Abstract
Background
Physical activity and cardiorespiratory fitness are not currently recognized as factors related to preventing gout, nor are risk factors for gout in physically active men well understood.
Objective
The objective was to identify risk factors for gout in ostensibly healthy, vigorously active men.
Design
Incident self-reported gout was compared with baseline diet, body mass index (BMI; in kg/m2), physical activity (in km/d run), and cardiorespiratory fitness (in m/s during 10-km footrace) prospectively in 28 990 male runners.
Results
Men (N=228; 0.79%) self-reported incident gout during 7.74 y of follow-up. The risk of gout increased with higher alcohol intake [per 10 g/d; relative risk (RR): 1.19; 95% CI: 1.12 to 1.26; P<0.0001], meat consumption (per servings/d; RR: 1.45; 95% CI: 1.06 to 1.92; P=0.002), and BMI (RR: 1.19; 95% CI: 1.15 to 1.23; P<0.0001) and declined with greater fruit intake (per pieces/d; RR: 0.73; 95% CI: 0.62 to 0.84; P<0.0001), running distance (per km/d; RR: 0.92; 95% CI: 0.88 to 0.97; P<0.001), and fitness (per m/s; RR: 0.55; 95% CI: 0.41 to 0.75; P<0.0001). The RR per 10 g alcohol/d consumed as wine (1.27; P=0.002), beer (1.19; P<0.0001), and mixed drinks (1.13; P=0.18) was not significantly different from each other. Men who consumed >15 g alcohol/d had 93% greater risk than abstainers, and men who averaged >2 pieces fruit/d had 50% less risk than those who ate <0.5 fruit/d. Risk of gout was 16-fold greater for BMI>27.5 than <20. Compared with the least active or fit men, those who ran ≥8 km/d or >4.0 m/s had 50% and 65% lower risk of gout, respectively. Lower BMI contributed to the risk reductions associated with distance run and fitness.
Conclusion
These findings, based on male runners, suggest that the risk of gout is lower in men who are more physically active, maintain ideal body weight, and consume diets enriched in fruit and limited in meat and alcohol.
INTRODUCTION
Gout results from the deposition of monosodium urate crystals in joints, causing an inflammatory response (1, 2). It is the most common inflammatory arthritis among men >40, affecting approximately 3.4 million American men (2). The US National Health Interview Survey found that the overall prevalence in 1996 of self-reported gout with age 45 y was 4.6% in men and 2% in women (3).
Hyperuricemia is the most important risk factor for gout (1, 3). The Normative Aging Study reported a 4.9% annual incidence for persons with urate concentrations >9 mg/dL compared with 0.1% in persons with urate concentrations <7 mg/dL (4). Higher uric acid concentrations probably explain the greater prevalence of gout in men than in women. However, most hyperuricemic patients do not develop gout (5). Other purported risk factors include hypertension, diuretic and low-dose aspirin use, total and intra-abdominal obesity, excessive weight gain in early adulthood, alcohol consumption, renal insufficiency, renal and other major organ transplantations, and family history (3). Although patients with gout are encouraged to avoid purine-rich foods, this recommendation is based primarily on increases of serum uric acid in animals and in humans fed purified purine, rather than purine’s direct affect on gouty arthritis (6–9). There have been few prospective studies of diet, adiposity, and hypertension to support their causal relation to incident gout.
Physical activity is largely ignored with respect to gout prevention (3). Cross-sectionally, serum concentrations of uric acid decline in relation to weekly distance run (10) and are lower in athletes than in healthy nonathletic controls (11). Whether postexercise increases in serum concentrations of uric acid because of decreased excretion might cause monosodium urate crystals to precipitate and trigger gouty attacks is not known (12). Cross-sectionally, persons with non-disabling arthritis are more likely to report no leisure-time physical activity and are less likely to report vigorous exercise than are persons without arthritis (13), but this could be due to arthritis limiting the exercise dose rather than to exercise producing a reduction in arthritis risk.
This report examines prospectively the dose-response relations of incident gout to baseline diet, body weight, hypertension, physical activity, and cardiorespiratory fitness. The National Runners’ Health Study was created to assess the health benefits of vigorous exercise (10, 14–17). Although other established cohorts have reported on the health effects of physical activity or fitness, all were selected with the use of geographical, occupational, or professional criteria and therefore include relatively few vigorously active men. This article also reports on cross-sectional relations of baseline serum concentrations of uric acid with baseline exercise, obesity, diet, hypertension, and aspirin use to determine their consistency with the prospective data.
SUBJECTS AND METHODS
The design and methods of the National Runners’ Health Study were described elsewhere (10, 14–17). Briefly, recruiting of this cohort took place between 1991 and 1993 by distributing a 2-page questionnaire nationally to runners identified through subscription lists to running magazine and among participants of footrace events. The questionnaire solicited information on demographics, running history, weight history, smoking habit, prior history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, and diabetes. I estimate that approximately 15% returned the questionnaires among the total number contacted. There were 36 152 nonsmoking, non-diabetic, non-vegetarian men who provided complete data on age, body mass index (BMI; in kg/m2), and weekly distance run. These included 5291 men who had their most recent uric acid concentrations forwarded to us by their physicians (10). The study protocol was approved by the University of California Berkeley Committee for the Protection of Human Subjects, and all participants signed committee-approved informed consents.
Follow-up questionnaires were mailed between 1998 and 2001 and requested updated information on the average distance run per week and other running history, body weight, and medical history. Participants reported whether a physician had told them they had gout since their baseline questionnaire and provided the year of diagnosis. Running distances were reported in usual miles run per week at baseline. Previously, I reported strong correlations between repeated questionnaires for self-reported running distance (r=0.89) (17) and between self-reported and clinically measured height (r=0.96) and weight (r=0.96) (17). In addition, self-reported running distance was shown to be significantly associated with a number of biomarkers traditionally associated with physical activity, including HDL-cholesterol, triacylglycerols, and LDL-cholesterol concentrations; systolic and diastolic blood pressures; fasting plasma concentrations of glucose; and self-reported BMI and body circumferences (10). Although other leisure-time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was added to the survey) show that running represented the majority of vigorously intense activity and total leisure time physical activity.
Intakes of meat, fish, and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat,” “ servings of fish do you eat,” and “ pieces of fruit do you eat?” Consumptions of fruit juice, vegetables, and specific fruit were not obtained. Alcohol intake was estimated from the corresponding questions for 4-oz (112 mL) glasses of wine, 12-oz (336 mL) bottles of beer, and mixed drinks and liqueurs. Alcohol was computed as 10.8 g/4-oz glass of wine, 13.2 g/12-oz bottle of beer, and 15.1 g/mixed drink. Correlations between these responses and values obtained from 4-d diet records in 110 men were r=0.65 for alcohol intake, r=0.46 for red meat, r =0.38 for fruit, and r=0.19 for fish. These values agree favorably with published correlations between food records and more extensive food-frequency questionnaires for red meat (r=0.50), wine (r=0.66), beer (r=0.70), and mixed drinks (r=0.72), somewhat less favorably for fruit intake (r=0.50), and less favorably for fish intake (r=0.51) (18). Errors in measuring the independent variable, as indicated by a low test-retest correlation, attenuate the magnitude and significance of the variable’s relation to gout (survival analyses) and uric acid concentrations (regression analyses). For this report, baseline cardiorespiratory fitness was defined as speed (in m/s) of the participant’s best 10-km race during the previous 5 y (reported as finish time in minutes). Published data support the use of running performance to estimate maximal oxygen consumption (19–23), and 10-km race performance times were shown to be more strongly correlated with the prevalence of diabetes, hypertension, and hypercholesterolemia than was running distance (24).
Cox proportional hazard model (JMP software version 5.0; SAS Institute, Cary, NC) was used to estimate the dose-response relations of incident gout to baseline body weight and circumferences, usual distances run, and cardiorespiratory fitness. Reported intakes of alcohol, meat, fish, and fruit, along with age, hypertension, and BMI, were used as covariates, with quadratic terms for age and BMI, because of their nonlinear relations to running distance and each other. All data were inspected for potential outliers before analyses. In addition, analyses were performed with the use of the risk factors as both continuous and categorical measures; although the statistical power to detect associations may be less for the categorical scale, re-expressing the exposure variables for categorical measures provides greater robustness to outliers.
RESULTS
There were 28 990 nonsmoking, non-vegetarian, and non-diabetic men who reported their average weekly running distance, height, body weight, and age at baseline, provided follow-up questionnaires, and did not report diagnosis of gout before their baseline survey (80.2% follow-up). Responders were somewhat older (mean±SD: 44.7±10.9 y compared with 42.2±10.8 y) and had a lower BMI (23.9±2.7 compared with 24.3±2.9) than did non-responders, but responders and non-responders ran similar baseline distances (3.3±2.0 km/d and 3.4±2.1 km/d). The distributions of the responder sample characteristics are shown in Table 1. These included 228 men (0.79%) who reported incident gout during 7.74±1.84 y of follow-up. Data from the women were not analyzed because only 8 of the 11,983 women surveyed reported a diagnosis of gout.
TABLE 1.
Distribution of covariates at baseline (N=28,990 men)
| Percentile | 10th | 25th | 50th | 75th | 90th |
|---|---|---|---|---|---|
| Age (y) | 32.15 | 37.90 | 44.63 | 51.36 | 58.62 |
| Aspirin (tablets/d) | 0 | 0 | 0 | 0.57 | 1.00 |
| Meat (servings/d) | 0 | 0.14 | 0.29 | 0.57 | 0.86 |
| Fish (servings/d) | 0 | 0.14 | 0.14 | 0.29 | 0.43 |
| Fruit (pieces/d) | 0.43 | 0.71 | 1.43 | 2.14 | 3.00 |
| Total alcohol (10 g/d) | 0 | 0 | 0.49 | 1.44 | 2.73 |
| Beer (10 g/d) | 0 | 0 | 0 | 0.15 | 0.77 |
| Wine (10 g/d) | 0 | 0 | 0.18 | 0.75 | 1.86 |
| Mixed drinks (10 g/d) | 0 | 0 | 0 | 0 | 0.43 |
| BMI (kg/m2) | 20.97 | 22.15 | 23.60 | 25.23 | 27.12 |
| Waist circumference (cm) | 76.20 | 81.28 | 83.82 | 86.36 | 91.44 |
| Chest circumference (cm) | 91.44 | 96.52 | 101.60 | 106.68 | 111.76 |
| BMIpreexercise (kg/m2) | 19.74 | 22.08 | 24.11 | 26.17 | 28.43 |
| Running distance (km/d) | 1.84 | 3.45 | 4.60 | 6.90 | 9.20 |
| 10-km Race performance (m/s) | 3.26 | 3.55 | 3.97 | 4.27 | 4.63 |
| Uric acid (mg/dL) | 4.0 | 4.7 | 5.5 | 6.3 | 7.1 |
There were 27,302 men who reported their waist circumferences, 23,651 men who reported their chest circumferences, 24,400 men who reported 10-km performance times, and 5,291 men whose physician provided their uric acid concentrations.
The distribution of physical activity, cardiovascular fitness, and BMI, in relation to each other and age and diet, is shown in Table 2. Men who were fitter, more physically active, and leaner tended to be younger and to eat less meat and more fruit. The more active men were also fitter and leaner, the fitter men were more active and leaner, and the leaner men were more active and more fit.
Table 2.
Distribution of selected covariates by physical activity, cardiorespiratory fitness, and BMI1
| Categories | |||||
|---|---|---|---|---|---|
| Lowest | Low | Intermediate | High | Highest | |
| Physical activity2 | |||||
| Age (y) | 45.41±10.99 | 45.96±10.34 | 45.26±10.00 | 44.80±10.40 | 42.78±10.40 |
| Cardiorespiratory fitness (m/s) | 3.70±0.55 | 3.70±0.49 | 3.88±0.47 | 4.05±0.46 | 4.31±0.50 |
| BMI (kg/m2) | 25.17±3.19 | 24.51±2.59 | 23.89±2.34 | 23.34±2.18 | 22.69±2.11 |
| Meat (servings/d) | 0.47±0.40 | 0.44±0.39 | 0.40±0.37 | 0.38±0.38 | 0.34±0.37 |
| Fish (servings/d) | 0.22±0.21 | 0.22±0.20 | 0.22±0.21 | 0.22±0.20 | 0.21±0.21 |
| Fruit (pieces/d) | 1.32±1.16 | 1.46±1.15 | 1.57±1.19 | 1.71±1.28 | 1.80±1.48 |
| Alcohol (10 g/d) | 0.88±1.28 | 1.05±1.44 | 1.04±1.41 | 0.98±1.41 | 0.97±1.43 |
| Cardiorespiratory fitness3 | |||||
| Age (y) | 53.51±11.51 | 49.51±10.07 | 46.18±9.29 | 42.69±8.62 | 37.43±8.87 |
| Physical activity (km/d) | 3.48±2.27 | 4.07±2.21 | 5.04±2.53 | 6.22±3.04 | 8.14±3.97 |
| BMI (kg/m2) | 25.62±3.19 | 24.95±2.67 | 24.02±2.36 | 23.25±2.08 | 22.24±1.90 |
| Meat (servings/d) | 0.41±0.39 | 0.42±0.38 | 0.41±0.38 | 0.38±0.37 | 0.39±0.40 |
| Fish (servings/d) | 0.26±0.23 | 0.24±0.22 | 0.22±0.20 | 0.21±0.19 | 0.20±0.20 |
| Fruit (pieces/d) | 1.55±1.17 | 1.51±1.22 | 1.55±1.22 | 1.65±1.30 | 1.67±1.33 |
| Alcohol (10 g/d) | 1.01±1.44 | 1.06±1.49 | 1.07±1.43 | 1.00±1.38 | 0.88±1.31 |
| BMI4 | |||||
| Age (y) | 40.74±13.06 | 43.94±11.39 | 45.38±10.07 | 45.73±9.33 | 45.27±8.78 |
| Physical activity (km/d) | 7.53±4.35 | 6.47±3.53 | 5.35±2.98 | 4.43±2.55 | 3.55±2.59 |
| Cardiorespiratory fitness (m/s) | 4.32±0.60 | 4.15±0.53 | 3.93±0.49 | 3.72±0.47 | 3.55±0.48 |
| Meat (servings/d) | 0.37±0.41 | 0.37±0.37 | 0.40±0.37 | 0.44±0.39 | 0.49±0.42 |
| Fish (servings/d) | 0.19±0.18 | 0.21±0.20 | 0.22±0.21 | 0.23±0.21 | 0.23±0.21 |
| Fruit (pieces/d) | 1.70±1.36 | 1.70±1.33 | 1.59±1.27 | 1.47±1.18 | 1.36±1.13 |
| Alcohol (10 g/d) | 0.73±1.13 | 0.92±1.30 | 1.03±1.40 | 1.13±1.56 | 0.98±1.54 |
All values are means± SD. ANOVA for significance across columns was P<0.0001 for all variables except fish intake by physical activity.
Physical activity was <2 km/d for the lowest category, 2–4 km/d for the low category, 4–6 km/d for the intermediate category, 6–8 km/d for the high category, and >8 km/d for the highest category.
Cardiorespiratory fitness was <3 m/s for the lowest category, 3–3.5 m/s for the low category, 3.5–4.0 m/s for the intermediate category, 4.0–4.5 m/s for the high category, and >4.5 m/s for the highest category.
BMI (in kg/m2) was <20 for the lowest category, 20–22.5 for the low category, 22.5–25 for the intermediate category, 25–27.5 for the high category, and >27.5 for the highest category.
Body weight and size
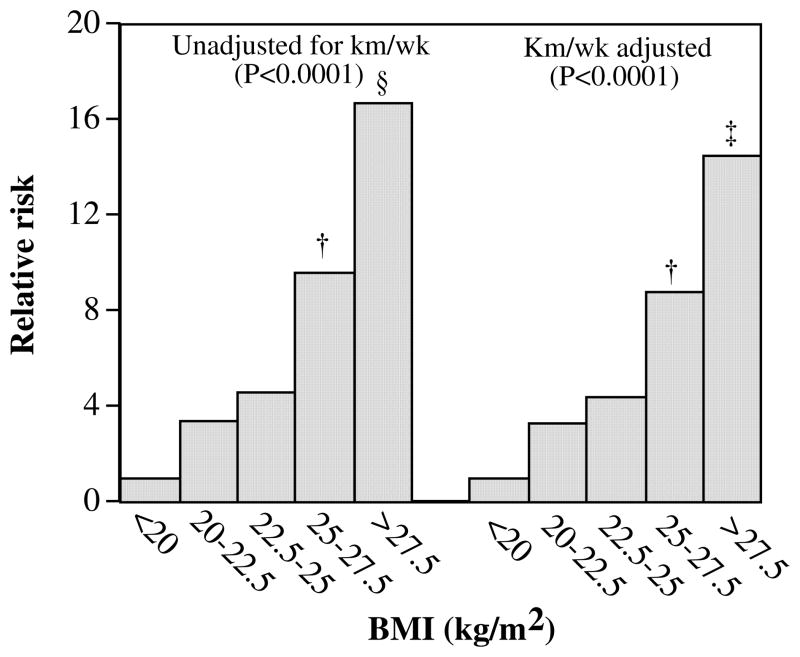
The significant increase in risk with increasing BMI, waist circumference, and chest circumference is shown in Table 3. BMI appeared to account for all of the significant associations between gout and waist circumference but only a portion of gout’s association with chest circumference. Figure 1, which displays the progressive increase in risk with increasing BMI, shows that men with BMI <20 kg/m2 had 85% lower risk of gout than did men with BMI >27.5 kg/m2. Adjustment for physical activity diminished only slightly the decline in risk associated with lower BMI.
Table 3.
Relative risk (95% CI) for incident self-reported physician-diagnosed gout during 7.74 y of follow-up in relation to BMI (n=28,990) and waist (n=27,302) and chest (n=23,651) circumferences1
| Unadjusted for daily running distance (km/d) and BMI | Adjusted for running distance (km/d) | Adjusted for BMI | |
|---|---|---|---|
| BMI, per kg/m2 | 1.19 (1.15, 1.23)2 | 1.18 (1.14, 1.23)2 | |
| Waist circumference, per cm | 1.06 (1.04, 1.08) 2 | 1.06 (1.04, 1.07) 2 | 1.01 (0.98, 1.04) |
| Chest circumference, per cm | 1.07 (1.05, 1.08)2 | 1.06 (1.04, 1.08)2 | 1.03 (1.00, 1.05)3 |
Cox proportional hazard model was adjusted for age; for daily intakes of meat, fish, fruit, alcohol, and aspirin; and for hypertension. Additional adjustment was made for physical activity and BMI where indicated.
P≤0.0001.
P≤0.01.
FIGURE 1.
Relative risk of incident gout by baseline BMI (in kg/m2) from Cox proportional hazard model in 28 990 men adjusted for age, hypertension, and daily intakes of meat, fish, fruit, alcohol, and aspirin. Additional adjustment was made for physical activity (in km/d) where indicated. P for trend is reported in parentheses. Bonferroni-corrected significant differences relative to BMI <20 kg/m2 are coded as follows: * P≤ 0.05, † P≤0.01, ‡ P≤0.001, and § P≤0.0001. The 95% CIs for the risk ratios for BMI unadjusted for physical activity are 0.71 to 58.02 for BMI 20–22.5 kg/m2, 1.00 to 79.00 for BMI 22.5–25.0 kg/m2, 2.01 to 158.09 for BMI 25–27.5 kg/m2, and 3.27 to 261.66 for BMI ≥27.5 kg/m2 without km/wk adjustment.
Diet
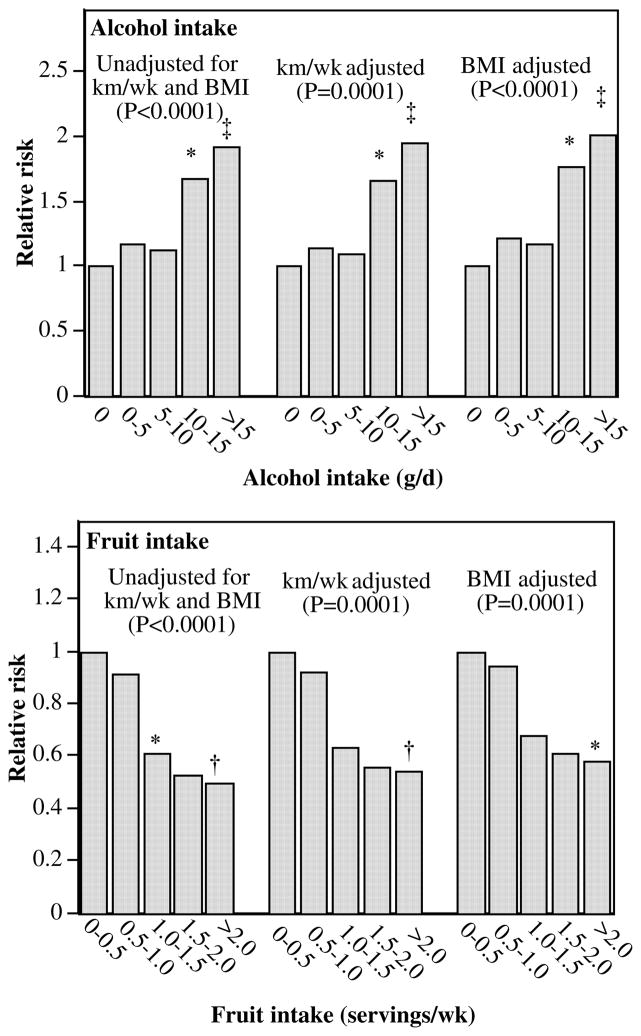
When adjusted for age, hypertension, aspirin use, and other dietary components, incident self-reported gout was associated with greater consumption of meat [per servings/d; relative risk (RR): 1.45; 95% CI: 1.06 to 1.92; P=0.002] and alcohol (per 10 g/d; RR: 1.19; 95%CI: 1.12 to 1.26; P≤0.0001) and less consumption of fruit (per pieces/d ;RR:0.73 ;95%CI: 0.62 to 0.84; P≤0.0001). When included simultaneously in the survival analyses, RRs per 10 g alcohol/d consumed in wine (1.27; 95% CI: 1.10 to 1.44; P=0.002), beer (1.19; 95% CI: 1.10 to 1.27; P≤0.0001), or mixed drinks (1.13; 95% CI: 0.94 to 1.31; P= 0.18) were not significantly different from each other. The associations were unaffected by adjustment for running distance or 10-km performance times, and adjustment for BMI reduced the significance of the association with meat intake (per serving/d; RR: 1.25; 95%CI: 0.90 to 1.68; P=0.17) but not fruit (per pieces/d; RR: 0.77; 95% CI: 0.66 to 0.88; P≤ 0.0001) or alcohol (per 10g/d; RR:1.19; 95%CI: 1.12 to 1.25; P ≤0.0001). The increase in incident gout with higher alcohol consumption and its decrease with higher fruit intake are shown in Figure 2. The risk of self-reported gout was 93% higher in men who consumed >15 g alcohol/d than in men who abstained from alcohol, and it was 50% lower in men that consumed 2 pieces of fruit/d than in men averaging 0.5 pieces/d. These relations were largely unaffected by adjustment for physical activity (in km/d) or BMI.
FIGURE 2.
Relative risk of incident gout by reported daily intakes of alcohol and fruit in 28 990 men from Cox proportional hazard model adjusted for age, hypertension, and weekly intakes of other foods and aspirin. Additional adjustment was made for physical activity and BMI where indicated. P for trend value is reported in parentheses. Bonferroni-corrected significant differences relative to the lowest consumptions are coded as follows: * P≤0.05, † P≤0.01, ‡ P≤0.001, and § P≤0.0001. The 95% CIs for the risk ratios for alcohol intake were 0.78 to 1.87 for 0–5 g alcohol/d, 0.70 to 1.81 for 5–10 g alcohol/d, 1.18 to 2.90 for 10–15 g alcohol/d, and 1.39 to 2.82 for >15 g alcohol/d and 0.62 to 1.33 for 0.5–1 piece fruit/d, 0.42 to 0.89 for 1–1.5 pieces fruit/d, 0.26 to 0.98 for 1.5–2.0 pieces fruit/d, and 0.33 to 0.74 for ≥2.0 pieces fruit/d unadjusted for physical activity or BMI.
Physical activity and cardiorespiratory fitness
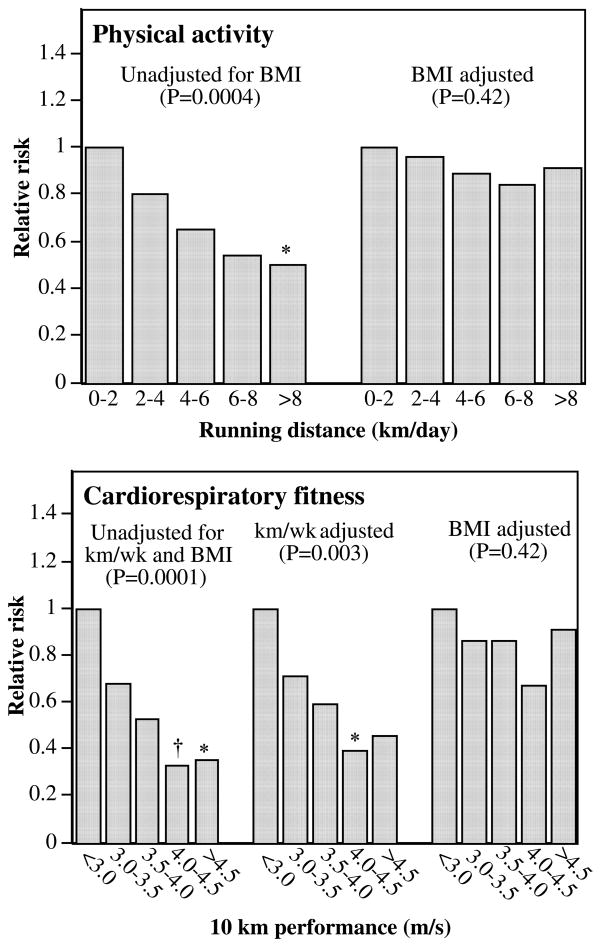
Both longer daily running distance and faster 10-km footrace performance speed predicted lower risk of incident gout (Table 4; Figure 3). The effect of fitness on incident gout was largely independent of the distance run per day. Men who on average ran >8 km/d had 50% lower risk of developing gout than did the least active runners (Figure 3). The findings suggest there were incremental improvements in risk from the slowest men to those who ran 4.0 m/s or faster (Figure 3). All of the risk reduction associated with distance run and three-fourths of the risk reduction associated with fitness were attributable to BMI (Table 4). Neither distance nor fitness predicted gout when adjusted for BMI (P=0.42 and P=0.42, respectively).
Table 4.
Relative risk (95% CI) for incident self-reported physician-diagnosed gout during an average of 7.74 y of follow-up in relation to physical activity and cardiorespiratory fitness1
| Cardiorespiratory fitness(10-km performance speed as m/s) | Physical activity(km/d running distance) | |||
|---|---|---|---|---|
| BMI unadjusted | BMI adjusted | BMI unadjusted | BMI adjusted | |
| All (n=28 990) | ||||
| Physical activity | 0.92 (0.88, 0.97)2 | 0.99 (0.94, 1.03) | ||
| Fitness subset (n= 24 400) | ||||
| Fitness | 0.55 (0.41, 0.75)3 | 0.89 (0.63, 1.24) | ||
| Physical activity | 0.92 (0.87, 0.97)4 | 0.98 (0.93, 1.04) | ||
| Fitness and activity | 0.61 (0.44, 0.86)4 | 0.90 (0.64, 1.29) | 0.95 (0.90, 1.00) | 0.99 (0.93, 1.05) |
Cox proportional hazard model adjusted for age; daily intakes of meat, fish, fruit, alcohol, and aspirin; and hypertension. Additional adjustment was made for physical activity, 10-km performance, and BMI where indicated. Fitness subset includes only those men who reported their 10-km performance time at baseline.
P≤0.001.
P≤0.0001.
P≤0.01.
FIGURE 3.
Relative risk of incident gout by physical activity (running distance in km/d; N= 28,990) and by cardiorespiratory fitness (10-km footrace performance in m/s; N=24,400) and from Cox proportional hazard model adjusted for age, diet, aspirin use, and hypertension. Additional adjustment was made for BMI where indicated. P for trend value is reported in parentheses. Bonferroni-corrected significant differences relative to the slowest, least active men are coded as follows: * P ≤0.05, † P≤0.01, ‡ P≤0.001, and § P≤0.0001. The 95% CIs were 0.38 to 1.29 for 3–3.5 m/s, 0.30 to 1.00 for 3.5–4 m/s, 0.17 to 0.66 for 4–4.5 m/s, and 0.17 to 0.75 for >4.5 m/s for cardiorespiratory fitness and 0.54 to 1.21 for 2–4 km/d, 0.43 to 0.98 for 4–6 km/d, 0.31 to 0.92 for 6–8 km/d, and 0.31 to 0.81 for ≥8 km/d for physical activity without BMI adjustment.
Physician-supplied serum concentrations of uric acid
Serum concentrations of uric acid were obtained from 5291 male runners at the time of their baseline survey. When adjusted for age, hypertension, aspirin use, and intakes of alcohol, meat, fish, and fruit, serum concentrations of uric acid (in mg/dL) were associated with BMI (regression slope±SE: 0.098±0.007 per kg/m2; P<0.0001), waist circumference (0.031±0.003 per cm; P<0.0001), and chest circumference (0.027±0.003 per cm; P<0.0001), and these slopes remained essentially unchanged when adjusted for average daily distance run. BMI accounted for all of uric acid’s association with waist circumference (adjusted slope±SE: 0.004±0.004 mg/dL per cm; P =0.31) and most of its association with chest circumferences (0.007±0.003 mg/dL per cm; P=0.03). Adjustment for BMI also accounted for one-half or more of the significant association of serum concentrations of uric acid with distance run (slope reduced from −0.04±0.01 mg/dL per km/d to −0.02±0.01 mg/dL per km/d, and correspondingly significance reduced from P=0.0001 to P=0.002) and cardiorespiratory fitness (slope reduced from −0.33±0.04 mg/dL per m/s to −0.14 ±0.04 mg/dL per m/s; P<0.0001 reduced to P=0.001).
When adjusted for age, hypertension, aspirin use, running distance, and BMI, serum concentrations of uric acid were also significantly related to intakes of meat (0.11±0.05 mg/dL per serving/d; P=0.02), fruit (−0.04±0.01 mg/dL per pieces/d, P=0.002), and alcohol (0.09±0.01 mg/dL per 10 g/d; P= 0.0001). These relations were also significant when adjusted for age alone. The uric acid concentrations increased significantly in association with alcohol consumed as beer (0.10±0.02 mg/dL per 10 g/d; P<0.0001), wine (0.07 ± 0.02 mg/dL per 10 g/d; P=0.006), or mixed drinks (0.09±0.03 mg/dL per 10 g/d; P=0.003). Moreover, no difference was observed between total alcohol intake and alcohol intake from beer (P=0.39 for difference), wine (P= 0.32), or mixed drinks (P =0.99).
DISCUSSION
Body weight
My analyses show that men with BMI >27.5 kg/m2 had a 16-fold greater risk of developing gout than did men with BMI <20 kg/m2 and 4-fold the risk as men with BMI <25 kg/m2. Greater waist and chest circumferences also increased the risk of gout during follow-up; however, the association with waist circumference was not independent of its association with BMI. The Health Professionals Follow-up Study also reported that risk increased incrementally with both BMI and waist-to-hip ratio (25). Other prospective studies that confirm the BMI relation include the Johns Hopkins Precursor Study (26) and the Normative Aging Study (27).
Higher serum concentrations of uric acid because of both increased production and decreased excretion probably account for the progressive increase in risk associated with adiposity (28, 29). Other evidence linking greater body weight with elevated serum concentrations of uric acid include 1) concordance between changes in BMI and uric acid concentrations (30), 2) reductions in uric acid concentrations in weight-loss studies (31, 32), and an inverse relation between visceral adiposity and renal clearance rates for urate (33). Serum concentrations of uric acid collected on a subset of the runners showed cross-sectional relations to adiposity that mirrored those observed for gout, namely 1) significant associations with BMI, waist circumference, and chest circumference that were largely independent of running distance and 2) adjustment for BMI that eliminated the association with waist circumference but not chest circumference.
Meat
I observed an increase in risk with meat consumption that is in agreement with the Health Professional Follow-up Study (34). That study reported an age-adjusted RR of 1.37 per serving/d, which is consistent with my estimate of 1.45 per serving/d. In their results, only beef, pork, or lamb consumed as main dishes increased the gout risk. In addition, it was reported that higher seafood intake was a risk factor, which I did not find, presumably because of the limited (fish intake only) and poorly reproducible seafood question (see Subjects and Methods). The prospective results are consistent with the baseline relation of serum concentrations of uric acid with reported meat (concordant) and fish consumption (none).
Fruit
Other studies have not reported that higher fruit intake reduces the risk of gout. The association I observed was statistically independent of alcohol and meat consumption, hypertension, and distance run and persisted when adjusted for weight. At baseline, uric acid concentrations were inversely related to reported pieces of fruit consumed. High fruit intake may characterize health-conscience diets rather than a specific effect of fruit per se. For example, men who ate more fruit might also consume more dairy products (34) or coffee, which were shown prospectively to reduce risk (35, 36) (neither coffee nor dairy products were asked about in this study).
Alcohol
In Figure 2, I show a significant dose-response relation between baseline alcohol consumption and incident self-reported gout. The intake did not need to be excessive to significantly increase risk. Alcohol is thought to increase uric acid concentration by both decreased urate excretion (37) and increased production (38). Metabolic studies show alcohol loading can induce hyperuricemia (38), and hyperuricemia was found to be a significant correlate of alcohol abuse among general hospital admissions (39). Alcohol intake was associated with gout in case-control comparisons (40, 41). There are, however, few prospective studies that verify their association. The Johns Hopkins Precursor Study (25, 42), the Meharry Cohort Study (42), and the Normative Aging Study (43, 44) all failed to show an increase in risk with consumption, although the latter did find an association between baseline alcohol intake and uric acid concentrations.
Alcohol significantly increased gout risk prospectively (P <0.0001) in the Health Professionals Follow-up Study (RR: 1.17 per 10 g/d), with as little as 15–30 g/d significantly increasing RR to abstainers (45). My data are consistent with the Health Professionals Follow-up Study estimate of RR (1.19 per 10 g/d), but they do not support their contention that wine does not increase serum concentrations of uric acid or the risk of gout (45, 46). I found that reported wine intake significantly increased risk independent of beer and mixed drink consumption, and no significant difference was observed between alcohols from wine compared with other sources. At baseline, higher serum concentrations of uric acid were also significantly and independently related to greater intakes of beer, wine, and mixed drinks, and there was no statistical evidence that wine had less affect than did beer or mixed drinks.
Physical activity and cardiorespiratory fitness
The analyses of Figure 3 and Table 4 show that men who ran ≥4 km/d or faster than 4.0 m/s had significantly lower risk of developing gout than slower, less active men. I have previously reported that serum concentrations of uric acid declined in runners from 5.60±0.07 mg/dL for 16 km/wk, 5.60±0.03 mg/dL for 16 km/wk to 32 km/wk, 5.50±0.03 mg/dL for 32 km/wk to 48 km/wk, 5.43±0.05 mg/dL for 48 km/wk to 64 km/wk, and 5.14±0.09 mg/dL for ≥64 km/wk (10). The average decline was −0.006±0.001 mg/dL per km/wk run (P <0.0001) (10). Additional analyses presented in this paper show that BMI accounted for more than one-half of the declines in serum concentrations of uric acid associated with running distance and speed cross-sectionally.
Physical inactivity and cardiorespiratory fitness are not recognized as risk factors for gout (3). In addition to showing prospectively that baseline running distance and speed predicted lower risk of gout, I also show that the lower risk is mediated by the leanness of the fitter, higher mileage runners (Table 4). A reduction in gout, mediated by body weight, would not diminish the public health significance promoting high levels of vigorous activity because the leanness of the runners is largely the consequence of running. Self-selection accounts for only 26% of the association of BMI with running levels in men (14). High mileage runners are leaner because they gain less weight as they age than do less-active men (15), in addition to weight lost when starting to run or increasing distance (16). Specifically, it was shown elsewhere that men who ran ≥48 km/wk had one-half the average annual weight gain of men who ran <24 km/wk (15). The prevention of age-related weight gain through running is disproportionately due to the prevention of more extreme gains in weight (15), which the nonlinear relation of Figure 1 suggests would have the greatest effect in preventing gout.
Limitations
The purposes of this study were 1) to assess the relation of gout risk to physical activity and cardiorespiratory fitness and 2) to confirm in physically active men the associations of incident gout with diet and adiposity that were previously reported for mostly sedentary men. In this regard my results are intended to complement rather than replicate other cohort studies that target geographical or occupational populations. These other studies tend to include few vigorously active men and may therefore miss relations with physical activity. The selection of runners who are generally leaner than the population in general is likely to account for the lower annual incidence of gout in this study (1.0 per 1000 person-years) than in the Health Professional Follow-up Study (24, 34, 45) or male physicians in the Johns Hopkins Precursor Study (2) (1.5 and 1.7 per 1000 person-years, respectively). Although the incidence of gout and the levels of physical activity of the National Runners’ Health Study may not be representative of US men sampled at random, the biological processes that relate gout to diet, exercise, and adiposity are likely to be similar. The leanness of my sample may have enhanced my ability to identify associations between gout and diet; i.e., others report stronger dietary associations with gout in lean rather than in overweight men (34).
The major limitation of my analyses is their use of self-reported gout. However, findings from the Health Professionals Follow-up Study suggest that there is either little difference in the dietary associations for self-reported gout compared with gout diagnosed from 6 of the 11 diagnostic criteria of the American College of Rheumatology (24, 34) or that the risk reduction is underestimated by self-report. Specifically, that study showed the RR for the highest compared with the lowest quintile of seafood consumption was 1.33 (95% CI: 1.11 to 1.60) for simple self-report (1332 cases) compared with 1.51 (95% CI: 1.17 to 1.95) for the diagnostic criteria of the American College of Rheumatology (730 men). The corresponding RRs for low-fat dairy products were 0.61 (95% CI: 0.50 to 0.73) compared with 0.58 (95% CI: 0.45 to 0.76), for drinking 2 glasses milk/d compared with <1 glass milk/mo were 0.59 (95% CI: 0.47 to 0.73) compared with 0.54 (95% CI: 0.40 to 0.73), and for intake of 50 g alcohol/d compared with abstainers was 1.93 (95% CI: 1.50 to 2.49) compared with 2.53 (95% CI: 1.73 to 3.70), respectively. For the association with alcohol intake, the magnitude of the association tended to increase with increasing specificity of the case definition. Thus, the significant findings reported here may underestimate the true strength of the relations. I also acknowledge that I did not collect data on intake of dairy, coffee, fructose, vegetables, or vitamin C, so I cannot exclude the possibility that these factors may have contributed to the associations given in this report. Finally, I note that cardiorespiratory fitness as measured by the participants’ best 10-km performance times within the previous 5 y may have differed somewhat from the actual baseline fitness, although inspection of the runners’ data showed relatively stable running levels preceding the baseline survey.
I believe that exercise reduced the risk of gout rather than the converse. Gout is episodic. In the absence of tophaceous gout or permanent joint damage, it is not clear that gout would limit the usual training distances or performance. Acute podagra or other gouty attacks usually peak in severity 1 or 2 d after the onset of symptoms and last ≤10 d (27). Low doses of non-steroidal anti-inflammatory drugs or colchicine are effective in preventing acute gouty attacks, and uricosuric drugs may be used to increase renal excretion of uric acid.
In conclusion, my data suggest that in ostensibly healthy, vigorously active men the prevention of weight gain through the promotion of vigorous physical activity may help prevent gout. Lower risk may also be attained through healthy diets characterized by the inclusion of fruit and limited meat intake. I find no evidence that consumption of wine rather than beer or mixed drinks reduces the risk of gout. I believe the effects of adiposity, alcohol, fruit, meat, exercise, and fitness on gout risk are likely to be mediated by serum concentrations of uric acid, which showed the same significant relations as observed for incident gout.
Acknowledgments
PTW designed and directed the study, obtained funding, wrote the paper, performed all statistical analyses, and takes responsibility for the results.
Footnotes
The author had no personal or financial conflict of interest.
References
- 1.Pascual E. Hyperuricemia and gout. Curr Opin Rheumatol. 1994;6:454–8. doi: 10.1097/00002281-199407000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA. 1991;266:3004–7. [PubMed] [Google Scholar]
- 3.Saag KG, Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. 2006;8(suppl 1):S2. doi: 10.1186/ar1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421–6. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 5.McCarty DJ. Gout without hyperuricemia. JAMA. 1994;271:302–3. [PubMed] [Google Scholar]
- 6.Clifford AJ, Riumallo JA, Young VR, Scrimshaw NS. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J Nutr. 1976;106:428–34. [Google Scholar]
- 7.Clifford AJ, Story DL. Levels of purines in foods and their metabolic effects in rats. J Nutr. 1976;106:435–42. [Google Scholar]
- 8.Zollner N. Influence of various purines on uric acid metabolism. Bibl Nutr Dieta. 1973;19:34–43. [PubMed] [Google Scholar]
- 9.Zollner N, Griebsch A. Diet and gout. Adv Exp Med Biol. 1974;41:435–42. doi: 10.1007/978-1-4757-1433-3_8. [DOI] [PubMed] [Google Scholar]
- 10.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G, Brocco G, Franchini M, Schena F, Guidi G. Comparison of serum creatinine, uric acid, albumin and glucose in male professional endurance athletes compared with healthy controls. Clin Chem Lab Med. 2004;42:644–7. doi: 10.1515/CCLM.2004.110. [DOI] [PubMed] [Google Scholar]
- 12.Kaya M, Moriwaki Y, Ka T, et al. Plasma concentrations and urinary excretion of purine bases (uric acid, hypoxanthine, and xanthine) and oxypurinol after rigorous exercise. Metabolism. 2006;55:103–7. doi: 10.1016/j.metabol.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Shih M, Hootman JM, Kruger J, Helmick CG. Physical activity in men and women with arthritis. National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385–93. doi: 10.1016/j.amepre.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Williams PT. Self-selection accounts for inverse association between weight and cardiorespiratory fitness. Obes Res. 2007;16:102–6. doi: 10.1038/oby.2007.5. [DOI] [PubMed] [Google Scholar]
- 15.Williams PT. Maintaining vigorous activity attenuates 7-yr weight gain in 8340 runners. Med Sci Sports Exerc. 2007;39:801–9. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams PT, Thompson PD. Dose-dependent effects of training and detraining on weight in 6406 runners during 7. 4 years. Obesity (Silver Spring) 2006;14:1975–84. doi: 10.1038/oby.2006.231. [DOI] [PubMed] [Google Scholar]
- 17.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obes Relat Metab Disord. 2004;28:120–8. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 19.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:875–88. [PubMed] [Google Scholar]
- 20.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann N Y Acad Sci. 1977;301:484–94. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 21.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–4. [PubMed] [Google Scholar]
- 22.Trank TV, Ryman DH, Minagawa RY, Trone DW, Schaefer RA. Running mileage, movement mileage, and fitness in male U.S. Navy recruits. Med Sci Sports Exerc. 2001;33:1033–8. doi: 10.1097/00005768-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Burger SC, Bertram SR, Stewart RI. Assessment of the 2. 4 km run as a predictor of aerobic capacity. S Afr Med J. 1990;78:327–9. [PubMed] [Google Scholar]
- 24.Williams PT, Frankin B. Vigorous exercise and diabetic, hypertensive, and hypercholesterolemia medication use. Med Sci Sports Exerc. 2007;39:1933–41. doi: 10.1249/mss.0b013e318145b337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;11:165:742–8. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 26.Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA. 1991;266:3004–7. [PubMed] [Google Scholar]
- 27.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421–6. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 28.Emmerson BT. The management of gout. N Engl J Med. 1996;334:445–51. doi: 10.1056/NEJM199602153340707. [DOI] [PubMed] [Google Scholar]
- 29.Fam AG. Gout, diet, and the insulin resistance syndrome. J Rheumatol. 2002;29:1350–5. [PubMed] [Google Scholar]
- 30.Glynn RJ, Campion EW, Silbert JE. Trends in serum uric acid levels 1961–1980. Arthritis Rheum. 1983;26:87–93. doi: 10.1002/art.1780260115. [DOI] [PubMed] [Google Scholar]
- 31.Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis. 2000;59:539–43. doi: 10.1136/ard.59.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita S, Matsuzawa Y, Tokunaga K, Fujioka S, Tarui S. Studies on the impaired metabolism of uric acid in obese subjects: marked reduction of renal urate excretion and its improvement by a low-calorie diet. Int J Obes. 1986;10:255–64. [PubMed] [Google Scholar]
- 33.Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Yamakita J, Higashino K. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism. 1997;46:1162–5. doi: 10.1016/s0026-0495(97)90210-9. [DOI] [PubMed] [Google Scholar]
- 34.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165:997–1003. doi: 10.1001/archinte.165.9.997. [DOI] [PubMed] [Google Scholar]
- 35.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 36.Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum. 2007;56:2049–55. doi: 10.1002/art.22712. [DOI] [PubMed] [Google Scholar]
- 37.Eastmond CJ, Garton M, Robins S, Riddoch S. The effects of alcoholic beverages on urate metabolism in gout sufferers. Br J Rheumatol. 1995;34:756–9. doi: 10.1093/rheumatology/34.8.756. [DOI] [PubMed] [Google Scholar]
- 38.Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med. 1982;307:1598–602. doi: 10.1056/NEJM198212233072602. [DOI] [PubMed] [Google Scholar]
- 39.Drum DE, Goldman PA, Jankowski CB. Elevation of serum uric acid as a clue to alcohol abuse. Arch Intern Med. 1981;141:477–9. [PubMed] [Google Scholar]
- 40.Sharpe CR. A case-control study of alcohol consumption and drinking behaviour in patients with acute gout. Can Med Assoc J. 1984;131:563–7. [PMC free article] [PubMed] [Google Scholar]
- 41.Gibson T, Rodgers AV, Simmonds HA, Court-Brown F, Todd E, Meilton V. A controlled study of diet in patients with gout. Ann Rheum Dis. 1983;42:123–7. doi: 10.1136/ard.42.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995;38:628–32. doi: 10.1002/art.1780380508. [DOI] [PubMed] [Google Scholar]
- 43.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia: risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421–6. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 44.Shadick NA, Kim R, Weiss S, Liang MH, Sparrow D, Hu H. Effect of low level lead exposure on hyperuricemia and gout among middle aged and elderly men: the normative aging study. J Rheumatol. 2000;27:1708–12. [PubMed] [Google Scholar]
- 45.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–81. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 46.Choi H, Curhan G. Beer, liquor, and wine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2004;51:1023–9. doi: 10.1002/art.20821. [DOI] [PubMed] [Google Scholar]