ABSTRACT
Resistance to phagocytosis is a crucial virulence property of Streptococcus equi (Streptococcus equi subsp. equi; Se), the cause of equine strangles. The contribution and interdependence of capsule and SeM to killing in equine blood and neutrophils were investigated in naturally occurring strains of Se. Strains CF32, SF463 were capsule and SeM positive, strains Lex90, Lex93 were capsule negative and SeM positive and strains Se19, Se1-8 were capsule positive and SeM deficient. Phagocytosis and killing of Se19, Se1-8, Lex90 and Lex93 in equine blood and by neutrophils suspended in serum were significantly (P ≤ 0.02) greater compared to CF32 and SF463. The results indicate capsule and SeM are both required for resistance to phagocytosis and killing and that the anti-phagocytic property of SeM is greatly reduced in the absence of capsule.
Keywords: capsule, phagocytosis, SeM, Streptococcus equi
Streptococcus equi (Streptococcus equi subsp. equi; Se), a Lancefield group C streptococcus causes strangles, a highly contagious disease of the upper respiratory tract of horses characterized by tonsillitis and metastatic abscessation of draining lymph nodes [9]. Se produces 4 virulence factors that affect uptake or killing of Se by neutrophils. These are SeM, factor H binding Se18.9, IdeE and a constitutively expressed hyaluronic acid capsule [1,2,3, 15, 16]. A commercial modified live attenuated non-encapsulated vaccine is of low virulence for mice and horses [6]. The capsule confers a characteristic wet mucoid colony morphology, whereas non-encapsulated variants form small dry colonies on solid media.
SeM, a 58 kDa cell wall anchored fibrillar protein, binds fibrinogen and limits deposition of C3b on the bacterial surface by an unknown mechanism [2]. Specific antibodies are opsonizing and mouse protective [4, 5, 11, 12]. Hyaluronic acid (HA), a polymer of glucuronic acid and N-acetylglucosamine repeating units is anti-phagocytic by mechanisms not well understood. It may interfere with phagocytic ingestion by steric interference or by charge repulsion [17].
Phagocytosis of Se has been correlated with the presence of capsule and with SeM [1, 3]. Hyaluronidase treatment of Se abolishes resistance to in vitro phagocytosis in a dose dependent manner [1]. However, hyaluronidase treatment does not abolish resistance to phagocytosis in the presence of fibrinogen to the extent it does in its absence [3]. This suggests the hyaluronic acid (HA) capsule is required for full functionality of SeM.
The aim of this study was to investigate the contribution of capsule and SeM to resistance to phagocytosis in three pairs of Se exhibiting the following phenotypes, HA+ SeM+; HA+ SeM– and HA– SeM+.
Se strains CF32, SF463, Lex 90, Lex 93 and Se19 had been isolated from abscesses or nasal swabs of horses as described in Table 1. All failed to ferment lactose, sorbitol and trehalose. Strains cultured overnight at 37°C on Columbia-colistin nalidixic acid (CNA) blood agar showed either a mucoid or non-mucoid colony phenotype (Fig. 1). Se1-8 is a SeM negative mutant of CF32 produced using Tn916 insertional mutagenesis [14].
Table 1. Strains of Streptococcus equi.
| Strain | Source | Description |
|---|---|---|
| CF32 | Mandibular abscess, NY, 1981 | SeM+, mucoid colony, SeM allele-2 |
| SF463 | Nasal swab, KY, 1993 | SeM+, mucoid colony, SeM allele-30 |
| Lex90 | Nasal swab, KY, 1990 | SeM+, non-mucoid colony, SeM allele-2 |
| Lex93 | Nasal swab, KY, 1993 | SeM+, non-mucoid colony, SeM allele-2 |
| Se1-8 | Tn916 insertional library of CF32 | SeM–, intact SeM sequence, mucoid colony |
| Se19 | Nasal swab, Ireland, 1985 | SeM–, intact SeM sequence, mucoid colony, SeM allele-1 |
Fig. 1.
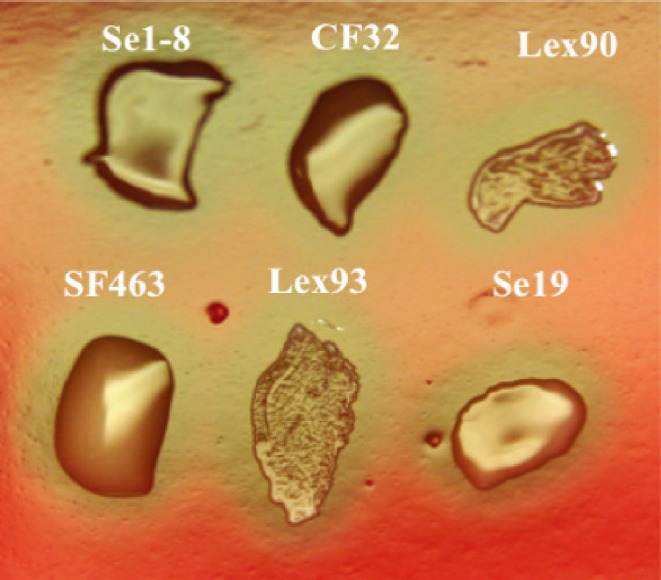
Mucoid and non-mucoid colonies of Streptococcus equi.
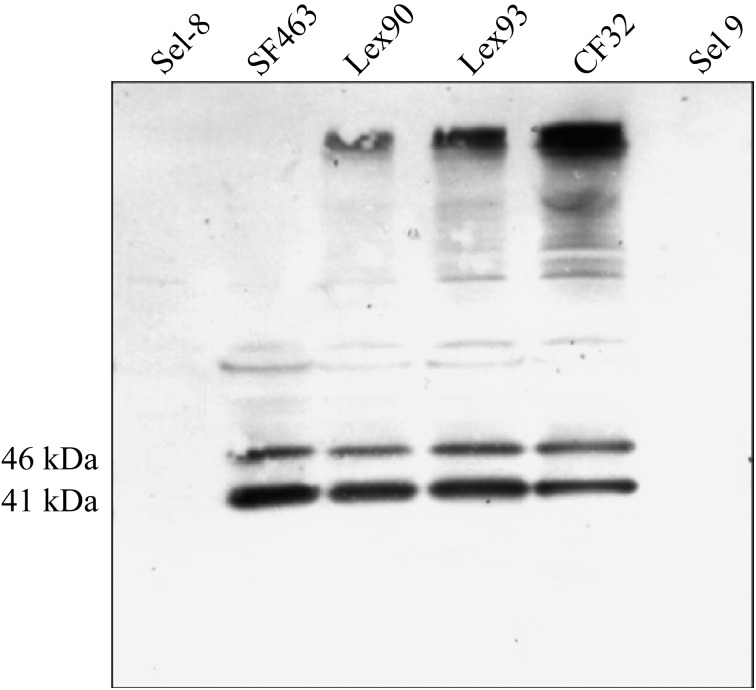
Proteins released by boiling acidified (pH – 2.5) bacterial pellets were separated on 12% SDS-PAGE gels and electrophoretically transferred to nitrocellulose membrane. The blots were sequentially incubated in SeM specific antiserum, peroxidase-conjugated protein G (1:4000) and developed in 4-chloro-1-naphthol.
The bactericidal assay was performed with heparinized whole blood and with neutrophils from Welsh ponies raised in isolation on the University of Kentucky Farm with no exposure to Se or its antigens. The assay consisted of 1 ml aliquots of blood mixed with 200 µl of each Se strain cultured overnight at 37°C in Todd-Hewitt broth with 0.2% yeast extract and diluted in phosphate buffered saline (PBS) to give a final concentration of 5.0 × 103 CFU/ml. After mixing, 600 µl were placed on ice (t0) and the remainder rotated for 60 min at 37°C in polypropylene tubes and then placed on ice (t60). Pour plates in triplicate were made by mixing 200 µl each t0 and t60 blood-bacteria suspension with 15 ml CNA agar and 1 ml heparinized equine blood. Plates were incubated overnight at 37°C and hemolytic colonies counted. % survival was calculated for each strain using CFU at t0 and t60. The assay was repeated 9 times for each strain and the counts pooled for each pair of strains of similar phenotype.
The assay was also done using equine neutrophils isolated from heparinized blood of Welsh ponies and suspended in Hank’s balanced salt solution (HBSS) as described by Sedgwick et al [8]. 200 µl of neutrophil suspension (1.0 × 106 cells/ml) were mixed with 1 ml fresh normal pony serum and with 200 µl of overnight culture of each Se strain diluted in PBS to contain 5.0 × 103 CFU/ml. The neutrophil-bacteria suspensions were then divided into 2 aliquots of 600 µl. One aliquot (t0) was placed in ice; the second was rotated in a polypropylene tube for 60 min and then placed on ice (t60). Pour plates in triplicates were prepared as described above for the bactericidal assay in blood, incubated overnight at 37°C and hemolytic colonies counted at t0 and t60. The assay was repeated 9 times for each strain and the counts pooled for each pair of phenotypically similar strains.
The Wilcoxon signed rank test was used to test for significance of difference in % survival between each pair of strains.
SeM-specific antiserum reacted strongly with 41 and 46 kDa SeM fragments of all strains except Se19 and Se1-8 (Fig. 2). Survival of CF32/SF463 in blood or in neutrophils suspended in serum was very significantly (P<0.001) greater than that of Lex90/Lex93 or Se19/1-8 (Table 2). The non-encapsulated SeM+ Lex90 and 93 showed moderately greater survival in blood (P=0.00096) and neutrophils (P=0.0002) than the SeM– encapsulated Se19 and Se1-8. Survival of Se19 and Se1-8 in blood was greater than in neutrophils (P=0.019).
Fig. 2.
Reactivities of hot acid extracts of encapsulated (Se1-8, SF463, CF32, Se19) and non-encapsulated (Lex90, Lex93) strains of Streptococcus equi with SeM specific rabbit antiserum. The immunoblot was prepared following SDS-PAGE and transfer of separated proteins to nitrocellulose.
Table 2. Effect of capsule and SeM protein on survival of Streptococcus equi in equine blood and in neutrophils suspended in equine serum.
| Strain | Phenotype | Median % Survival (Range) |
|
|---|---|---|---|
| Blood (n=18) | Neutrophil Suspension (n=18) |
||
| CF32 | HA+, SeM+ | 14.0 (7.0–20.0) | 14.0 (6.0–35.0) |
| SF463 | |||
| Lex90 | HA–, SeM+ | 6.0 (3.0–9.0) | 4.0 (1.0–8.0) |
| Lex93 | P=0.00096a | P=0.0002a | |
| Se 1-8 | HA+, SeM– | 4.0 (1.0–8.0) | 2.0 (1.0–7.0) |
| Se19 | P=0.09b | P=0.019b | |
Percentages were calculated from triplicate data from 9 separate experiments for each Se strain. a, P values calculated using Wilcoxon’s signed rank test of significance of difference in survival between HA+ SeM+ and HA– SeM+ strains. P values for the comparison of HA+ SeM+ and HA+ SeM– were also 0.00096 (blood) and 0.0002 (neutrophils). b, P values calculated as above for differences in % survival between HA– SeM+ and HA+ SeM– strains. Survival of Se1-8 and Se19 was significantly greater (P=0.019) in blood than in neutrophils.
Absence of detectable SeM from Se19 and Se1-8 was confirmed by immunoblot analysis with SeM-specific antiserum (Fig. 2). Conversely, CF32, SF463, Lex90 and Lex93 were positive for the 41 and 46 kDa fragments of SeM. Sequence analysis of sem in both Se19 and Se1-8 revealed an intact gene in each strain. Lack of expression involved down-regulation at either the transcriptional or translational levels. Resolution of these possibilities is the subject of current investigation.
Absence of capsule rendered both SeM-positive strains susceptible to phagocytosis/killing, an indication that the anti-phagocytic activity of SeM requires capsule. Therefore, the conclusion of previous investigators that capsule is an important virulence factor, is fully valid only when capsule and SeM are present together [1, 3, 10]. Loss of SeM is associated with much reduced pathogenicity for the mouse and horse consistent with its crucial role in virulence of Se [7, 14].
Efficient phagocytosis/killing of the non-encapsulated Lex90 and 93 expressing normal amounts of SeM is consistent with previous studies [1]. SeM is the major fibrinogen binding protein of Se. Bound fibrinogen greatly enhances resistance of Se to killing by equine neutrophils possibly by binding complement control factor H [2]. However, the ability of SeM to reduce deposition of the opsonic forms of C3 on the surface of encapsulated organisms is not affected by fibrinogen binding [2]. It is tempting to speculate that optimal binding of fibrinogen as well as inhibition of C3 deposition requires the 3-dimensional conformation of SeM available only in the presence of capsule. In the absence of the hydrophilic capsule, Lex90 and 93 aggregate and sediment in THB, an effect of hydrophobic surface proteins including SeM. The greater survival of Se19 and Se1-8 in blood compared to neutrophils in serum may be explained either by reduction of phagocytosis due to binding of fibrinogen to SzPSe [13] or by loss of viability of neutrophils during isolation from blood.
It must be stressed that strains in this study with the exception of Se1-8 were naturally occurring clinical isolates. It is therefore possible that undetected differences other than SeM and capsule in each strain might have affected susceptibility to phagocytosis and killing. However, this is very unlikely given that the Se population is almost clonal and isolation of mutants with these altered phenotypes is extremely rare. Supporting this argument was the similarity of mean survival rates of strains within each pair i.e. CF32 and SF463, Lex90 and Lex93 and Se1-8 and Se19. It is likely these mutants were generated de novo in the horses from which they were isolated since their lack of virulence would not favor successful infection of a new host and onward transmission. Finally, Se1-8 was derived by Tn916 mutagenesis by which a single copy of the transposon was inserted in CF32 with no discernible effect on its proteome other than loss of SeM expression (Artiushin S. and Timoney J.F, Unpublished data).
Acknowledgments
The study was supported by income from the Endowment of the Keeneland Association Endowed Chair in Infectious Diseases.
References
- 1.Anzai T., Timoney J.F., Kuwamoto Y., Fujita Y., Wada R., Inoue T.1999. In vivo pathogenicity and resistance to phagocytosis of Streptococcus equi strains with different levels of capsule expression. Vet. Microbiol. 67: 277–286 [DOI] [PubMed] [Google Scholar]
- 2.Boschwitz J.S., Timoney J.F.1994. Inhibition of C3 deposition on Streptococcus equi subsp. equi by M protein: a mechanism for survival in equine blood. Infect. Immun. 62: 3515–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanter N., Collin N.C., Mumford J.A.1994. Resistance of Streptococcus equi in vitro to equine polymorphonuclear leucocytes. pp. 201–205. In: Proceedings of seventh International Conference on Equine Infectious Diseases. (Plowright, W., and Nakajima, H. eds.), R and W publications, Newmarket.
- 4.Galán J.E., Timoney J.F.1988. Immunologic and genetic comparison of Streptococcus equi isolates from the United States and Europe. J. Clin. Microbiol. 26: 1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jean-François M.J.B., Poskitt D.C., Turnbull S.J., Macdonald L.M., Yasmeen D.1991. Protection against Streptococcus equi infection by monoclonal antibodies against an M-like protein. J. Gen. Microbiol. 137: 2125–2133 [DOI] [PubMed] [Google Scholar]
- 6.Li W., Fiala S., Gibson N., Timoney J.F., Cairns R., Acree W.M., Chu H.J.1999. Efficacy of a modified live Streptococcus equi vaccine in an equine experimental model. pp. 357–358. In: Proceedings of eight International Conference on Equine Infectious Diseases. (Wernery, U., Wade, J.F., Mumford, J.A., and Kaaden, O.R. eds.), R and W publications, Newmarket.
- 7.Meehan M., Lynagh Y., Woods C., Owen P.2001. The fibrinogen-binding protein (FgBP) of Streptococcus equi subsp. equi additionally binds IgG and contributes to virulence in a mouse model. Microbiology 147: 3311–3322 [DOI] [PubMed] [Google Scholar]
- 8.Sedgwick A.D., Morris T., Russell B.A., Lees P.1986. Single step purification procedure for the rapid separation of equine leucocytes. Vet. Res. Commun. 10: 445–452 [DOI] [PubMed] [Google Scholar]
- 9.Timoney J.F.1993. Strangles. pp. 365–374. In: The Veterinary Clinics of North America: Equine practice. (Traub-Dargatz, J.L. ed.), W.B. Saunders, Philadelphia. [Google Scholar]
- 10.Timoney J.F., Galan J.E.1985. The protective response of the horse to an avirulent strain of Streptococcus equi. pp. 294–295. In: Recent Advances in Streptococci and Streptococcal Diseases: Proceedings of the ninth Lancefield International symposium on Streptococci and Streptococcal Diseases. (Kimura, Y., Kotami, S., and Shiokawa, Y. eds.), Reedbooks Ltd., Berkshire, England.
- 11.Timoney J.F., Guan M.1996. Characterisation of murine monoclonal antibodies recognising opsonic, mouse-protective, chaining and mucosally relevant epitopes on the M protein of Streptococcus equi subspecies equi. Res. Vet. Sci. 60: 76–81 [DOI] [PubMed] [Google Scholar]
- 12.Timoney J.F., Trachman J.1985. Immunologically reactive proteins of Streptococcus equi. Infect. Immun. 48: 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timoney J.F., Artiushin S.C., Boschwitz J.S.1997. Comparison of the sequences and functions of Streptococcus equi M-like proteins SeM and SzPSe. Infect. Immun. 65: 3600–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timoney J.F., Wang J., Artiushin S., Sheoran A., Nally J., Kumar P.2000. Virulence and immunogenicity of a clone of Streptococcus equi in which expression of SeM is blocked by Tn916 mutagenesis. pp. 699–702. In: Streptococci and Streptococcal Diseases Entering the New Millinium. (Martin, D.R., and Tagg, J.R. eds.) XIV Lancefield International symposium on Streptococci and Streptococcal Diseases. C/-ESR, PD. Box 50-348, Porirua.
- 15.Timoney J.F., Yang J., Liu J., Merant C.2008. IdeE reduces the bactericidal activity of equine neutrophils for Streptococcus equi. Vet. Immunol. Immunopathol. 122: 76–82 [DOI] [PubMed] [Google Scholar]
- 16.Tiwari R., Qin A., Artiushin S., Timoney J.F.2007. Se18.9, an anti-phagocytic factor H binding protein of Streptococcus equi. Vet. Microbiol. 121: 105–115 [DOI] [PubMed] [Google Scholar]
- 17.Whitnack E., Bisno A.L., Beachey E.H.1981. Hyaluronate capsule prevents attachment of group A streptococci to mouse peritoneal macrophages. Infect. Immun. 31: 985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]