Abstract
Purpose
The objective of this paper was to evaluate the performance of the built-in MR-based attenuation correction method (MRAC) included in the combined whole-body Ingenuity TF PET/MR scanner and compare it to the gold standard CT-based attenuation correction (CTAC).
Methods
Twenty-six patients undergoing clinical whole body FDG-PET/CT imaging were subsequently scanned on the PET/MR (mean delay 100min). Patients were separated in two groups: alpha group (N=14) with no MR coils during PET/MR imaging and beta group (N=12) including MR coils (Neuro-Vascular, Spine, Cardiac or Torso coils). All images were coregistered to the same space (PET/MR). Voxel- and region-based (10 regions of interest, ROIs) comparisons were made between the 2 PET images from PET/MR: using MRAC and CTAC. Additional comparison of lesions performed by an experienced clinician was also reported.
Results
Body mass index (BMI) and lung density showed significant differences between alpha and beta groups. Right vs. left lung density was also significantly different within each group. Overall the beta group (with coils) presented higher MRAC PET values than the alpha group when compared to the CTAC (alpha: −0.2±33.6%, R2=0.98, p<0.001, beta: 10.31±69.86%, R2=0.97, p<0.001).
Conclusion
In comparison to CTAC, PET values with MRAC method were underestimated by less than 10% on average, although some ROIs and lesions do differ by more (such as spine, lung or heart). The beta group (with coils) showed increased overall PET quantification as well as increased variability when compared to the alpha group (no coils). PET reconstructed with MRAC showed some differences when compared to PET reconstructed with CTAC, mostly due to air pockets, metallic implants and attenuation differences in big bone areas (such as pelvis and spine) due to the segmentation limitation of the MRAC method.
Keywords: PET/MR, attenuation correction, MRAC, CTAC
Introduction
CT-based attenuation correction (CTAC) is presently accepted as the “gold standard” for PET attenuation correction in PET/CT scanners. CT images are directly related to the electron density of the tissues and organs and hence proportional to the attenuation coefficient at PET energy levels (511 keV). On the contrary, MR images are related to the unpaired proton density of the tissues and organs and their relaxation times [1]. MR-based attenuation correction (MRAC) methods consist of creating approximate attenuation coefficient maps from the MR images. Additionally the use of the MR instead of a CT imposes a series of specific challenges in terms of attenuation correction to allow full quantitative PET imaging. The most important ones are: the challenge to obtain accurate lung and bone segmentation; MR artefacts (implants, special devices, etc); the lack of MR signal for the patient's table and the additional MR coils and finally the field inhomogeneities and limited field of view (FOV) leading to image truncation.
Several approaches have been described in the literature to obtain close approximations of the attenuation maps [2]. Mostly, MRAC approaches could be divided into three main strategies: segmentation-based, atlas-based and PET-based methods. The first group focuses on segmenting the MR images into tissue/organs classes and assigning their respective estimated linear attenuation coefficients (LACs) [3-6]. In general, the better the segmentation, the closer the attenuation map approximates the gold standard (CTAC). The second group, the atlas-based methods, are based on obtaining a coregistered pair of atlases: MR and corresponding attenuation map (either CT- or transmission-based). When a new MR image is obtained, it is then coregistered to the MR atlas [7-10]. The third and last group includes those techniques where the attenuation correction maps are generated from the PET emission data directly. These techniques follow in general an iterative approach where emission and attenuation maps are updated repeatedly under certain constraints [11-13]. Despite these techniques are showing promising results in time of flight (TOF) systems its usefulness and stability is still to be proven and further investigations are needed to evaluate their application in non-TOF scanners. Whole-body techniques are scarce because of the particularities and challenges imposed in both atlas-and segmentation-based methods: misregistration problems and tissue segmentation/classification failures due to the difficulties in lung segmentation and the proximity of bone and air [4, 9], as well as the inter- and intra-subject variability of the lungs’ density [14]. Finally a new methodology based on simultaneous emission and transmission image acquisition has been recently developed [15]. The method, requiring a low dose transmission source ring and PET detectors with TOF capabilities, is showing promising results.
The objective of this study was to evaluate the performance, in terms of PET quantification, of the MRAC method implemented on a sequential Whole-body PET/MR scanner for whole body imaging. MRAC and CTAC reconstructed PET images from PET/MR were compared in two patient setups, one including MR coils and one without MR coils within the PET FOV.
Materials and Methods
Patients
Twenty-six patients were included in this study. All patients had been referred for a clinical PET/CT with 18F-fluorodeoxyglucose (FDG). Patients were scanned first on the PET/CT and subsequently on the combined PET/MR scanner. Subjects received and injection of FDG with the standard clinical doses specified at our Institution, 578.3±55.8 MBq (mean±SD). Patients were organized in two groups: the alpha group including only subjects who did not have any MR coils during their PET/MR image acquisition and the beta group, including subjects who had MR coils during PET/MR image acquisition. The alpha group (N=14) was composed of 7 males and 7 females aged 61±11 (mean±SD). The beta group (N=12) was composed of 7 males and 5 females aged 64±10. See table 1 for a summary of the patient details and imaging times post-FDG injection. The study was approved by the Institutional Review Board of the Mount Sinai School of Medicine. All patients gave written informed consent.
TABLE 1.
Summary of the patient details.
| Patient | Diagnostic | Sex * | Age (yr) | Weight (kg) | BMI | Dose (MBq) | PET/MR † (min p.inj.) |
|---|---|---|---|---|---|---|---|
| Alpha group | |||||||
| 1 | Colon Ca | M | 80 | 70 | 21.6 | 564.2 | 206 |
| 2 | Retromolar Ca | M | 60 | 75 | 23.9 | 589.0 | 129 |
| 3 | Pancreatic Ca | M | 67 | 90 | 27.8 | 507.3 | 163 |
| 4 | Head-Neck Ca | F | 48 | 63 | 25.6 | 507.9 | 128 |
| 5 | Lymphoma | F | 44 | 77 | 30.1 | 604.8 | 148 |
| 6 | Myeloma | M | 70 | 96 | 31.3 | 415.5 | 237 |
| 7 | Breast Ca | 70 | 58 | 21.3 | 568.1 | 212 | |
| 8 | Head-Neck Ca | M | 64 | 71 | 23.2 | 539.8 | 208 |
| 9 | Lymphoma | F | 43 | 58 | 19.6 | 605.8 | 130 |
| 10 | Lymphoma | F | 69 | 65 | 24.8 | 623.2 | 149 |
| 11 | Melanoma | M | 57 | 111 | 28.9 | 588.5 | 193 |
| 12 | Lymphoma | F | 67 | 78 | 28.0 | 630.0 | 140 |
| 13 | Pharyngeal Ca | M | 50 | 66 | 22.1 | 507.5 | 158 |
| 14 | Myeloma | F | 63 | 68 | 23.5 | 604.2 | 157 |
| Mean α N/A‡ | N/A‡ | 60.9 | 74.7 | 25.1 | 561.1 | 168.4 | |
| SD a N/A‡ | N/A‡ | 11.1 | 15.1 | 3.6 | 59.6 | 35.9 | |
| Beta group | |||||||
| 1 | Tongue Ca | F | 49 | 66 | 26.8 | 616.1 | 132 |
| 2 | Tonsil Ca | M | 59 | 84 | 29.1 | 602.2 | 179 |
| 3 | Lung Ca | F | 67 | 84 | 29.8 | 603.4 | 168 |
| 4 | Lymphadenopathy | M | 76 | 107 | 33.0 | 643.6 | 168 |
| 5 | Liver Ca | M | 58 | 86 | 26.5 | 609.0 | 207 |
| 6 | Myeloma | F | 68 | 70 | 27.3 | 606.5 | 147 |
| 7 | Myeloma | M | 64 | 80 | 23.9 | 628.4 | 212 |
| 8 | Myeloma | F | 72 | 67 | 31.0 | 610.0 | 336 |
| 9 | Myeloma | M | 48 | 95 | 29.3 | 642.8 | 288 |
| 10 | Thymoma | F | 66 | 73 | 28.5 | 475.6 | 161 |
| 11 | Head-Neck Ca | M | 79 | 69 | 25.3 | 553.3 | 144 |
| 12 | Melanoma | M | 57 | 111 | 28.9 | 588.5 | 142 |
| Mean β N/A‡ | N/A$ | 63.6 | 82.7 | 28.3 | 598.3 | 190.3 | |
| SD β N/A‡ | N/A$ | 9.8 | 15.2 | 2.5 | 45.5 | 62.7 | |
| Mean All N/A‡ | N/A$ | 62.1 | 78.4 | 26.6 | 578.3 | 178.5 | |
| SD All N/A‡ | N/A$ | 10.4 | 15.4 | 3.5 | 55.8 | 50.2 | |
M=Male, F=Female;
PET/CT and PET/MR imaging times in min. post injection;
N/A=Not applicable.
The same patient supports for head and legs were used in both scanners in order to place the patient in the same position during both scans and therefore facilitating image coregistration of CT and PET/MR images. These holders were however outside the analysed PET FOV (from pelvis to shoulders) and therefore did not contribute to photon attenuation.
CT Imaging
CT Images were obtained from the combined PET/CT images (16 slices multidetector CT) scanner (GE Discovery LS, Waukesha, WI). A non-contrast low dose CT was acquired. The final matrix size of the CT images was 512×512 voxels in-plane with 1.37×1.37×3.75 mm3 voxel size.
PET/MR Scanner
Immediately after their PET/CT session, patients were taken to the PET/MR facility. PET/MR images were acquired on the combined whole-body PET/MR system, Ingenuity TF PET/MR (Philips Healthcare, Cleveland) [16]. PET images were acquired in 3D mode, using TOF information, standard for this system. Whole-body and partial-body protocols were acquired on the PET/MR: 2 to 3 minutes per bed position (159.4±59.7 sec), 7 to 11 bed positions, with 45 slices per bed and a 55% overlap between beds (standard for this system). Images were reconstructed with a matrix size of 144×144, with 4×4×4 mm3 voxel size, using a TOF, list-mode, blob-based OSEM algorithm with 3 iterations and 33 subsets using corrections for normalization, dead time, attenuation, scatter, random coincidences, sensitivity and decay.
PET/MR attenuation correction
MRAC
Since the objective of this study was to compare the manufacturer built-in MRAC method with the gold standard CTAC, all PET images followed the built-in method for attenuation correction implemented on the Ingenuity TF PET/MR (v3.7). Full details of this method are provided in [1]. Briefly, a specific MR sequence (called atMR, for MR attenuation correction) was run prior to any PET acquisition. The atMR sequence, acquired only with the integrated body coil of the MR scanner, matches the PET dimensions and allows both anatomical detail and attenuation correction, similarly to a low-dose CT image in a standard PET/CT camera. The atMR image was segmented into 3 tissue classes, air, soft tissue and lungs and pre-determined LACs were assigned to each class (0, 0.095 and 0.022 cm−1 respectively). An attenuation template of the patient table and of those MR coils for which the manufacturer provides an attenuation template were incorporated into the attenuation map in order to correct for their attenuation. As the objective of this study was to evaluate the global effect of the presence of clinical MR coils in the PET FOV, 4 different coils were used on the beta group: Cardiac coil (32 channel), Torso Sense XL (16 elements), Neuro-Vascular Sense (16 elements) and Sense Spine coil (15 elements). The built-in MRAC method developed by the manufacturer provided templates for both fix-positioned coils, Neuro-Vascular and Spine coils, however, by default the standard procedure of the PET/MR scanner does not provide an attenuation template for the flexible coils (cardiac and torso). While the posterior parts of these flexible coils remain on the patient table, the standard procedure requires the anterior parts of the flexible coils to be detached from the patient before starting the PET acquisition. Examples of the attenuation templates of the coils used in this study could be found in [17]. No truncation compensation method was used for the MRAC method.
CTAC
In order to provide PET/MR with CT-based attenuation correction (CTAC), the CT images were rigidly coregistered to PET/MR space, similar to [18], first using SPM8 software (Statistical Parametric Mapping 8, Wellcome Trust Centre for Neuroimaging, UCL, London, UK). To avoid possible mis-registration on the arms and legs areas that could bias the results, coregistration and therefore PET analysis was only enforced from shoulders to the pelvic area. Fine manual adjustments based on anatomical features were applied when needed. The coregistered CT images were then masked to exclude the CT table and then smoothed with a Gaussian kernel (4 mm) to match the PET resolution before reslicing them into PET/MR image space (4×4×4 mm3 voxel size). The resliced images were then converted into attenuation coefficients following the bi-linear method implemented in the GE Discovery LS PET/CT scanner [19]. The CTAC maps were finally included into the normal reconstruction procedure of the PET/MR scanner in which the scanner automatically adds the PET/MR patient table and the coil templates (when present) onto the final AC map.
PET Quantification
PET images from PET/MR images were reconstructed with MRAC and CTAC for comparison. These PET reconstructed images are called here MRAC and CTAC images respectively. Comparisons were made per patient following a voxel-based and a region-based method. Differences (Mean±SD) were expressed in terms of percentage, taking CTAC as gold standard (denominator of the equation).
Voxel-based comparison
An in-house program was developed in Matlab (Mathworks, Natick, USA) to provide voxel-based comparison of the PET images. A patient specific mask, automatically generated on the PET/MR, was used to avoid noise-only voxels that could bias the comparison. Comparisons were made for the coregistered area only, between shoulders and pelvic area. Bland-Altman [20] and correlation plots were generated for all patients in each group, using standardized uptake values (SUV) to quantify the PET images [21].
Region-based comparison
Ten regions of interest (ROIs) were drawn following the anatomy on the coregistered CT images: aortic arch, thoracic aorta, heart, liver, left and right kidney, left and right lung, spine and soft tissue. Soft tissue ROIs were placed in muscle and soft tissue areas at the back of shoulders (above the lungs) and the pelvic area. Spine ROIs were placed just above the pelvis. A total of 10 slices per ROI were drawn on consecutive axial images. These ROIs were then applied to the coregistered PET images from PET/MR (both MRAC and CTAC). Mean and maximum SUV (SUVmean and SUVmax respectively) were reported from PET images. An average of the SUVmean and SUVmax of all slices was calculated per ROI for all patients. Bland-Altman [20] and correlation plots were generated for all patients in each group for both SUVmean and SUVmax. Known for their variability in their density [14], CT quantification of the lungs was also calculated to compare their variability and the potential impact of assigning them a unique LAC (0.022 cm−1).
Evaluation of lesions
An experienced clinician (J.M, Director of Nuclear Medicine Division, >20y of experience) reported the same lesions of the PET images (MRAC and CTAC) following the same standard clinical procedures applied for PET images at Mount Sinai Hospital using SUVmax. The clinician reported n=168 lesions in total, defined as areas with abnormal (increased) FDG uptake. Lesions were then classified into 3 types following the criteria of the clinician according to the degree of malignancy: normal (healthy) tissue with increased uptake (described as normal/increased uptake), inflammatory/degenerative lesions and tumor lesions. Bland-Altman [20] and correlation plots were also generated for all patients in each group. Additional comments reported by the clinician of the image comparisons (in terms of differences observed) were also included for this study.
Statistical Analysis
Results are presented as mean±SD in percentage from the gold standard, CTAC. Pearson's correlation, and appropriate Student's t-tests were evaluated using the Statistical Package for the Social Sciences (SPSS) software, ver. 21.0 (SPSS Inc, IBM Inc, Chicago). Statistical significance was considered for p < 0.05.
Results
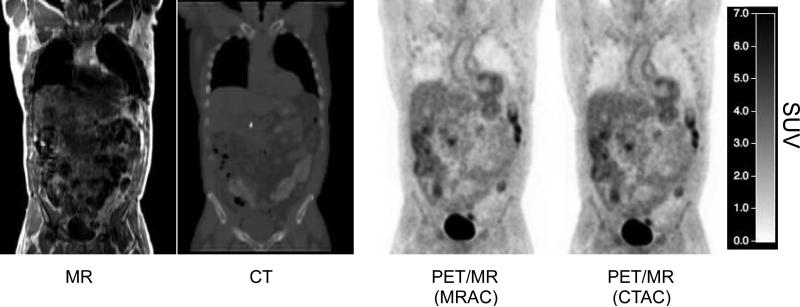
Table 1 presents a summary of the patient details for both alpha and beta groups. There were no statistically significant differences in patient age, sex, weight, height or injected dose between groups. However significant differences were found on body mass index (BMI) between both groups (p=0.02). Figure 1 shows an example of all images of a representative subject (atMR, CT, and PET with MRAC and CTAC reconstruction) coregistered into the same space (PET/MR image space). There is no statistically significant differences between injection time and the start time of the PET/MR scan time between alpha and beta groups.
Figure 1.
Examples of images for 1 subject (from left to right): MR, CT and PET images reconstructed with MRAC and CTAC, coregistered for the same patient. Colorbar shows SUV values for PET images.
Voxel-based comparison
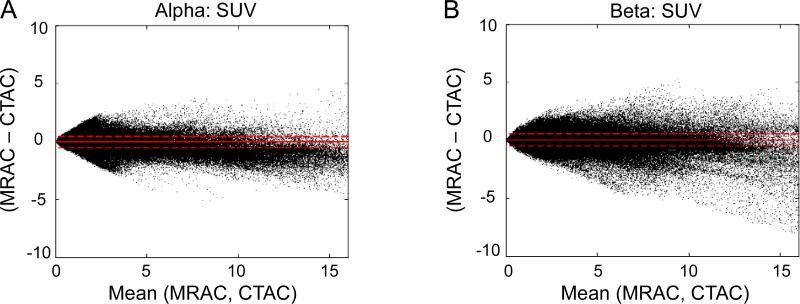
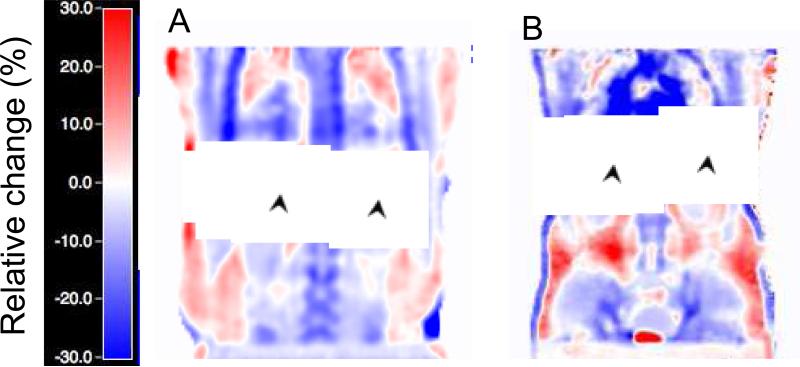
Figure 2 presents the Bland-Altman plots for the voxel-based comparison of PET MRAC vs. CTAC for both alpha (A) and beta (B) groups. Correlation plots are shown on supplementary figure 1. Table 2 shows a summary of all voxel-based results. All differences were statistically significant. Figure 3 displays an image of a typical subject showing the bias from MRAC to CTAC PET reconstructed data (in % difference from CTAC) for both alpha (A) and beta groups (B). Bigger variability is displayed on the beta case confirming what the numbers of table 2 and figure 2 suggested.
Figure 2.
Voxel-based comparison plots for the PET MRAC vs. CTAC for alpha (A) and beta (B) groups. The solid lines represent the mean difference and the dotted lines represents the 95% C.I. (Mean±1.96SD).
TABLE 2.
Summary of the MRAC vs. CTAC voxel-based comparison results.
| SUV Mean±SD % | R2 | |
|---|---|---|
| α | −0.2±33.6 | 0.98 |
| β | 10.31±69.86 | 0.97 |
Figure 3.
Relative change of PET MRAC images (in % relative to PET reconstructed with CTAC) showing positive (red-coloured) and negative (blue-coloured) bias on a coronal plane for a typical subject of the alpha (A) and beta (B) groups. The black arrows point to changes due to different lung position during the MR and CT acquisitions.
Region-based comparison
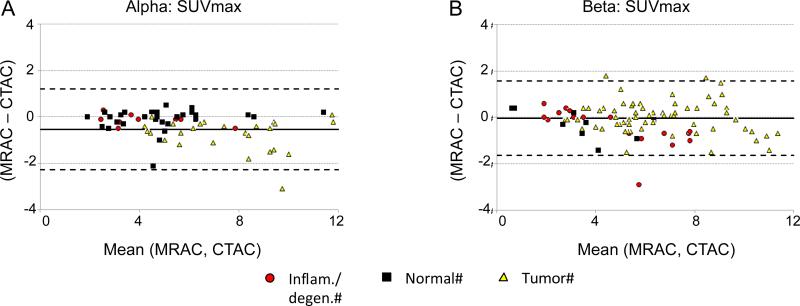
Figure 4 shows the results of the region-based PET MRAC vs. CTAC Bland-Altman comparison for all 10 ROIs (colour coded).
Figure 4.
Region-based Bland-Altman plots for PET MRAC vs. CTAC. Top row shows SUVmean plots for alpha (A) and beta (B) groups. Bottom row shows SUVmax plots for alpha (C) and beta (D) groups. The solid line in each plot represents the mean difference and the dotted lines represents the 95% C.I. (Mean±1.96SD).
SUVmean values for MRAC over all ROIs were lower compared to CTAC for both alpha and beta groups (alpha: −8.05±7.37%, p<0.001 and beta: −3.02±11.76%, p=0.03 respectively) (table 3). SUVmax followed a similar pattern (table 3). Similarly to the voxel case, the beta group showed increased variability with respect to the alpha group.
TABLE 3.
Summary of the MRAC vs. CTAC comparison for ROI-based and lesions results.
| ROI | SUVmean Mean±SD % | R2 | SUVmax Mean±SD % | R2 | |
|---|---|---|---|---|---|
| All | α | −8.05±7.37 | 0.99 | −9.07±8.85 | 0.99 |
| ROIs | β | −3.02±11.76 | 0.97 | −3.16±13.0 | 0.96 |
| All | α (n=74 lesions) | − | −6.01±9.31 | 0.96 | |
| lesions | β (n=94 lesions) | − | 1.76±18.46 | 0.94 | |
For individual ROIs, all ROIs on both alpha and beta groups were underestimated when comparing MRAC vs. CTAC by less than 10%, except for spine (−12.03%), right lung (−10.51%) and heart (−10.45%) for SUVmean, and right lung (−22.68%) and spine (−10.83%) for SUVmax, all for the alpha group (supplementary table 1). All of these differences for alpha group were significantly different (p<0.01). In the case of the beta group, only the right lung SUVmax was higher than 10% (-14.73%), but this just failed to achieve statistical significance (p=0.06). Overestimation occurred only on the left and right kidneys for the beta group when comparing MRAC vs. CTAC, for both SUVmean (LK, 3.28%; RK, 1.95%) and SUVmax (LK, 4.65%; RK, 2.36%), however these differences were not statistically significant.
In terms of lung's density, measured in Hounsfield units (HU) on the coregistered CT images, our results showed that significant differences occurred between the alpha and beta groups, with large variability within the groups (table 4). Moreover, within the same group, right and left lungs showed also significant differences (table 4).
TABLE 4.
Summary of the CT Lung ROI results (in Hounsfield Units, HU).
| α Mean±SD [min max] | β Mean±SD [min max] | ||||
|---|---|---|---|---|---|
| All | −693.9±157.4 [-819.7-535.9] | −656.0±199.8 [-781.3 -468.2] | p<0.001 | ||
| R vs. L* | R | −685.3±160.5 [-819.7 -535.9] | p<0.001 | −654.0±194.8 [-762.3 -470.0] | p=0.03 |
| L | −702.7±154.2 [-787.2 -564.6] | −658.2±205.2 [-781.3 -468.2] | |||
R vs. L = Right versus Left.
Evaluation of lesions
Figure 5 presents the Bland-Altman plots for the PET MRAC vs. CTAC comparative of the relevant lesions reported by the clinician. A total of 168 lesions were reported (alpha: 74; beta: 94). When compared to CTAC, MRAC SUVmax values were lower in the alpha group (−6.01±9.31%, p<0.001) while for the beta group MRAC SUVmax were higher but not statistically different from CTAC values (1.76±18.46%, p=0.64) (table 3). The three lesion types were underestimated when comparing MRAC to CTAC, except in the case of normal/increased uptake group (11.85±45.74%) and the tumor lesions (1.65±11.7%) both in the beta group (supplementary table 2).
Figure 5.
Bland-Altman plots for the relevant lesions reported (SUVmax) for alpha (A) and beta (B) groups. The solid lines represent the mean difference and the dotted lines represents the 95% C.I. (Mean±1.96SD).
The comparison of all images showed some differences between PET CTAC and MRAC images. Most of these differences were due to air pockets in the colon area (n=12), differences around big bone areas such as pelvis and spine (n=17), differences in liver (n=4) and differences due to metallic implants (n=2). Three patients had no visible differences between CTAC and MRAC images. The atMR images showed flow artefacts that created visible phantom replica of the aortic arch in the lungs (n=23), respiratory motion artefacts in the chest (n=15), metallic implant artefacts (n=8) and body truncation (n=7) (figure 6). Finally, the MRAC attenuation map showed metallic artefacts (n=9), errors in lung segmentation (n = 5), air in soft tissue areas (n=5), body truncation (n=6) and other artefacts due to noise in the atMR image (n=6). Five patients had no MRAC attenuation map errors. In general, not all the artefacts and errors in the atMR images were translated into the attenuation maps or the final PET MRAC image (supplementary table 3).
Figure 6.
Example of artefacts on the atMR sequence: flow (A); motion respiration (B); metallic implant (C) and body truncation (D).
Discussion
This study presented a comparison of whole body PET images for two groups, one with coils and one without coils within the PET FOV, with different attenuation correction methods for the combined whole body PET/MR system. Although other studies have already shown MRAC to CTAC or transmission-based AC [1, 18] to our knowledge this is the first study showing at the same time results of a combined PET/MR scanner using MRAC and CTAC methods including groups with and without coils and performing evaluation of lesions for all the images.
Both the voxel- and ROI-based comparisons also showed that in general the beta group overestimates the PET MRAC quantification and increases the variability (Voxel-based: alpha: −0.2±33.6%, beta: 10.31±69.86%; ROI-based: alpha: −8.05±7.37%, beta: −3.02±11.76%) (figures 2, 3, 4 and table 3). It has been previously reported that differences in BMI could have an important impact in PET quantification [22]. This could explain some of the differences between both groups since we have found that BMI is significantly higher for the beta group (table 1). Differences in lung density between the alpha and beta groups could also explain some of the results, since only one single LAC (0.022 cm−1, corresponding to HU=−770) was assigned for the whole lung, while in reality lungs showed a large range of HU values that are directly translated to LACs (table 4). Our results also showed that significant differences appear even within the same group between the right and left lungs, which could also explain some of the difference in their ROI results (supplementary table 1). The increased attenuation and scatter fractions due to the presence of the coils could also explain the higher variability between both groups. Additionally the small physical tolerances in placing the coil in the patient table slightly misplaced from where the template is calculated as well as the fact that the posterior parts of the cardiac and torso coils were not corrected may also affect PET quantification and have an impact in both bias and variability [23, 24]. However, since the scope of this study was not to evaluate particular effects of specific coils but rather to compare two groups of patients with and without MR coils, further studies are required to evaluate the effects of particular coils in the final PET reconstructed image. It is also worth noting that our mean relative differences (in %) are averaging positive and negative values together, and as shown in figure 3, local differences may potentially be higher than the average values presented here.
In terms of the lesions reported, comparisons of MRAC vs. CTAC results showed that the beta group presented again higher PET values than the alpha group (alpha: −6.01±9.31%, beta: 1.76±18.46%), similarly to the voxel- and ROI-based case (figure 5). In general, all lesion types classified in 3 groups following malignancy (normal with increased uptake, inflammatory/degenerative and tumor lesions), were underestimated for the alpha group and overestimated for the beta group except for the inflammatory/degenerative lesions that were also slightly underestimated (−3.13±16.35%). These results also agree with the overall PET overestimation of the beta group. Image artefacts such as flow ghosting, metallic implants or body truncation (figure 6) may potentially bias the clinical interpretation of the PET/MR images in a similar fashion to CT artefacts (produced by metallic implants for instance). Therefore these effects need to be known and addressed properly.
The quantitative results presented in this study are in accordance to previous results presented in the literature. In particular, the studies by Schulz et al. [1] and by Schramm et al. [18] followed similar MRAC and CTAC comparisons with the same AC method implemented here. However our study differs from theirs in several aspects. First, Schulz et al. used a standard clinical PET/CT scanner while we used a PET/MR scanner to acquire the PET data, since the objective of this study was to evaluate the feasibility of this new PET/MRI technology. Secondly we introduced a second group of subjects that include MR coils while none of the two studies does. This is of great interest since most future PET/MR studies will indeed include coils to improve the quality of the clinical MR images. Finally, our study provided additional lesion evaluation of PET MRAC and CTAC images, which was not present in their studies. Martinez-Moller et al. and Eiber et al. reported an underestimation of less than 8% of bone lesions when using CT-segmented images using 4 classes (background, lungs, fat and soft tissue) compared with CTAC [4, 25]. Keereman et al. reported underestimation between 10% to 15% for spine and femur lesions when spongious bone was considered as soft tissue and 10% to 20% when cortical bone was considered as soft tissue [26]. Similarly, Hofmann et al. presented results that showed underestimation of more than 10% in almost 85% of the ROIs placed in bone areas when using an MR segmentation method with 5 tissue classes (not including bone) [27].
This study has however some limitations. First, the study has not compared MRAC methods that include bone segmentation to evaluate whether bone segmentation could improve PET quantification neither what is the impact of potential misclassification errors. Second, this study has been limited to the analysis of whole-body protocols. Brain imaging, known to be more prone to MRAC underestimation when bone segmentation is not included [28], has not been included on this study. Third, the study does not address the effect of potential misregistration that could lead to differences in PET quantification. Although non-rigid coregistration appears in principle as a natural option that achieves very good quality results in brain imaging [10, 29], whole-body non-rigid coregistration is still non optimal [8] and as explained by Beyer et al. it could in fact lead to increased quantification bias due to incorrect repositioning of certain challenging areas [9]. For this reason we have followed rigid registration, similarly to [18], limiting the effect of non-rigid misregistration by enforcing the use of the same supports to position the patient similarly on both scanners (figure 1). Additionally the distribution of bias shown on figure 3 does not suggest global misregistration errors. Our group has also successfully proved a similar strategy in pre-clinical images, showing coregistration errors smaller than the resolution of the PET scanner (5mm) [30]. Despite these efforts some of the differences between MRAC and CTAC images could still potentially be due to this effect. Another limitation is that the results shown on this study cannot be extrapolated to other PET/MR systems as the technology and AC methods implemented differ considerably. Finally, this study does not address the use of the MR images for further clinical evaluation (other than just its use for attenuation correction and anatomical fusion similarly to a low dose CT in clinical standard PET/CT). The potential of MRI for clinical evaluation could be of great advantage compared to CT in many cases due to its superior soft-tissue contrast although this point is yet to be proved. However, we have limited our study to the impact of attenuation correction methods to the final PET quantification. This study evaluates the global effect that the use of different clinical MR coils could produce in the final PET quantification rather than focusing on the effects of a particular MR coil. As we previously discussed, more in detail studies should focus to investigate the precise effects on specific MR coils on PET quantification, similarly to the studies presented in [23, 24, 31-33]. Despite these known limitations, the study showed that the MRAC method implemented in the Ingenuity TF PET/MR scanner differs overall by less than 10% compared to CTAC. However some ROIs and lesions presented higher differences, in particular, spine, lung and heart ROIs. Further studies would be required to address these known limitations.
Conclusion
The results of this study showed that the built-in MRAC strategy on the PET/MR scanner, correcting for the presence of MR coils except for flexible coils, tended to increase variability of PET quantification when comparing a group containing coils to a group without coils. Lesions were underestimated overall by -6% when compared to PET CTAC in the alpha group (no MR coils) and overestimated by 2% in the beta group (MR coils included). Potential MR artefacts need to be properly addressed to minimize their impact in the clinical evaluation. In summary, the MRAC method implemented in this combined PET/MR scanner showed MRAC images that were underestimated overall by less than 10% in comparison with CTAC. However, some ROIs and lesions presented higher differences (large bone areas like spine for instance) for both SUVmean and SUVmax.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the staff members from the Nuclear Medicine division (Ash Rafique and Melissa Quispe) as well as the Translational and Molecular Imaging Institute (Frank Macaluso and Charles Adapoe) for their help and support with the study. We would also like to thank Philips for their help and for providing support for CTAC reconstruction on the PET/MR, in particular, to Kevin Kilroy and Gary Muswick. We would like to specially thank Dr. Ciprian Catana from the Athinoula A. Martinos Center for his guidance and expert review of this paper. Finally we would also like to thank the NIH for partial support NIH/NHLBI R01 HL071021 and R01 HL078667 (D. I.-G. and Z.A.F.).
Footnotes
Disclaimer: None
References
- 1.Schulz V, Torres-Espallardo I, Renisch S, Hu Z, Ojha N, Bornert P, et al. Automatic, three-segment, MR-based attenuation correction for whole-body PET/MR data. Eur J Nucl Med Mol Imaging. 2011;38:138–52. doi: 10.1007/s00259-010-1603-1. doi:10.1007/s00259-010-1603-1. [DOI] [PubMed] [Google Scholar]
- 2.Bezrukov I, Mantlik F, Schmidt H, Scholkopf B, Pichler BJ. MR-Based PET Attenuation Correction for PET/MR Imaging. Semin Nucl Med. 2013;43:45–59. doi: 10.1053/j.semnuclmed.2012.08.002. doi:10.1053/j.semnuclmed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Le Goff-Rougetet R, Frouin V, Mangin J- F, Bendriem B. Segmented MR images for brain attenuation correction in PET. Proceedings of SPIE. 1994;2167:725–36. [Google Scholar]
- 4.Martinez-Moller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd'hotel C, Ziegler SI, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–6. doi: 10.2967/jnumed.108.054726. doi:jnumed.108.054726 [pii] 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 5.Catana C, van der Kouwe A, Benner T, Michel CJ, Hamm M, Fenchel M, et al. Toward implementing an MRI-based PET attenuation-correction method for neurologic studies on the MR-PET brain prototype. J Nucl Med. 2010;51:1431–8. doi: 10.2967/jnumed.109.069112. doi:10.2967/jnumed.109.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keereman V, Fierens Y, Broux T, De Deene Y, Lonneux M, Vandenberghe S. MRI-based attenuation correction for PET/MRI using ultrashort echo time sequences. J Nucl Med. 2010;51:812–8. doi: 10.2967/jnumed.109.065425. doi:51/5/812 [pii] 10.2967/jnumed.109.065425. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann M, Pichler B, Scholkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S93–104. doi: 10.1007/s00259-008-1007-7. doi:10.1007/s00259-008-1007-7. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, et al. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med. 2008;49:1875–83. doi: 10.2967/jnumed.107.049353. doi:jnumed.107.049353 [pii] 10.2967/jnumed.107.049353. [DOI] [PubMed] [Google Scholar]
- 9.Beyer T, Weigert M, Quick HH, Pietrzyk U, Vogt F, Palm C, et al. MR-based attenuation correction for torso-PET/MR imaging: pitfalls in mapping MR to CT data. Eur J Nucl Med Mol Imaging. 2008;35:1142–6. doi: 10.1007/s00259-008-0734-0. doi:10.1007/s00259-008-0734-0. [DOI] [PubMed] [Google Scholar]
- 10.Kops ER, Herzog H. Alternative methods for attenuation correction for PET images in MR-PET scanners. Nuclear Science Symposium Conference Record. 2007;6:4327–30. doi:10.1109/NSSMIC.2007.4437073. [Google Scholar]
- 11.Salomon A, Goedicke A, Schweizer B, Aach T, Schulz V. Simultaneous reconstruction of activity and attenuation for PET/MR. IEEE Trans Med Imaging. 2011;30:804–13. doi: 10.1109/TMI.2010.2095464. doi:10.1109/TMI.2010.2095464. [DOI] [PubMed] [Google Scholar]
- 12.Nuyts J, Bal G, Kehren F, Fenchel M, Michel C, Watson C. Completion of a Truncated Attenuation Image from the Attenuated PET Emission Data. IEEE Trans Med Imaging. 2012 doi: 10.1109/TMI.2012.2220376. doi:10.1109/TMI.2012.2220376. [DOI] [PubMed] [Google Scholar]
- 13.Defrise M, Rezaei A, Nuyts J. Time-of-flight PET data determine the attenuation sinogram up to a constant. Phys Med Biol. 2012;57:885–99. doi: 10.1088/0031-9155/57/4/885. doi:10.1088/0031-9155/57/4/885. [DOI] [PubMed] [Google Scholar]
- 14.Heremans A, Verschakelen JA, Van fraeyenhoven L, Demedts M. Measurement of lung density by means of quantitative CT scanning. A study of correlations with pulmonary function tests. Chest. 1992;102:805–11. doi: 10.1378/chest.102.3.805. [DOI] [PubMed] [Google Scholar]
- 15.Mollet P, Keereman V, Clementel E, Vandenberghe S. Simultaneous MR-compatible emission and transmission imaging for PET using time-of-flight information. IEEE Trans Med Imaging. 2012;31:1734–42. doi: 10.1109/TMI.2012.2198831. doi:10.1109/TMI.2012.2198831. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi H, Ojha N, Morich M, Griesmer J, Hu Z, Maniawski P, et al. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys Med Biol. 2011;56:3091–106. doi: 10.1088/0031-9155/56/10/013. doi:S0031-9155(11)76486-7 [pii] 10.1088/0031-9155/56/10/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalemis A, Delattre BM, Heinzer S. Sequential whole-body PET/MR scanner: concept, clinical use, and optimisation after two years in the clinic. The manufacturer's perspective. MAGMA. 2013;26:5–23. doi: 10.1007/s10334-012-0330-y. doi:10.1007/s10334-012-0330-y. [DOI] [PubMed] [Google Scholar]
- 18.Schramm G, Langner J, Hofheinz F, Petr J, Beuthien-Baumann B, Platzek I, et al. Quantitative accuracy of attenuation correction in the Philips Ingenuity TF whole-body PET/MR system: a direct comparison with transmission-based attenuation correction. MAGMA. 2013;26:115–26. doi: 10.1007/s10334-012-0328-5. doi:10.1007/s10334-012-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger C, Goerres G, Schoenes S, Buck A, Lonn AH, Von Schulthess GK. PET attenuation coefficients from CT images: experimental evaluation of the transformation of CT into PET 511-keV attenuation coefficients. Eur J Nucl Med Mol Imaging. 2002;29:922–7. doi: 10.1007/s00259-002-0796-3. doi:10.1007/s00259-002-0796-3. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 21.Izquierdo-Garcia D, Davies JR, Graves MJ, Rudd JH, Gillard JH, Weissberg PL, et al. Comparison of methods for magnetic resonance-guided [18-F]fluorodeoxyglucose positron emission tomography in human carotid arteries: reproducibility, partial volume correction, and correlation between methods. Stroke. 2009;40:86–93. doi: 10.1161/STROKEAHA.108.521393. doi:STROKEAHA.108.521393 [pii] 10.1161/STROKEAHA.108.521393. [DOI] [PubMed] [Google Scholar]
- 22.Halpern BS, Dahlbom M, Auerbach MA, Schiepers C, Fueger BJ, Weber WA, et al. Optimizing imaging protocols for overweight and obese patients: a lutetium orthosilicate PET/CT study. J Nucl Med. 2005;46:603–7. [PubMed] [Google Scholar]
- 23.Delso G, Martinez-Moller A, Bundschuh RA, Ladebeck R, Candidus Y, Faul D, et al. Evaluation of the attenuation properties of MR equipment for its use in a whole-body PET/MR scanner. Phys Med Biol. 2010;55:4361–74. doi: 10.1088/0031-9155/55/15/011. doi:10.1088/0031-9155/55/15/011. [DOI] [PubMed] [Google Scholar]
- 24.Wollenweber SD, Delso G, Deller T, Goldhaber D, Hullner M, Veit-Haibach P. Characterization of the impact to PET quantification and image quality of an anterior array surface coil for PET/MR imaging. MAGMA. 2013 doi: 10.1007/s10334-013-0388-1. doi:10.1007/s10334-013-0388-1. [DOI] [PubMed] [Google Scholar]
- 25.Eiber M, Martinez-Moller A, Souvatzoglou M, Holzapfel K, Pickhard A, Loffelbein D, et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging. 2011;38:1691–701. doi: 10.1007/s00259-011-1842-9. doi:10.1007/s00259-011-1842-9. [DOI] [PubMed] [Google Scholar]
- 26.Keereman V, Holen RV, Mollet P, Vandenberghe S. The effect of errors in segmented attenuation maps on PET quantification. Med Phys. 2011;38:6010–9. doi: 10.1118/1.3651640. doi:10.1118/1.3651640. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann M, Bezrukov I, Mantlik F, Aschoff P, Steinke F, Beyer T, et al. MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation- and atlas-based methods. J Nucl Med. 2011;52:1392–9. doi: 10.2967/jnumed.110.078949. doi:10.2967/jnumed.110.078949. [DOI] [PubMed] [Google Scholar]
- 28.Berker Y, Franke J, Salomon A, Palmowski M, Donker HC, Temur Y, et al. MRI-based attenuation correction for hybrid PET/MRI systems: a 4-class tissue segmentation technique using a combined ultrashort-echo-time/Dixon MRI sequence. J Nucl Med. 2012;53:796–804. doi: 10.2967/jnumed.111.092577. doi:10.2967/jnumed.111.092577. [DOI] [PubMed] [Google Scholar]
- 29.Malone IB, Ansorge RE, Williams GB, Nestor PJ, Carpenter TA, Fryer TD. Attenuation correction methods suitable for brain imaging with a PET/MRI scanner: a comparison of tissue atlas and template attenuation map approaches. J Nucl Med. 2011;52:1142–9. doi: 10.2967/jnumed.110.085076. doi:10.2967/jnumed.110.085076. [DOI] [PubMed] [Google Scholar]
- 30.Bini J, Izquierdo-Garcia D, Mateo J, Machac J, Narula J, Fuster V, et al. Preclinical Evaluation of MR Attenuation Correction Versus CT Attenuation Correction on a Sequential Whole-Body MR/PET Scanner. Invest Radiol. 2013 doi: 10.1097/RLI.0b013e31827a49ba. doi:10.1097/RLI.0b013e31827a49ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald LR, Kohlmyer S, Liu C, Lewellen TK, Kinahan PE. Effects of MR surface coils on PET quantification. Med Phys. 2011;38:2948–56. doi: 10.1118/1.3583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulus DH, Braun H, Aklan B, Quick HH. Simultaneous PET/MR imaging: MR-based attenuation correction of local radiofrequency surface coils. Med Phys. 2012;39:4306–15. doi: 10.1118/1.4729716. doi:10.1118/1.4729716. [DOI] [PubMed] [Google Scholar]
- 33.Tellmann L, Quick HH, Bockisch A, Herzog H, Beyer T. The effect of MR surface coils on PET quantification in whole-body PET/MR: results from a pseudo-PET/MR phantom study. Med Phys. 2011;38:2795–805. doi: 10.1118/1.3582699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.