Abstract
Background
Platinum resistance is a major limitation in the treatment of advanced non-small cell lung cancer (NSCLC). We previously demonstrated that low tissue platinum concentration in NSCLC specimens was significantly associated with reduced tumor response. Furthermore, low expression of the copper transporter CTR1, a transporter of platinum uptake was associated with poor clinical outcome following platinum-based therapy in NSCLC patients. We investigated the relationship between tissue platinum concentrations and CTR1 expression in NSCLC specimens.
Methods
We identified paraffin-embedded NSCLC tissue blocks of known tissue platinum concentrations from 30 patients who underwent neoadjuvant platinum-based chemotherapy at MD Anderson Cancer Center. Expression of CTR1 in tumors and normal adjacent lung specimens was determined by immunohistochemistry with adequate controls.
Results
Tissue platinum concentration significantly correlated with tumor response in 30 patients who received neoadjuvant platinum-based chemotherapy (P<0.001). CTR1 was differentially expressed in NSCLC tumors. A subset of patients with undetectable CTR1 expression in their tumors had reduced platinum concentrations (P=0.058) and tumor response (P=0.016) compared to those with any level of CTR1 expression. We also observed that African Americans had significantly reduced CTR1 expression scores (P=0.001), tissue platinum concentrations (P=0.009) and tumor shrinkage (P=0.016) compared to Caucasians.
Conclusions
To our best knowledge this is the first study investigating the function of CTR1 in clinical specimens. CTR1 expression may be necessary for therapeutic efficacy of platinum drugs, consistent with previous preclinical studies. A prospective clinical trial is necessary to develop CTR1 into a potential biomarker for platinum drugs.
INTRODUCTION
Platinum-based chemotherapy is widely used in advanced non-small cell lung cancer (NSCLC). However, first-line platinum-containing doublets yield response rates in NSCLC of only 20–30%,1,2 since a significant portion of tumors express intrinsic or de novo resistance. A better understanding of molecular mechanisms of platinum resistance is necessary to develop new therapeutic approaches that induce greater platinum sensitivity and more durable responses.
There are several mechanisms by which NSCLC cells may express resistance to platinum, such as, but not limited to, drug inactivation by detoxifying factors, alterations in checkpoint and apoptotic proteins, and alteration in intracellular drug accumulation.3 Despite the multifactorial nature of platinum resistance, reduced intracellular drug accumulation is one of the most consistently identified features of cisplatin-resistant cell lines,4,5 including resistant NSCLC cell lines.6–10 To clinically validate reduced drug accumulation as an important mechanism of platinum resistance, we previously demonstrated that lower tissue platinum concentrations from NSCLC specimens following platinum-based chemotherapy significantly correlated directly with reduced tumor response and shorter survival time.11 The role of copper transporter CTR1, a significant transporter of platinum uptake, may be implicated in modulation of intratumoral platinum concentrations.
CTR1 regulates uptake of copper which is a vital micronutrient for eukaryotic development. CTR1 also plays a significant role in platinum uptake. Deletion of the Ctr1 gene in yeast and murine cells resulted in reduced accumulation of cisplatin and increased cisplatin resistance.12 Conversely, enhanced uptake of carboplatin and oxaliplatin was seen when the Ctr1 gene was transfected into small cell lung cancer cell lines, supporting the importance of CTR1 in uptake of a variety of platinum drugs.13 Furthermore, low levels of CTR1 mRNA level in platinum-treated ovarian tumors were associated with poor clinical outcome.14 In NSCLC, low expression of CTR1 by immunohistochemistry (IHC), but not expression of the efflux transporters ATP7A and ATP7B, is associated with poor prognosis in patients treated with platinum-based therapy.15 Therefore, CTR1 has a significant potential to become a biomarker for intracellular platinum uptake and tumor response. To date, however, no study has compared tumor CTR1 expression with intratumoral platinum concentration in any tumor type. We hypothesized that a defect in tumor CTR1 expression is associated with reduced tissue platinum accumulation and tumor response in NSCLC following platinum-based chemotherapy.
METHODS
Patients and Tissue Specimens
This study (approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center [UTMDACC]) used UT Lung Cancer Specialized Program of Research Excellence Tissue Bank archived fresh-frozen NSCLC tumor specimens. We identified paraffin-embedded NSCLC tissue blocks from 30 of our 44 UTMDACC NSCLC patients who had received neoadjuvant platinum-based chemotherapy and with known tissue platinum concentrations obtained from our previous study that measured intratumoral platinum levels.11 Paraffin-embedded blocks were not available for 14 patients. Of those 30 tumor specimens, 25 specimens had matching normal adjacent lung tissues which were included in this study. Various histopathologic features including percentage of residual viable tumor cells, necrosis and fibrosis were assessed in all tumor samples as described previously.16
Immunohistochemistry for CTR1
IHC to determine post-treatment expression of CTR1 was performed on representative paraffin-embedded blocks of tumor and normal adjacent lung specimens with adequate controls as previously described.15 Primary antibody for CTR1 (Novus Biologicals, Littleton, CO) we used has been validated by us and several others.15,17–19 The antibody was diluted at 1:500 and incubated overnight at 4°C. Expression of CTR1 was scored by assessing the intensity (on a 0–3 scale) by UTMDACC thoracic pathologists who were blinded to clinical information and platinum concentrations; 0 = undetectable; 1+ = barely detectable staining; 2+ = readily appreciable staining; and 3+ = dark brown staining. Percentage of positive cytoplasmic staining cells was also determined but most of the specimens with scores of 1+ or greater demonstrated diffuse cytoplasmic staining (>75%). Thus, semi-quantitative scores are not reported separately in this article.
Tissue Platinum Measurement
Approximately 30mg of tumor from each patient was weighed and digested overnight in benzethonium hydroxide at 55°C to achieve homogeneity.11,20,21 After acidification, each sample was analyzed by flameless atomic absorption spectrophotometry (FAAS) to measure absorbance unit associated with platinum content, as previously described.11,20 Validity of the assay was ensured with a linear standard curve that was generated from serial dilutions of certified stock platinum standard (Sigma, 987μg/ml). Most specimens were analyzed in at least two independent experiments where samples were taken from different parts of the tumor. The averaged platinum concentration was reported as absorbance unit per mg of tissue.
Statistical Analysis
The main objective of this study was to study the relationship between CTR1 expression level and intratumoral platinum concentration in NSCLC patients. Tissue platinum concentrations in tumors from 30 NSCLC patients were obtained from our previous study that showed a significant correlation between tissue platinum concentration and percent reduction in tumor size in 44 patients who received neoadjuvant platinum-based chemotherapy.11 This correlation was re-estimated based on the subsample of 30 patients, for comparison. Four group Kruskal-Wallis tests were used to compare the distributions of both platinum concentrations and percent reduction in tumor size by IHC-based CTR1 expression score (0, 1+, 2+, 3+). Mann-Whitney non-parametric tests were used to make all two group comparisons such as comparison of the distributions of CTR1 expression score, tumor response, and intratumoral platinum concentration for Caucasian Americans (CAs) versus African Americans (AAs).
RESULTS
Patient characteristics
Table 1 shows the patient characteristics of 30 evaluable patients with early stage NSCLC with known intratumoral platinum concentrations who received neoadjuvant platinum-based chemotherapy prior to undergoing surgical resection. Median age was 63, with 60% males and 40% females. There were 23 Caucasians (77%) and 6 African Americans (20%). A majority of patients had either stage IIB (37%) or IIIA (43%) disease. All 30 patients received a doublet consisting of cisplatin (N=11) or carboplatin (N=19). Most received taxanes as the second agent. Median time from last dose of chemotherapy to surgery was 37 days. There were 16 adenocarcinomas (53%), 7 squamous cell (23%) and 7 other histology types (23%).
Table 1.
Medical and demographic characteristics of 30 evaluable patients.
| Total number of evaluable patients | 30 | |
|---|---|---|
| Age, median (range) | 63(44–78) | |
| Characteristic | N | % |
| Gender | ||
| Male | 18 | 60 |
| Female | 12 | 40 |
| Ethnicity | ||
| Caucasian | 23 | 77 |
| African American | 6 | 20 |
| Hispanic | 1 | 3 |
| Clinical stage | ||
| IB | 2 | 7 |
| IIA | 1 | 3 |
| IIB | 11 | 37 |
| IIIA | 13 | 43 |
| IIIB | 3 | 10 |
| Histology | ||
| Adenocarcinoma | 16 | 53 |
| Squamous cell carcinoma | 7 | 23 |
| Other | 7 | 23 |
| Neoadjuvant chemotherapy | ||
| Cisplatin | 11 | 37 |
| + Taxane | 9 | |
| + Other | 2 | |
| <3 cycles | 5 | |
| ≥3 cycles | 6 | |
| Carboplatin | 19 | 63 |
| + Taxane | 17 | |
| + Other | 2 | |
| <3 cycles | 8 | |
| ≥3 cycles | 11 | |
| Smoking status | ||
| Current smoker | 10 | 33 |
| Former smoker | 10 | 33 |
| Never smoker | 5 | 17 |
| Undocumented | 5 | 17 |
| Tumor response by RECIST | ||
| Stable disease | 22 | 73 |
| Partial response | 8 | 27 |
| Tobacco pack years | 32 (0–145) | |
| Time lapse from last chemotherapy (days) | 37 (22–71) | |
Abbreviations: RECIST= Response Evaluation Criteria in Solid Tumors
Correlation between tissue platinum concentration and tumor response
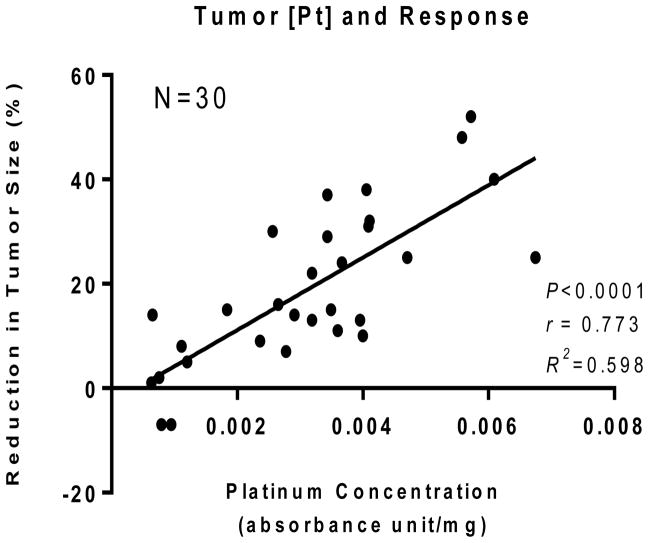
In our previous study, we reported a significant correlation between tissue platinum concentration and tumor response in 44 patients.11 To confirm if the same correlation is seen in the 30 evaluable patients with available paraffin blocks, tissue platinum concentration and percent reduction in tumor size following platinum-based chemotherapy were correlated. As shown in Figure 1, all except 2 platinum-treated patients had at least some degree of tumor shrinkage with therapy, and there was a significant correlation (Pearson r=0.773, P<0.0001) between tumor platinum concentration and % change in tumor size.
Figure 1.
Correlation between tissue platinum concentration and tumor response in 30 patients whose tumor specimens were adequate for immunohistochemistry. The data was abstracted from the previous publication demonstrating correlation between tissue platinum concentration and tumor response in 44 patients.
CTR1 expression by immunohistochemistry in NSCLC and normal lung tissues
Figure 2 demonstrates CTR1 expression in representative specimens of each IHC score group. CTR1 expression scores for tumor and adjacent normal lung tissues are shown in Table 2. Of 30 tumor specimens, 50% of specimens demonstrated scores of 2+. There were 2 patients (7%) with undetectable CTR1 expression by IHC. The specimens from 6 patients (20%) demonstrated intense 3+ staining. Of 30 tumor specimens, there were 25 matching normal adjacent lung specimens. There was no significant relationship in CTR1 expression scores between tumor and normal specimens (P=0.12).
Figure 2.
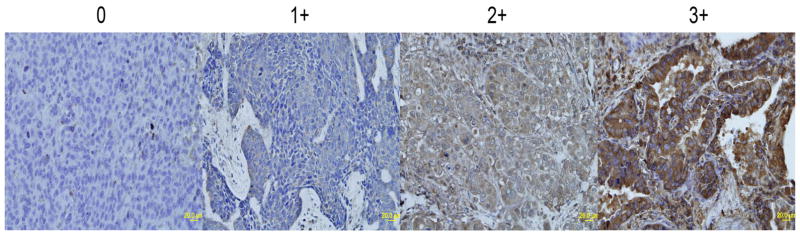
Differential expression of CTR1 by immunohistochemistry in NSCLC. (0 = no appreciable staining; 1+ = barely detectable staining; 2+ = readily appreciable staining; and 3+ = dark brown staining)
Table 2.
CTR1 expression scores in tumor and normal lung epithelium
| Specimen | Tumor Pt Concentration (absorbance units/mg) | CTR1 in Tumors | CTR1 in Normal Epithelium |
|---|---|---|---|
| 1 | 0.00120 | 0 | 3+ |
| 2 | 0.00095 | 0 | 2+ |
| 3 | 0.00470 | 1+ | N/A* |
| 4 | 0.00076 | 1+ | 2+ |
| 5 | 0.00112 | 1+ | 2+ |
| 6 | 0.00609 | 1+ | 2+ |
| 7 | 0.00065 | 1+ | N/A |
| 8 | 0.00572 | 1+ | 3+ |
| 9 | 0.00349 | 1+ | N/A |
| 10 | 0.00079 | 2+ | 3+ |
| 11 | 0.00409 | 2+ | 3+ |
| 12 | 0.00291 | 2+ | 3+ |
| 13 | 0.00184 | 2+ | 2+ |
| 14 | 0.00256 | 2+ | 0 |
| 15 | 0.00319 | 2+ | 3+ |
| 16 | 0.00344 | 2+ | 2+ |
| 17 | 0.00406 | 2+ | 2+ |
| 18 | 0.00343 | 2+ | 2+ |
| 19 | 0.00278 | 2+ | 2+ |
| 20 | 0.00674 | 2+ | 2+ |
| 21 | 0.00396 | 2+ | 2+ |
| 22 | 0.00366 | 2+ | N/A |
| 23 | 0.00064 | 2+ | 3+ |
| 24 | 0.00400 | 2+ | 2+ |
| 25 | 0.00236 | 3+ | 0 |
| 26 | 0.00265 | 3+ | 2+ |
| 27 | 0.00410 | 3+ | 0 |
| 28 | 0.00319 | 3+ | 2+ |
| 29 | 0.00360 | 3+ | 3+ |
| 30 | 0.00558 | 3+ | N/A |
N/A = specimen not available
Abbreviations: Pt=Platinum
Relationship between CTR1 expression and tissue platinum concentration
As shown in Figure 3A, there was insufficient evidence of differences in tissue platinum concentration by individual CTR1 expression score groups (P=0.44). However, the specimens with undetectable CTR1 expression (score of 0) by IHC, in particular, had extremely low mean intratumoral platinum concentrations of 0.0011 absorbance units/mg of tissue compared to the mean platinum concentration of the rest of specimens (score of 1+ or higher) at 0.0033 absorbance units/mg (Figure 3B). This represents approximately a 3-fold difference that has a considerable trend towards significance (P=0.058). Furthermore, there was a significant difference in % reduction in tumor size between the group with undetectable CTR1 expression and the rest (P=0.016) which translated to response rates of 0% and 29%, respectively (Figure 3C). The characteristics of the two patients with undetectable CTR1 expression were most notable for African American ethnicity and cisplatin therapy, and neither of them had adenocarcinomas (squamous cell carcinoma and NSCLC with sarcomatoid features). Ethnic difference in CTR1 expression is further discussed below. CTR1 expression scores were significantly higher in patients who received carboplatin compared to those who received cisplatin (P=0.004). Furthermore, CTR1 expression was higher in adenocarcinomas compared to squamous cell carcinomas (P=0.018). Of note, all 6 patients who demonstrated 3+ staining had adenocarcinomas. However, there was no significant difference in tumor platinum concentrations between adenocarcinoma vs. squamous cell carcinoma and cisplatin vs. carboplatin, consistent with our previous report.11
Figure 3.
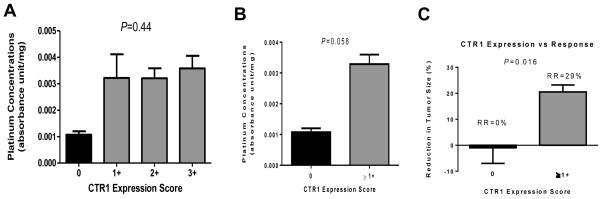
Relationship between tumor platinum concentration and the level of CTR1 expression in NSCLC. There was no significant difference in platinum concentration when compared across the four different groups based on immunohistochemistry scores for CTR1 expression (A). However, tumors with no expression of CTR1 (score of 0) had significantly lower platinum concentration compared to the rest of the groups combined (B). Patients with no expression of CTR1 had response rate (RR) of 0% whereas those with any level of CTR1 expression demonstrated RR of 29% (C). The bars represent the standard error of the mean.
Ethnic differences in CTR1 expression, tissue platinum concentration and tumor response
The fact that both patients with null CTR1 expression in their tumors were AAs led to additional analysis. There was a total of 23 CAs and 6 AAs. As shown in Figure 4A, we observed a significant difference in CTR1 expression score between CAs and AAs (P=0.0013). This observation translated to the finding that AAs had significantly reduced intratumoral platinum concentrations (P=0.009) and tumor response (P=0.016) compared to CAs (Figure 4B and 4C, respectively). However, there was no significant difference in CTR1 expression score between CAs and AAs in normal adjacent epithelial specimens (P=0.28).
Figure 4.
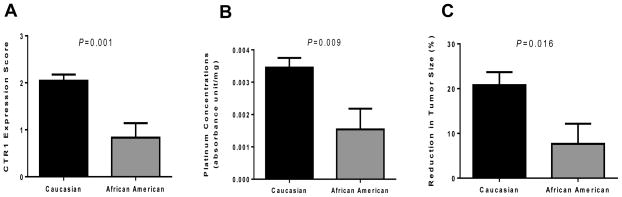
Ethnic variations in CTR1 expression and tumor platinum concentrations. Caucasian patients had higher level of CTR1 expression (A) and tissue platinum concentration (B) compared to African American patients. There was also a significant difference in tumor response between Caucasian and African American patients. The bars represent the standard error of the mean.
DISCUSSION
Despite extensive preclinical investigation of CTR1 as an important platinum transporter, there has been no investigation of CTR1’s function in clinical specimens to date, possibly due to difficulty in obtaining adequate amount of fresh post-treatment biopsy to permit tissue platinum measurement. To our best knowledge, this is the first study in any tumor type investigating the relationship between copper transporter CTR1 expression and intratumoral platinum concentrations in clinical specimens. We demonstrated that CTR1 is differentially expressed in NSCLC tumors with no apparent concordance with CTR1 expression in normal adjacent lung tissues. Even though there was no directly proportional relationship between CTR1 expression scores and intratumoral platinum concentrations, the subgroup of patients with undetectable CTR1 expressions had markedly lower tissue platinum concentrations and reduced tumor response compared to the rest. While we were limited by a small sample size to conduct robust statistical analysis in our clinical specimens, we believe that this could be of substantial clinical significance supporting several preclinical studies to date that suggest the role of CTR1 as an important uptake transporter of platinum drugs and as a major determinant of platinum sensitivity in tumor cells.12–14 Previous studies also demonstrated that low expression of CTR1 was associated with poor clinical outcome following platinum therapy in NSCLC and ovarian cancer patients.14,15 Our data offer a potential explanation for these clinical studies demonstrating low CTR1 expression as a poor prognosticator following platinum-based chemotherapy. We can speculate that intact CTR1 function prior to treatment may be required for platinum drug’s therapeutic efficacy. However, our sample size was inevitably small because only a small proportion of all patients with NSCLC are candidates for neoadjuvant chemotherapy which currently is not a standard of care. Despite these small patient numbers, we had access to 30 highly-characterized, matching fresh-frozen and paraffin-embedded neoadjuvant NSCLC specimens to study correlation between CTR1 expression and tumor platinum concentrations. Even though we could not address the influence of potential confounding variables relating to a small sample size, this is the first report to our knowledge investigating the function of CTR1 in any clinical specimens. This may set a foundation for future studies with a larger number of patients to permit independent validation.
When we looked in detail the characteristics of the two patients with undetectable CTR1 expressions, we were surprised to learn that both patients were AAs. Further analysis led to the observation that AAs had significantly lower levels of CTR1 expression in their tumors but not in normal tissues compared to CAs which corresponded to significantly reduced intratumoral platinum concentrations and tumor response in AAs. Our finding is further supported by a recent study using ethnically-defined Human Variation Panel lymphoblastoid cell lines from 100 CAs and 100 AAs which demonstrated that the cell lines derived from CAs were significantly more sensitive to cisplatin compared to those derived from AAs.22 Pharmacoethnicity, defined as ethnic diversity in drug response or toxicity, is being recognized as an important contributing factor for inter-individual variation in response to anti-tumor agents.23 A few examples in NSCLC include higher prevalence of sensitizing EGFR mutations to tyrosine kinase inhibitors in Asians24,25 and activity of cetuximab in exclusively CAs.26 Although pharmacoethnicity can be both genetic and environmental, ethnic variations in several cancer drug-related genes have been reported.23 For example, previous studies reported ethnic differences in allelic frequencies of drug transporter genes ABCG2 and MDR1.27,28 AAs with lung cancer in which platinum-based therapy remains as standard of care have lower survival rates compared to CAs.29 Furthermore, Gynecologic Oncology Group study involving 428 patients with advanced cervical cancer who received cisplatin-based therapy revealed that African-American ethnicity is independently prognostic of poor tumor response to cisplatin.30 Platinum pharmacoethnicity due to ethnic variations in transporter expression could certainly be a contributing factor as demonstrated by our evaluation of a key platinum transporter CTR1.
Our finding that tumor CTR1 expression scores did not directly correlate with tissue platinum concentration could be explained by several limitations besides a small sample size. Our study population was fairly heterogeneous especially in terms of histology and type of platinum drugs received. Patients who received carboplatin or had adenocarcinomas demonstrated higher tumor CTR1 expressions. It is uncertain if adenocarcinoma cells generally express a higher level of CTR1 proteins at baseline and what percentage of these would be actively functioning as transporters. It is possible that CTR1 may be acting as an adaptive response/resistance-inducing transport factor that limits further net platinum accumulation after initial exposure to platinum drugs. Our data represent only post-treatment CTR1 expression levels as we did not have pre-treatment slides available. Thus, we were not able to assess the potential modulation of CTR1 by platinum drugs. Our observation that patients who received cisplatin had lower CTR1 expression could be due to cisplatin-mediated down-regulation of CTR1, as previously demonstrated by Holzer and colleagues.31 A future study prospectively investigating both pre- and post-treatment CTR1 expression in the same patient population would be important. Lastly, it is possible that other mechanisms (i.e. efflux transporters) may also be at play to contribute to modulation of intratumoral platinum concentrations.
In conclusion, this is the first translational study to our knowledge evaluating relationship between drug transporter expression and intratumoral tissue platinum concentration in any tumor type. Our data suggest CTR1’s role as a platinum uptake transporter as evidenced by our finding that NSCLC patients with undetectable CTR1 expression in their tumors had reduced intratumoral platinum concentration and tumor response compared to patients with any level of CTR1 expression. In addition, we report a novel finding that AAs had reduced CTR1 expression, tissue platinum concentration and tumor response compared to CAs. Our data, together with an independent study suggesting reduced CTR1 as a poor prognostic marker for platinum-based therapy in NSCLC15 warrant further investigation of CTR1 as a potential biomarker for platinum therapy in NSCLC. This is a single institution study with a small patient population that requires independent validation with a larger number of patients.
Acknowledgments
Supported by ASCO Conquer Cancer Foundation Young Investigator Award, National Foundation of Cancer Research 90088436, Department of Defense W81XWH-07-1-0306, National Institute of Health CA127263, CA160687, CA16672, Specialized Program of Research Excellence in Lung Cancer P50CA70907
Footnotes
Presented in part at 2013 ASCO Annual Meeting, Chicago, IL
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoang T, Xu R, Schiller JH, et al. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–83. doi: 10.1200/JCO.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit Rev Oncol Hematol. 2010;75:173–234. doi: 10.1016/j.critrevonc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993;67:1171–6. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews PA, Howell SB. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990;2:35–43. [PubMed] [Google Scholar]
- 6.Shellard SA, Fichtinger-Schepman AM, Lazo JS, et al. Evidence of differential cisplatin-DNA adduct formation, removal and tolerance of DNA damage in three human lung carcinoma cell lines. Anticancer Drugs. 1993;4:491–500. doi: 10.1097/00001813-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Shimura M, Saito A, Matsuyama S, et al. Element array by scanning X-ray fluorescence microscopy after cis-diamminedichloro-platinum(II) treatment. Cancer Res. 2005;65:4998–5002. doi: 10.1158/0008-5472.CAN-05-0373. [DOI] [PubMed] [Google Scholar]
- 8.Kawai H, Kiura K, Tabata M, et al. Characterization of non-small-cell lung cancer cell lines established before and after chemotherapy. Lung Cancer. 2002;35:305–14. doi: 10.1016/s0169-5002(01)00430-5. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura T, Takigawa N, Kiura K, et al. Determinants of cisplatin and irinotecan activities in human lung adenocarcinoma cells: evidence of cisplatin accumulation and topoisomerase I activity. In Vivo. 2005;19:717–21. [PubMed] [Google Scholar]
- 10.Bungo M, Fujiwara Y, Kasahara K, et al. Decreased accumulation as a mechanism of resistance to cis-diamminedichloroplatinum(II) in human non-small cell lung cancer cell lines: relation to DNA damage and repair. Cancer Res. 1990;50:2549–53. [PubMed] [Google Scholar]
- 11.Kim ES, Lee JJ, He G, et al. Tissue platinum concentration and tumor response in non-small-cell lung cancer. J Clin Oncol. 2012;30:3345–52. doi: 10.1200/JCO.2011.40.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida S, Lee J, Thiele DJ, et al. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song IS, Savaraj N, Siddik ZH, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3:1543–9. [PubMed] [Google Scholar]
- 14.Ishida S, McCormick F, Smith-McCune K, et al. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574–83. doi: 10.1016/j.ccr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HH, Yan JJ, Chen WC, et al. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–34. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pataer A, Kalhor N, Correa AM, et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer After Neoadjuvant Chemotherapy. J Thorac Oncol. 2012 doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzer AK, Varki NM, Le QT, et al. Expression of the human copper influx transporter 1 in normal and malignant human tissues. J Histochem Cytochem. 2006;54:1041–9. doi: 10.1369/jhc.6A6970.2006. [DOI] [PubMed] [Google Scholar]
- 18.More SS, Akil O, Ianculescu AG, et al. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010;30:9500–9. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jandial DD, Farshchi-Heydari S, Larson CA, et al. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res. 2009;15:553–60. doi: 10.1158/1078-0432.CCR-08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddik ZH, Boxall FE, Harrap KR. Flameless atomic absorption spectrophotometric determination of platinum in tissues solubilized in hyamine hydroxide. Anal Biochem. 1987;163:21–6. doi: 10.1016/0003-2697(87)90087-x. [DOI] [PubMed] [Google Scholar]
- 21.Verschraegen CF, Kumagai S, Davidson R, et al. Phase I clinical and pharmacological study of intraperitoneal cis-bis-neodecanoato(trans- R, R-1, 2-diaminocyclohexane)-platinum II entrapped in multilamellar liposome vesicles. J Cancer Res Clin Oncol. 2003;129:549–55. doi: 10.1007/s00432-003-0481-3. [DOI] [PubMed] [Google Scholar]
- 22.Tan XL, Moyer AM, Fridley BL, et al. Genetic variation predicting cisplatin cytotoxicity associated with overall survival in lung cancer patients receiving platinum-based chemotherapy. Clin Cancer Res. 2011;17:5801–11. doi: 10.1158/1078-0432.CCR-11-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009;15:4806–14. doi: 10.1158/1078-0432.CCR-09-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–20. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 25.Takano T, Ohe Y, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829–37. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 26.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 27.Xie HG, Kim RB, Wood AJ, et al. Molecular basis of ethnic differences in drug disposition and response. Annu Rev Pharmacol Toxicol. 2001;41:815–50. doi: 10.1146/annurev.pharmtox.41.1.815. [DOI] [PubMed] [Google Scholar]
- 28.Kim RB, Leake BF, Choo EF, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 29.U.S. National Institutes of Health. National Cancer Institute: SEER Cancer Statistics Review. 1973–2006. [Google Scholar]
- 30.Moore DH, Tian C, Monk BJ, et al. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116:44–9. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 2006;66:10944–52. doi: 10.1158/0008-5472.CAN-06-1710. [DOI] [PubMed] [Google Scholar]