports Exerc. 2012;44:1728–1737. doi: 10.1249/MSS.0b013e318258af3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams PT. Non-exchangeability of running vs. other exercise in their association with adiposity, and its implications for public health recommendations. PLOSOne. 2012;7:e36360. doi: 10.1371/journal.pone.0036360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PT. Distance walked and run as improved metrics over time-based energy estimation in epidemiological studies and prevention; evidence from medication use. PLoS One. 2012;7:e41906. doi: 10.1371/journal.pone.0041906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams PT, Thompson PD. Walking versus running for hypertension, cholesterol, and diabetes mellitus risk reduction. Arterioscler Thromb Vasc. Biol. 2013;33:1085–1091. doi: 10.1161/ATVBAHA.112.300878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams PT. Reduced diabetic, hypertensive, and cholesterol medication use with walking. Med Sci Sports Exerc. 2008;40:433–443. doi: 10.1249/MSS.0b013e31815f38f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams PT. Reductions in incident coronary heart disease risk above guideline physical activity levels in men. Atherosclerosis. 2010;209:524–527. doi: 10.1016/j.atherosclerosis.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. World Health Organization; Geneva: [accessed Oct 5, 2012]. 1992. http://www.cdc.gov/nchs/data/dvs/Volume-1-2005.pdf. [Google Scholar]
- 21.Diep L, Kwagyan J, Kurantsin-Mills J, Weir R, Jayam-Trouth A. Association of physical activity level and stroke outcomes in men and women: a meta-analysis. J Womens Health (Larchmt). 2010;19:1815–1822. doi: 10.1089/jwh.2009.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Tuomilehto J, Jousilahti P, Antikainen R, Mähönen M, Katzmarzyk PT, Hu G. Occupational, commuting, and leisure-time physical activity in relation to heart failure among finnish men and women. J Am Coll Cardiol. 2010;56:1140–1148. doi: 10.1016/j.jacc.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 25.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 26.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC, Jr, American College of Cardiology. American Heart Association ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 27.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103:1572–1577. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–912. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 30.Sowers JR, Bakris GL. Antihypertensive therapy and the risk of type 2 diabetes mellitus. N Engl J Med. 2000;342:969–970. doi: 10.1056/NEJM200003303421310. [DOI] [PubMed] [Google Scholar]
- 31.Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: A potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology. 2007;22:252–260. doi: 10.1152/physiol.00012.2007. [DOI] [PubMed] [Google Scholar]
- 32.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 33.Usui I, Fujisaka S, Yamazaki K, Takano A, Murakami S, Yamazaki Y, Urakaze M, Hachiya H, Takata M, Senda S, Iwata M, Satoh A, Sasaoka T, Ak ND, Temaru R, Kobayashi M. Telmisartan reduced blood pressure and HOMA-IR with increasing plasma leptin level in hypertensive and type 2 diabetic patients. Diabetes Research and Clinical Practice. 2007;77:210–214. doi: 10.1016/j.diabres.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Wendelhag I, Fagerberg B, Hulthe J, Bokemark L, Wikstrand J. Endothelium-dependent flow-mediated vasodilatation, insulin resistance and the metabolic syndrome in 60-year-old men. European Journal of Internal Medicine. 2002;252:305–313. doi: 10.1046/j.1365-2796.2002.01036.x. [DOI] [PubMed] [Google Scholar]
- 35.Vasdev S, Gill V, Singal P. Role of advanced glycation end products in hypertension and atherosclerosis: Therapeutic implications. Cell Biochemistry and Biophysics. 2007;49:48–63. doi: 10.1007/s12013-007-0039-0. [DOI] [PubMed] [Google Scholar]
- 36.Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation. 2005;12:83–92. doi: 10.1080/10739680590960241. [DOI] [PubMed] [Google Scholar]
- 37.Ruilope LM, Bakris GL. Renal function and target organ damage in hypertension. Eur Heart J. 2011;32:1599–604. doi: 10.1093/eurheartj/ehr003. [DOI] [PubMed] [Google Scholar]
- 38.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim PO, MacFadyen RJ, Clarkson PB, MacDonald TM. Impaired exercise tolerance in hypertensive patients. Ann Intern Med. 1996;124:41–55. doi: 10.7326/0003-4819-124-1_part_1-199601010-00008. [DOI] [PubMed] [Google Scholar]
- 40.Lakkireddy DR, Gowda MS, Murray CW, Basarakodu KR, Vacek JL. Death certificate completion: how well are physicians trained and are cardiovascular causes overstated? Am J Med. 2004;117:492–498. doi: 10.1016/j.amjmed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 41.McEwen LN, Kim C, Haan M, Ghosh D, Lantz PM, Mangione CM, Safford MM, Marrero D, Thompson TJ, Herman WH, TRIAD Study Group Diabetes reporting as a cause of death: results from the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2006;29:247–253. doi: 10.2337/diacare.29.02.06.dc05-0998. [DOI] [PubMed] [Google Scholar]
- 42.Messite J, Stellman SD. Accuracy of death certificate completion: the need for formalized physician training. JAMA. 1996;13(275):794–796. [PubMed] [Google Scholar]
- 43.Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 44.Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is “no pain, no gain” passé? JAMA. 2001;285:1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- 45.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr. Cardiovascular Health Study Collaborative Research Group Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 46.Goodman JM, McLaughlin PR, Plyley MJ, Holloway RM, Fell D, Logan AG, Liu PP. Impaired cardiopulmonary response to exercise in moderate hypertension. Can J Cardiol. 1992;8:363–731. [PubMed] [Google Scholar]
- 47.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589(Pt 5):1209–1220. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Williams PT, Franklin BA. Reduced incidence of cardiac arrhythmias in middle-aged and older walkers and runners. PLOS One. 2013 doi: 10.1371/journal.pone.0065302. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams PT. Vigorous exercise, fitness and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams PT. Advantage of distance-versus time-based estimates of walking in predicting adiposity. Med Sci Sports Exerc. 2012;44:1728–37. doi: 10.1249/MSS.0b013e318258af3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams PT. Non-exchangeability of running vs. other exercise in their association with adiposity, and its implications for public health recommendations. PLOSOne. 2012;7:e36360. doi: 10.1371/journal.pone.0036360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams PT. Distance walked and run as improved metrics over time-based energy estimation in epidemiological studies and prevention; evidence from medication use. PLoS One. 2012;7:e41906. doi: 10.1371/journal.pone.0041906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams PT, Thompson PD. Walking versus running for hypertension, cholesterol, and diabetes mellitus risk reduction. Arterioscler Thromb Vasc Biol. 2013;33:1085–91. doi: 10.1161/ATVBAHA.112.300878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams PT. Reduced diabetic, hypertensive, and cholesterol medication use with walking. Med Sci Sports Exerc. 2008;40:433–43. doi: 10.1249/MSS.0b013e31815f38f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams PT. Reductions in incident coronary heart disease risk above guideline physical activity levels in men. Atherosclerosis. 2010;209:524–7. doi: 10.1016/j.atherosclerosis.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics . National Death Index user’s guide. Hyattsville, MD: [Accessed May 10, 2013]. 2013. Available from: http://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf. [Google Scholar]
- 11.Sathiakumar N, Delzell E, Abdalla O. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med. 1998;40:808–13. doi: 10.1097/00043764-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. World Health Organization; Geneva: [accessed Oct 5, 2012]. 1992. http://www.cdc.gov/nchs/data/dvs/Volume-1-2005.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


