ABSTRACT
We previously isolated the commensal bacteria lactobacilli and bifidobacteria from the Thoroughbred intestine and prepared the horse probiotics LacFiTM, consisting of Lactobacillus ruminis KK14, L. equi KK 15, L. reuteri KK18, L. johnsonii KK21, and Bifidobacterium boum HU. Here, we found that the five LacFiTM constituent strains remarkably suppressed pro-inflammatory interleukin-17 production in mouse splenocytes stimulated with interleukin-6 and transforming growth factor-β. The protective effects of the probiotic on impaired intestinal barrier function were evaluated in Caco-2 cells treated with tumor necrosis factor-α. Evaluation of transepithelial resistance showed that all the strains exhibited intestinal barrier protective activity, with significant suppression of barrier impairment by L. reuteri KK18. The LacFiTM constituent strains were detected in neonatal LacFiTM-administered Thoroughbred feces using polymerase chain reaction denaturing gradient gel electrophoresis and culture methods. These five strains were found to be the predominant lactobacilli and bifidobacteria in the intestinal microbiota of LacFiTM-administered Thoroughbreds. Administration of LacFiTM to neonatal Thoroughbreds decreased diarrhea incidence from 75.9% in the control group (n=29 neonatal Thoroughbreds) to 30.7% in the LacFiTM-administered group (n=101 neonatal Thoroughbreds) immediately after birth to 20 weeks after birth. LacFiTM treatment also prevented diarrhea especially at and around 4 weeks and from 10 to 16 weeks. The duration of diarrhea was also shorter in the probiotics-administered group (7.4 ± 0.8 days) than in the control group (14.0 ± 3.2 days). These results indicate that the LacFiTM probiotics regulates intestinal function and contributes to diarrhea prevention.
Keywords: diarrhea prevention, interleukin-17, probiotics, Thoroughbred
The incidence of diarrhea in Thoroughbred yearlings is very high, sometimes resulting in severe maldevelopment and even death. As physical maldevelopment and mental stress may negatively affect Thoroughbred performance, the avoidance of diarrhea in Thoroughbred yearlings is important for breeders and veterinarians.
Lactobacilli and bifidobacteria are important constituents of the healthy gastrointestinal tract of mammals and humans, and some strains of lactobacilli and bifidobacteria are frequently administered as probiotics because of their beneficial roles in mammal and human health and diarrhea prevention [1, 5, 6, 19]. The existence of lactobacilli in the intestinal flora of Thoroughbreds has long been suggested [9]. Morotomi et al. [15], Endo et al. [4], and Morita et al. [13, 14] isolated lactobacilli from horse fecal samples, including Thoroughbreds. We also isolated Bifidobacterium boum from the intestinal flora of Thoroughbreds [3]. Based on the above studies, a horse probiotics consisting of Lactobacillus ruminis KK14, L. equi KK15, L. reuteri KK18, L. johnsonii KK21 [13], and B. boum HU [4] was prepared and termed LacFiTM. LacFiTM has been recognized as “Generally Recognized As Safe (GRAS)” following use for >11 years.
It has recently been reported that some probiotic strains reduce the incidence of antibiotics and Clostridium difficile-associated diarrhea [7, 26]. These bacteria have been shown to increase beneficial intestinal bacteria and to modulate immune function. Several intestinal toxins induce epithelial barrier dysfunction through changes in tight junction (TJ) proteins that contribute to diarrhea [17]. We reported that Enterococcus hirae, an intestinal bacterium in the adjacent mucosa (mucosal bacterium), ameliorates tumor necrosis factor (TNF)-α-induced barrier impairment in the human epithelial TJ [10]. Therefore, immunomodulation, such as suppression of inflammation, and regulation of TJ function are beneficial for preventing diarrhea.
In this study, we investigated the regulative effects of the five LacFiTM constituent bacterial strains (four lactobacilli and one bifidobacterium) on splenocytes and intestinal epithelial cells to evaluate the effect of the probiotics on diarrhea prevention. The LacFiTM constituent strains were detected in neonatal Thoroughbred feces by polymerase chain reaction denaturing gradient gel electrophoresis (PCR-DGGE) and culture methods. We also evaluated the incidence of diarrhea in neonatal Thoroughbreds after the administration of LacFiTM, with a focus on the anti-inflammatory and the intestinal barrier–protective activities.
Materials and Methods
Reagents
RPMI 1640 and Dulbecco’s modified Eagle’s medium, nonessential amino acids, penicillin, streptomycin, and gentamycin were all obtained from Life Technologies (Forster City, CA, USA). Fetal bovine serum (FBS) was obtained from MP Biomedicals, (Osaka, Japan). Both recombinant human transforming growth factor (TGF)-β and mouse interleukin (IL)-6 were obtained from R&D Systems (Minneapolis, MN, USA). TNF-α was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). MRS broth and MRS agar were obtained from Becton, Dickinson and Company (Sparks, MD, USA). All other chemicals were of reagent grade.
Bacterial culture preparation for studies on anti-inflammatory and intestinal barrier-protective activities
Bacteria were cultured in MRS broth and incubated under anaerobic conditions by using AnaeroPackTM (Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan) at 37°C for 24 hr. The cell suspensions were washed with distilled water, incubated at 100°C for 50 min [10], and then lyophilized. Subsequently, the heat-killed bacteria were added to murine splenocyte or Caco-2 cell culture.
Splenocyte culture
The suppressive effects of bacteria on IL-17 production were evaluated according to previously described methods [18, 23]. Briefly, 6-week-old female Balb/c mice were obtained from Charles River Laboratories International, Inc. (Kanagawa, Japan) and killed by cervical dislocation. Spleens were removed from three mice for each experiment and the pooled splenocytes (107 cells) were incubated with TGF-β (2 ng/ml) and IL-6 (20 ng/ml) at 37°C for 72 hr in 1 ml of RPMI 1640 medium supplemented with 10% FBS, 10 μM 2-mercaptoethanol, 10 mM HEPES, penicillin, and streptomycin. Heat-killed bacteria (107 cells) were added to the culture. A culture without the addition of TGF-β, IL-6, or heat-killed bacteria was included as a control. Culture supernatants were harvested to measure IL-17 concentrations by sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
Caco-2 cell culture
Caco-2 cells were purchased from the American Type Culture Collection (Rockville, MD, USA). In this study, Caco-2 cells were used between passages 35 and 45. The growth medium consisted of Dulbecco’s modified Eagle’s medium with 10% FBS, 1% nonessential amino acids, and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamycin). Cells were cultured at 37°C under a humidified 5% CO2 atmosphere in 75-cm2 tissue culture flasks to approximately 80% confluence and seeded into a 12-well Transwell cell culture chamber (0.4-μm pore size, 12-mm diameter) (Corning Inc., Tewksbury, MA, USA) at a density of 5 × 104 cells/cm2. After 14 days of culture, transepithelial resistance (TER) was measured using a Millicell-ERS instrument with Ag/AgCl electrodes (EMD Millipore Corporation, Billerica, MA, USA). Caco-2 cell monolayers were used when their TER values were >300 ohm•cm2. Each well was placed in a cluster plate with an external medium (basolateral side, 1.5 ml) and an internal medium (apical side, 0.5 ml). The cell monolayers were fed fresh medium every 24 hr.
Evaluation of epithelial barrier function
The protective effects of bacteria on epithelial impairment were evaluated according to previously described methods [10, 11]. Briefly, bacteria (105 cells/well) were added to the apical side of the Caco-2 cell monolayers. One hour later, the cells were treated with TNF-α (100 ng/ml) on the basolateral side of the cell monolayers and cultured for 48 hr. After the 48-hr incubation, the TER value was measured to assess epithelial barrier function.
Bacterial culture preparation for administration to neonatal Thoroughbreds
L. ruminis KK14, L. equi KK15, L. reuteri KK18, L. johnsonii KK21 [13], and B. boum HU [4] strains produced by Crossfield-Bio, Inc. (Tokyo, Japan) were combined to prepare the probiotic LacFiTM in this study. Using 10 Sprague-Dawley rats and 10 Balb/c mice (Charles River Laboratories International, Inc.), these bacterial strains (1010 colony forming units (CFU)/head) had been previously evaluated to elicit no sign of illness and toxicity (such as full blood count, hematocrit, mean corpuscular volume [MCV], erythrocyte sedimentation rate [ESR], aspartate transaminase [AST], alkaline phosphatase [ALP], lactate dehydrogenase [LDH], glutamic oxaloacetic transaminase [GOT], and glutamic pyruvate transaminase [GPT]) over 6 months by veterinarians. All animal experiments were performed in accordance with guidelines for the care and use of laboratory animals. Each strain was cultured separately in MRS broth (Kanto Chemical Co., Inc., Tokyo, Japan) and incubated at 37°C for 24 hr. Bacterial cells were washed twice with saline by centrifugation. The cell pellets were resuspended with sterilized 10% skim milk to the final concentration of CFU/ml shown in Table 1 and stored at −80°C until administration. Each thawed suspension contained more than 109 CFU/ml of living bacteria.
Table 1. LacFiTM-constituent strains and cell counts before/after freezing at –80°C.
| Species | Strain | Cell counts before freezing (CFU/ml) |
Cell counts after freezing (CFU/ml) |
|---|---|---|---|
| Lactobacillus reuteri | KK18 | 2.0 × 109 | 1.6 × 109 |
| Lactobacillus ruminis | KK14 | 1.2 × 109 | 0.9 × 109 |
| Lactobacillus equi | KK15 | 5.8 × 109 | 4.8 × 109 |
| Lactobacillus johnsonii | KK21 | 6.4 × 109 | 5.9 × 109 |
| Bifidobacterium boum | HU | 7.9 × 109 | 6.1 × 109 |
| Mixture of the above five strains* | 9.8 × 109 | 8.6 × 109 |
* The mixture was administered to neonatal Thoroughbreds as LacFiTM in this study.
Neonatal Thoroughbreds: Observation of animals and clinical evaluation
One hundred and thirty healthy neonatal Thoroughbreds at ages ranging from immediately after birth to 20 weeks of age were included in the study from January to May 2008. This evaluation was carried out with sufficient attention given to animal protection. The neonatal Thoroughbreds belonged to the research herd at Northern Farm in Hokkaido, Japan. Prior to study commencement, the neonatal Thoroughbreds had been determined to have no signs of illness by veterinarians. The LacFiTM-administered group (n=101) was administered 50 ml of probiotics in skim milk, and the control group (n=29) was untreated. Following study commencement, the neonatal Thoroughbreds were evaluated for signs of illness and diarrhea every 2 weeks by veterinarians for up to 20 weeks after birth. In this study, although we did not evaluate subjects with the blind study, we evaluated them with the random control group study.
Polymerase chain reaction denaturing gradient gel electrophoresis (PCR-DGGE)–based detection of lactobacilli
Meconium and feces were collected on the 7th, 14th, and 21st days after birth from five neonatal Thoroughbreds of both the control and LacFiTM-administered groups. DNA was extracted from the fecal samples and PCR-DGGE analysis was performed using a previously described method [12, 16]. Approximately 340 bp of the 16S rRNA gene of Escherichia coli No. 341–534 were amplified by PCR using the Lac1 and Lac2GC primer sets [25]. The primer set used for PCR-DGGE comprised the forward primer Lac1 (5′-AGCAGTAGGGAATCTTCCA-3′) and the reverse primer Lac2GC (5′-CGCCCGGGGCGCGCCCCGGGCGGCCCGGGGGCACCGGGGGATTYCACCGCTACACATG-3′). The PCR fragments were separated by DGGE using the DCode system (Bio-Rad Laboratories, Hercules, USA) with the following modifications: For the DGGE analysis of bacteria, 8% (wt/vol) polyacrylamide gels were prepared using a denaturing gradient ranging from 35 to 50%, and electrophoresis was performed in Tris-acetate-EDTA (TAE) buffer for 14 hr at a constant voltage of 60 V. A 100% denaturant corresponded to 7 M urea and 40% formamide. After electrophoresis, the gels were stained for 15 min using an ethidium bromide solution (250 ml TAE buffer + 25 µl of 10 mg/ml ethidium bromide solution). The PCR products derived from the DGGE bands were purified with the Wizard SV Gel and PCR Clean-up system (Promega Co., Madison, USA). The primers used for sequencing were the same as those used for the re-amplification of DNAs from DGGE bands. Sequence similarities between the DNAs were determined using Standard Nucleotide BLAST (BLASTN) (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analyses
Differences between measured values were analyzed using Tukey-Kramer’s test, Student’s t-test and Fisher’s exact test, and P-values <0.05 were considered statistically significant.
Results and Discussion
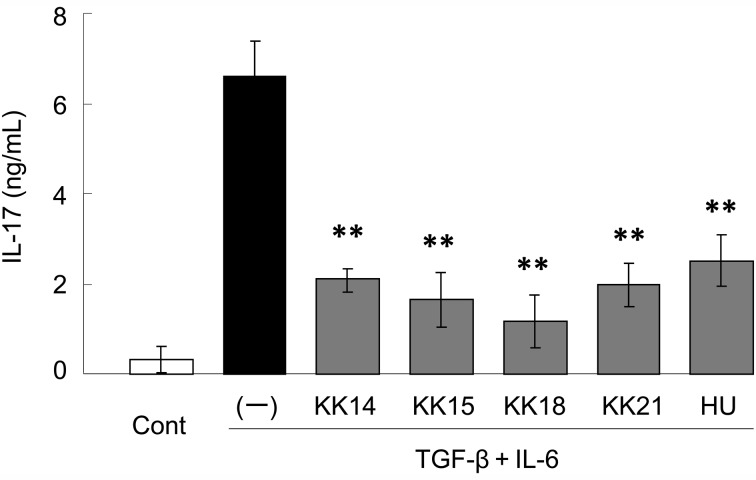
The pathogenesis of diarrhea, including the effects of physiological and mental stress, in Thoroughbred yearlings is complex, and the resulting intestinal inflammation is responsible for the occurrence of diarrhea. The anti-inflammatory activity of the five LacFiTM constituent strains, L. ruminis KK14, L. equi KK 15, L. reuteri KK18, L. johnsonii KK21, and B. boum HU, was examined in vitro. Elevated levels of pro-inflammatory cytokines such as IL-17 and chemokines, a subset of chemoattractant cytokines, are observed in the inflamed intestine and are critically involved in the pathogenesis of intestinal inflammation [8, 22]. In this study, IL-17 production by mouse splenocytes was evaluated. The addition of TGF-β and IL-6 drastically enhanced IL-17 production by splenocytes (Fig. 1); however, all five strains in LacFiTM significantly (P<0.01) suppressed the production of IL-17. Among the five strains, L. reuteri KK18 suppressed IL-17 production most potently. We have reported the similar suppressive effects of Streptococcus thermophilus ST28 and its genomic DNA fraction on Th17 response in murine splenocytes stimulated with TGF-β plus IL-6 [24]. Several studies showed that Toll-like receptor (TLR) 9-signalling had an important role for modulating experimental allergic encephalomyelitis and colitis. For example, it was reported that TLR9 ligands mediated the anti-inflammatory effects of probiotic bacteria in murine experimental colitis [20]. Therefore, it was likely that genomic DNA of the LacFiTM constituent strains suppressed IL-17 production, at least in part, via TLR9.
Fig. 1.
Suppressive effects of LacFiTM constituent bacteria on pro-inflammatory interleukin (IL)-17 production in murine splenocytes. Splenocytes were stimulated with TGF-β and IL-6. Bacteria were added to the media and cultured for 72 hr. Culture supernatants were harvested and assayed for IL-17 concentrations. The culture alone (Cont) and culture without the addition of bacteria (–) were included as controls. Results are expressed as mean ± SD values (n=3). **P<0.01 compared to control (–) (Tukey-Kramer).
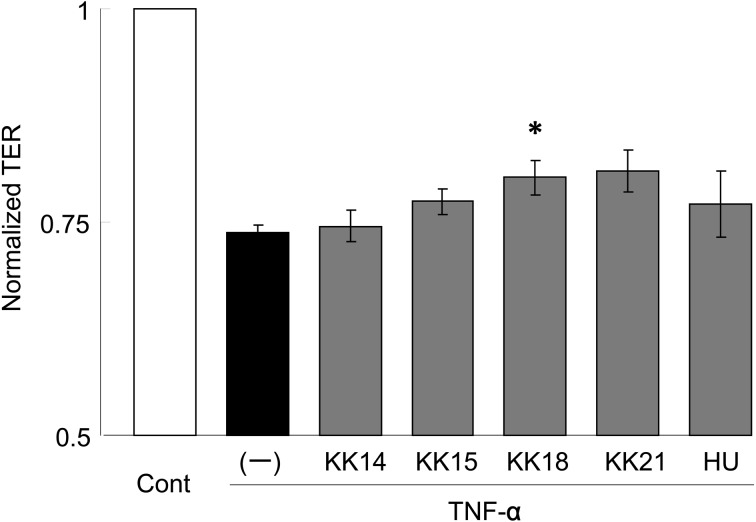
Next, the protective effects of the LacFiTM constituent strains on epithelial barrier impairment were evaluated, since intestinal barrier impairment is also involved in diarrhea. TNF-α is an essential mediator of inflammation in the gut. Several pro-inflammatory actions have been proposed for TNF-α, such as the production and stimulation of pro-inflammatory cytokines and activation of the acute-phase response. TNF-α induces an increase in intestinal TJ permeability [2]. In this study, TJ barrier impairment was induced by TNF-α in the human epithelial Caco-2 cells, and the protective effects of LacFiTM constituent bacteria on the impairment were evaluated by measuring the TER value, reflecting TJ permeability. As shown in Fig. 2, TNF-α decreased the TER value by approximately 25%; however, pretreatment with L. reuteri KK18 significantly (P<0.05) suppressed the TNF-α-induced decrease in TER. L. johnsonii KK21 also suppressed the decrease in TER to some extent. We have reported that Enterococcus hirae ATCC 9790T and its cell wall fractions protected against intestinal impairment by regulation of epithelial tight junction via TLR2 signaling. In addition, Cario et al. [3] reported that Pam3Cys-Ser-(Lys)4, a TLR2 ligand, ameliorated colonic inflammation in mice with colitis induced by dextran sodium sulfate. It was probable that cell wall fractions of the LacFiTM constituent strains suppressed the intestinal barrier impairment in the present study, although further investigations are necessary to clarify this.
Fig. 2.
Protective effects of LacFiTM constituent bacteria with respect to intestinal barrier impairment. Caco-2 cells were treated with the bacterium or medium alone (–) for 1 hr and exposed to tumor necrosis factor-α for 48 hr. After incubation, the transepithelial resistance value was measured to assess intestinal barrier function. The culture alone (Cont) and culture without the addition of bacteria (–) were included as controls. Results are expressed as the relative values and mean ± SD values (n=3). *P<0.05 compared to control (–) (Tukey-Kramer).
A defective intestinal epithelial TJ barrier characterized by an increase in intestinal permeability is an important pathogenic factor contributing to the development of intestinal inflammation resulting in diarrhea. Animal studies have shown that enhancement of the intestinal TJ barrier prevents cytokine-mediated development of intestinal inflammation and diarrhea [2]. Clinical studies have also shown that anti–TNF-α therapy recovers the intestinal barrier and that normalization of intestinal permeability is associated with long-term clinical remission [21]. In this regard, it was hypothesized that LacFiTM constituent probiotics would prevent diarrhea through modulation of the immune response and improvement in TJ function.
Considering the interesting results obtained in vitro, we evaluated the in vivo effect of LacFiTM constituent probiotics on the incidence of diarrhea in neonatal Thoroughbreds after the administration of LacFiTM. The frozen bacterial mixture LacFiTM was administered to neonatal Thoroughbreds orally at a dose of 8.6 × 109 CFU/50 ml (Table 1) on the 2nd, 3rd, 4th, and 5th days after birth; LacFiTM administration was then continued with one administration/week up to 4 weeks. After the 20-week experimental period, oral administration of LacFiTM to 101 neonatal Thoroughbreds did not result in reduced body weight. Also, no signs of toxicity were observed in any animal and no animals died during the test period.
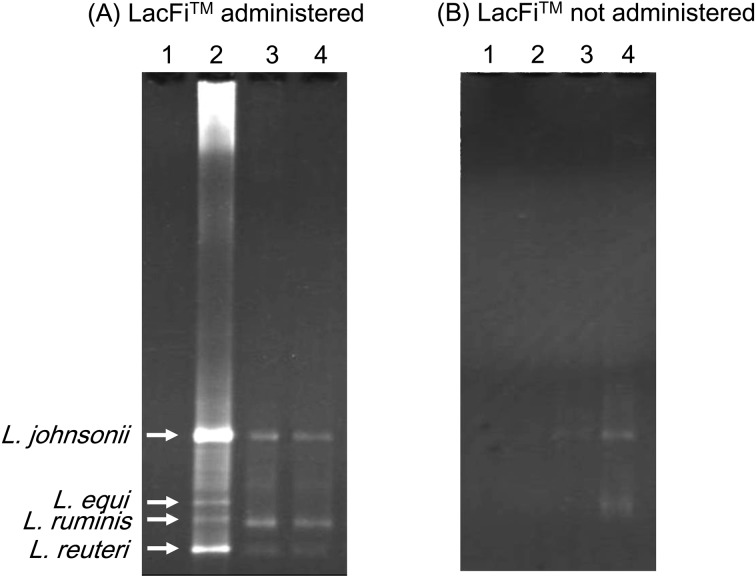
PCR-DGGE analysis confirmed that L. ruminis, L. equi, L. reuteri, L. johnsonii, possibly derived from LacFiTM, colonized the neonatal Thoroughbred intestine immediately after birth under germ-free conditions (Fig. 3-A). The broad band (Fig. 3-B) corresponding to the LacFiTM species was not detected in the analysis of the control group (LacFiTM not administered) fecal DNA.
Fig. 3.
DGGE analysis of the PCR products of lactic acid bacteria present in the neonatal Thoroughbred feces by lactic acid bacteria-specific primers [25]. Approximately 200 bp 16S rDNA of E. coli No. 341–534 were amplified by PCR. Lane 1: meconium, lane 2: feces obtained on the 7th day after birth, lane 3: feces obtained on the 14th day after birth, lane 4: feces obtained on the 21st day after birth. Band a: L. johnsonii (100% similarity), band b: L. equi (100% similarity), band c: L. ruminis (99.9% similarity), and band d: L. reuteri (99.8% similarity).
B. boum was isolated from the feces of LacFiTM-administered neonatal Thoroughbreds by culture methods using MRS agar under anaerobic conditions at 37°C for 48 hr but not in the feces of the control group. As bifidobacteria comprise a minority of the fecal microbiota of the Thoroughbred [3], it is likely that the Bifidobacterium isolated from the intestinal tract originated from the probiotics.
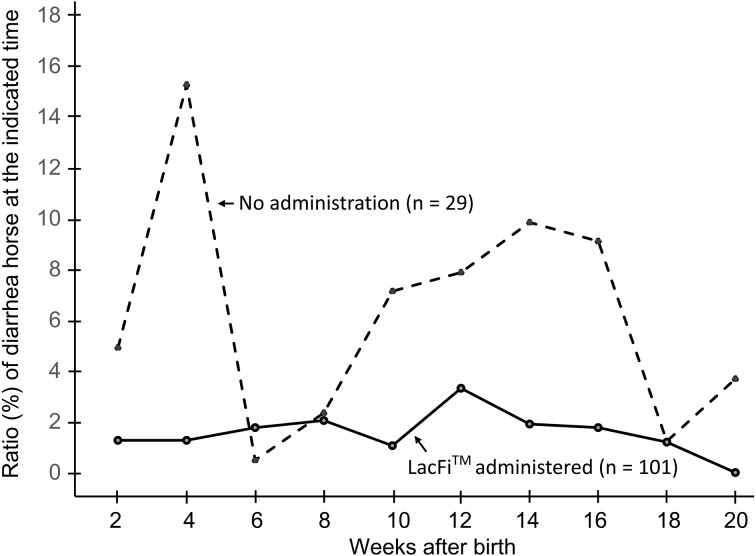
The symptom of diarrhea was also compared between the LacFiTM-administered and control groups. The Thoroughbreds were evaluated for diarrhea every 2 weeks, and the ratio (%) of horses which were suffered from diarrhea at 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 weeks were shown in Fig. 4. Administration of LacFiTM to the neonatal Thoroughbreds decreased the incidence of diarrhea from 75.9% in the control group (n=29 neonatal Thoroughbreds) to 30.7% in the LacFiTM-administered group (n=101 neonatal Thoroughbreds). The control group, which was not administered LacFiTM, exhibited diarrhea after birth up to 2 weeks, and the LacFiTM-treated group had markedly lesser diarrhea at all these timepoints, especially at and around 4 weeks and from 10 to 16 weeks (Fig. 4). The duration of diarrhea was also shorter in the probiotics-administered group (7.4 ± 0.8 days) than in the control group (14.0 ± 3.2 days) (Table 2).
Fig. 4.
Comparison of the number of neonatal Thoroughbreds showed the symptom of diarrhea in the LacFiTM-administered and control groups. The ratio (%) of horses which were suffered from diarrhea at 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 weeks were shown.
Table 2. Comparison of clinical manifestations of diarrhea in the LacFiTM-administered and control groups.
| LacFiTM-administered | Not administered (control) |
|
|---|---|---|
| Total number of neonatal Thoroughbreds | 101 | 29 |
| Number of neonatal Thoroughbreds exhibiting diarrhea * | 31 | 22 |
| Ratio of diarrhea in neonatal Thoroughbreds (%) | 30.7 | 75.9 |
| Duration of diarrhea (days) (mean ± standard error of the mean) ** | 7.4 ± 0.8 | 14.0 ± 3.2 |
* Number of neonatal Thoroughbreds exhibiting diarrhea in LacFiTM-administered group was significantly (P<0.01) lower than that in control group, judged by Fisher’s exact test. ** Duration of diarrhea of LacFiTM-administered group was significantly (P<0.001) shorter than that of control group, judged by Student’s t-test.
These results indicate that the horse probiotic LacFiTM regulates intestinal function and contributes to diarrhea prevention. LacFiTM treatment may be useful even if diarrhea develops, considering the impact of medical treatments administered at this important time of Thoroughbred growth on future race performance.
The intestine not only digests and absorbs food but also functions as a part of the immune system critical for host-defense. In addition, the intestinal barrier acts as the first defense against a vast amount of food, exogenous antigens, and commensal bacteria; therefore, it is important to regulate the cytokine balance and maintain the intestinal barrier. Diarrhea prevention by LacFiTM might be, at least in part, attributed to the improvement in cytokine balance and to the enforcement of the TJ barrier and subsequent maintenance of intestinal integrity [11, 18, 22,23,24].
References
- 1.Allen S.J., Wareham K., Wang D., Bradley C., Hutchings H., Harris W., Dhar A., Brown H., Foden A., Gravenor M.B., Mack D.2013. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 382: 1249–1257 [DOI] [PubMed] [Google Scholar]
- 2.Al-Sadi R., Guo S., Ye D., Ma T.Y.2013. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 183: 1871–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cario E., Gerken G., Podolsky D.K.2007. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374 [DOI] [PubMed] [Google Scholar]
- 4.Endo A., Okada S., Morita H.2007. Molecular profiling of Lactobacillus, Streptococcus, and Bifidobacterium species in feces of active racehorses. J. Gen. Appl. Microbiol. 53: 191–200 [DOI] [PubMed] [Google Scholar]
- 5.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., Taylor T.D., Itoh K., Kikuchi J., Morita H., Hattori M., Ohno H.2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547 [DOI] [PubMed] [Google Scholar]
- 6.Hempel S., Newberry S.J., Maher A.R., Wang Z., Miles J.N.V., Shanman R., Johnsen B., Shekelle P.G.2012. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 307: 1959–1969 [DOI] [PubMed] [Google Scholar]
- 7.Hickson M., D’Souza A.L., Muthu N., Rogers T.R., Want S., Rajkumar C., Bulpitt C.J.2007. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 335: 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koelink P.J., Overbeek S.A., Braber S., de Kruijf P., Folkerts G., Smit M.J., Kraneveld A.D.2012. Targeting chemokine receptors in chronic inflammatory diseases: an extensive review. Pharmacol. Ther. 133: 1–18 [DOI] [PubMed] [Google Scholar]
- 9.Mitsuoka T., Kaneuchi C.1977. Ecology of the bifidobacteria. Am. J. Clin. Nutr. 30: 1799–1810 [DOI] [PubMed] [Google Scholar]
- 10.Miyauchi E., Morita H., Okuda J., Sashihara T., Shimizu M., Tanabe S.2008. Cell wall fraction of Enterococcus hirae ameliorates TNF-alpha-induced barrier impairment in the human epithelial tight junction. Lett. Appl. Microbiol. 46: 469–476 [DOI] [PubMed] [Google Scholar]
- 11.Miyauchi E., Morita H., Tanabe S.2009. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 92: 2400–2408 [DOI] [PubMed] [Google Scholar]
- 12.Morita H., Nakajima F., Murakami M., Endo A., Suzuki T., Shiratori C., Kato Y., Okatani T.A., Akita H., Masaoka T.2007. Molecular monitoring of the main changes in bacterial floral diversity in the gastrointestinal tract of a thoroughbred foal with catarrhal enteritis by using PCR-DGGE. J. Equine Vet. Sci. 27: 14–19 [Google Scholar]
- 13.Morita H., Nakano A., Shimazu M., Toh H., Nakajima F., Nagayama M., Hisamatsu S., Kato Y., Takagi M., Takami H., Akita H., Matsumoto M., Masaoka T., Murakami M.2009. Lactobacillus hayakitensis, L. equigenerosi and L. equi, predominant lactobacilli in the intestinal flora of healthy thoroughbreds. Anim. Sci. J. 80: 339–346 [DOI] [PubMed] [Google Scholar]
- 14.Morita H., Shimazu M., Shiono H., Toh H., Nakajima F., Akita H., Takagi M., Takami H., Murakami M., Masaoka T., Tanabe S., Hattori M.2010. Lactobacillus equicursoris sp. nov., isolated from the faeces of a thoroughbred racehorse. Int. J. Syst. Evol. Microbiol. 60: 109–112 [DOI] [PubMed] [Google Scholar]
- 15.Morotomi M., Yuki N., Kado Y., Kushiro A., Shimazaki T., Watanabe K., Yuyama T.2002. Lactobacillus equi sp. nov., a predominant intestinal Lactobacillus species of the horse isolated from faeces of healthy horses. Int. J. Syst. Evol. Microbiol. 52: 211–214 [DOI] [PubMed] [Google Scholar]
- 16.Muyzer G., Smalla K.1998. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie van Leeuwenhoek 73: 127–141 [DOI] [PubMed] [Google Scholar]
- 17.Ngendahayo Mukiza C., Dubreuil J.D.2013. Escherichia coli heat-stable toxin b impairs intestinal epithelial barrier function by altering tight junction proteins. Infect. Immun. 81: 2819–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogita T., Nakashima M., Morita H., Saito Y., Suzuki T., Tanabe S.2011. Streptococcus thermophilus ST28 ameliorates colitis in mice partially by suppression of inflammatory Th17 cells. J. Biomed. Biotechnol. 2011: 378417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohland C.L., Macnaughton W.K.2010. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 298: G807–G819 [DOI] [PubMed] [Google Scholar]
- 20.Rachmilewitz D., Katakura K., Karmeli F., Hayashi T., Reinus C., Rudensky B., Akira S., Takeda K., Lee J., Takabayashi K., Raz E.2004. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126: 520–528 [DOI] [PubMed] [Google Scholar]
- 21.Suenaert P., Bulteel V., Lemmens L., Noman M., Geypens B., Van Assche G., Geboes K., Ceuppens J.L., Rutgeerts P.2002. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am. J. Gastroenterol. 97: 2000–2004 [DOI] [PubMed] [Google Scholar]
- 22.Tanabe S.2013. The effect of probiotics and gut microbiota on Th17 cells. Int. Rev. Immunol. 32: 511–525 [DOI] [PubMed] [Google Scholar]
- 23.Tanabe S., Kinuta Y., Saito Y.2008. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int. J. Mol. Med. 22: 181–185 [PubMed] [Google Scholar]
- 24.Ogita T., Tanii Y., Morita H., Suzuki T., Tanabe S.2011. Suppression of Th17 response by Streptococcus thermophilus ST28 through induction of IFN-γ. Int. J. Mol. Med. 28: 817–822 [DOI] [PubMed] [Google Scholar]
- 25.Walter J., Hertel C., Tannock G.W., Lis C.M., Munro K., Hammes W.P.2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67: 2578–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong S., Jamous A., O’Driscoll J., Sekhar R., Weldon M., Yau C.Y., Hirani S.P., Grimble G., Forbes A.2014. A Lactobacillus casei Shirota probiotic drink reduces antibiotic-associated diarrhoea in patients with spinal cord injuries: a randomised controlled trial. Br. J. Nutr. 111: 672–678 [DOI] [PubMed] [Google Scholar]