Abstract
The inflammasome is a protein complex that is comprised of an intracellular sensor that is typically an NLR protein, the pro-protein, procaspase-1 and adaptor molecule ASC. Inflammasome activation leads to caspase-1 maturation and the processing of its substrate, IL-1β and IL-18. Although initially the inflammasome was described as a complex that affects infection and inflammation, recent evidence suggests that inflammasome activation influences a host of metabolic disorders including atherosclerosis, type 2 diabetes, gout and obesity. Another aspect regarding inflammation in general and inflammasome in specific is that the activation process has a profound effect on aerobic glycolysis, or the Warburg effect. How the Warburg effect might be link to inflammation and inflammasome activation is a novel concept to contemplate.
The incidence of metabolic disorders such as obesity, type 2 diabetes (T2D) and atherosclerosis has increased dramatically during recent decades, and these diseases constitute some of the most serious threats to public health. Chronic inflammation is a key common feature of metabolic disorders. Many inflammatory mediators such as tumor necrosis factor (TNF), interleukins and cytokine-like proteins known as adipokines have been linked to the development of multiple forms of metabolic disorders 1, 2. Interleukin (IL)-1β is a prominent pro-inflammatory cytokine since it can efficiently cause the generation of other inflammatory mediators through IL-1 receptor signaling, thus initiating a self-amplifying cytokine network 3. As a result, IL-1β is postulated to play an important role in the pathogenesis of metabolic disorders. Indeed, studies employing the recombinant IL-1 receptor antagonist (IL-1RA) anakinra have been tested in T2D, with some encouraging results 4. Recent clinic trials also suggest that non-inflammasome cytokines TNF contributes to impaired glucose homeostasis and insulin resistance in patients with T2D 5–7. Therefore, it is highly possible that both IL-1β and TNF may drive chronic inflammation in a cooperative manner, which highlights the importance of combined immunotherapy against multiple inflammatory cytokines. This review will focus on the possible role of the inflamamsome complex which is important for the maturation of IL-1β in a variety of metabolic disorders. We will also examine how metabolic alterations in cells including the so-called “Warburg effect” of aerobic glycolysis contribute to inflammatory processes relevant for these diseases. While most of the data are based on animal models, the potential translational relevance will be underscored.
The inflammasome in diabetes and obesity
Inflammasome is a large multimeric danger-sensing platform, which promotes auto-catalytic activation of the cysteine protease, caspase-1, and mediates the cleavage of inactive pro-IL-1β and IL-18 among other proteins into their active forms 8, 9. Several recent studies have provided strong evidence to suggest a critical role of the NLRP3 inflammasome in the development of insulin resistance using gene-deletion mice 10–14. Genetic ablation of NLRP3 (Nlrp3−/−) or the NLRP3 inflammasome-associated molecules such as ASC (also known as PYCARD; Pycard−/−) and caspase-1 (Casp1−/−) resulted in improved glucose tolerance and insulin sensitivity after high-fat diet (HFD) feeding, therefore linking the NLRP3 inflammasome to insulin resistance in a number of studies. Several studies have explored how a HFD in mice might contribute to T2D and the role played by inflammasome proteins. Ceramide, the specific product of long-chain saturated fatty acid metabolism, can cause caspase-1 activation and IL-1β release in macrophages from wild-type (WT) mice but not in macrophages from Nlrp3−/− mice 12. The saturated free fatty acid (FFA) palmitate, but not unsaturated FFA, induces the activation of caspase-1 and the cleavage of IL-1β and IL-18 in an NLRP3- and ASC-dependent manner 13. Furthermore, palmitate signals through an AMP-activated protein kinase (AMPK)-autophagy-mitochondrial reactive oxygen species (mROS) pathway to activate the NLRP3 inflammasome. An NLRP3 inflammasome-dependent process affects insulin target tissues such as liver, muscle and adipose tissues (Fig 1). Therefore, these studies explore the mechanisms of NLRP3 inflammasome activation by danger signal molecules associated with saturated FFA metabolism, and highlight the importance of NLRP3 inflammasome activation in the development of insulin resistance. Interestingly, a widely used insulin secretagogue (Glyburide) provides a linkage between insulin homeostasis and the NLRP3 inflammasome activation. For example, Glyburide can inhibit NLRP3 inflammasome-mediated caspase-1 activation and both IL-1β and IL-18 release 15.
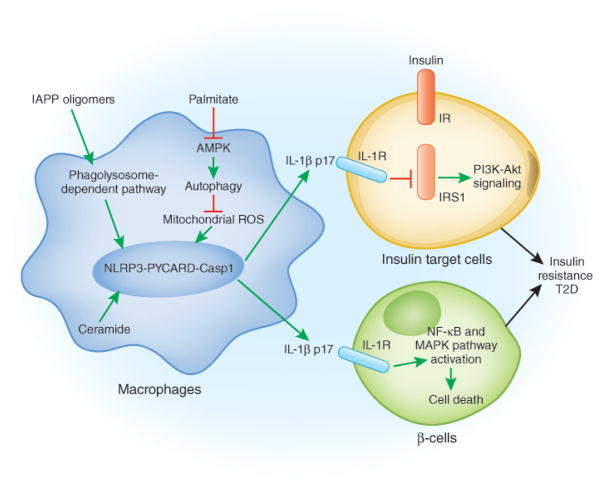
Fig 1.
A model for the pathogenesis of T2D: adipose tissue, the pancreas, NLRP3 and T2D. In adipose tissue, elevated levels of saturated free fatty acids decrease the activity of AMPK, a central regulator of energy biosynthesis and lipid metabolism, leading to defective autophagy of mitochondria (Mitophagy). The accumulation of dysfunctional mitochondria then enhances mitochondrial ROS generation and the release of mitochondrial DNA into cytosol, both of which promotes NLRP3 inflammasome activation and IL-1β release. The active IL-1β induces the activation of JNK and IKK through IL-1 receptor, which impair the insulin-insulin receptor (IR), insulin receptor substrate-1 (IRS-1), and PI3K-Akt signaling pathway. In the pancreas, the accumulation of IAPP activates the NLRP3 inflammasome and promotes IL-1β release from macrophages, which causes β-cell dysfunction and death.
In addition to the essential role of the NLRP3 inflammasome and IL-1β in the impairment of insulin signaling in insulin target liver, muscle and adipose tissues, IL-1β also promotes β-cell dysfunction and cell death directly 16. Islet amyloid polypeptide (IAPP; also known as amylin) has been identified as a key inducer of NLRP3 inflammasome activation and IL-1β cleavage 17. IAPP, a hormone that is secreted together with insulin, is deposited in the islet interstitium of patients with T2D and is considered to be a significant danger risk for T2D 18. IAPP forms amyloid structures, and amyloid particles have previously been shown to activate the NLRP3 inflammasome 8. Therefore, it has been proposed that IAPP oligomers activate the NLRP3 inflammasome and IL-1β cleavage in a manner similar to other crystalline activators of the NLRP3 inflammasome 17. Other studies suggest that a high level of glucose induces β-cell production and release of IL-1β in vitro, which then promotes functional impairment and apoptosis of β-cell in an autocrine manner 10, 19. However, this effect seems to be islets specific, since high level of glucose fails to promote IL-1β release induced by IAPP in bone marrow-derived dendritic cells. Instead, sufficient glucose is required for NF-κB-dependent, but NLRP3 inflammasome-independent, pro-IL-1β and IL-6 production 17. The molecular mechanism underlying glucose-induced IL-1β release by islets remains to be determined, and will be discussed later.
Another potential regulatory mechanism of inflammasome activation during obesity and insulin resistance is autophagy. Autophagy is a cell intrinsic mechanism for the degradation and recycling of cellular components 20. Genetic variants of the autophagy gene ATG16L1 have been linked to Crohn’s disease 21–24. Deletion of either the Atg16l1 or Atg7 autophagic gene in mouse macrophages leads to increased caspase-1 activation and IL-1β release, but not TNF and IL-6 generation. This was observed in response to treatment with either lipopolysaccharide (LPS) alone or LPS in conjunction with NLRP3 inflammasome activators such as ATP and monosodium urate (MSU) 25. These findings have been recently confirmed and more detailed molecular mechanisms involving disrupted mitochondrial homeostasis have been explored 13, 26, 27. Deletion of autophagy gene 18, 19 or palmitate treatment 13 inhibits autophagy, which subsequently leads to the accumulation of dysfunctional mitochondria and increased mitochondrial ROS (mROS) generation. These events activate the NLRP3 inflammasome. Nakahira et al. suggests that the leakage of mitochondrial DNA into cytosol upon autophagy inhibition may serve as a coactivator for inflammasome activation 26. These studies provide evidence that autophagy negatively regulates NLRP3 inflammasome activation by maintaining mitochondrial homeostasis. Accordingly, defective autophagy has been observed in the liver of both genetic (ob/ob) and diet-induced (HFD feeding) obesity animal models 28. Although the mechanism by which an obese condition leads to defective autophagy has not been characterized, AMPK and/or mTOR signaling pathways may be potential candidate of upstream modulators, since these two evolutionally conserved signaling pathways have been shown to regulate autophagy by direct phosphorylation of ATG1 29, 30. Therefore, a strong possibility is that defective autophagy associated with obesity and insulin resistance may promote inflammasome activation and amplify inflammatory network in insulin target tissues.
In addition to a direct effect of the NLRP3 inflammasome on innate immunity, recent studies show that IL-1β and IL-18 play an essential role in shaping adaptive immune responses in several animal models such as experimental autoimmune encephalomyelitis (EAE), arthritis and cytotoxic T cell (CTL)-mediated anti-tumor responses 31. Specifically, IL-1 promotes the differentiation of T helper 17 (TH17) lineage by increasing the expression of IRF4 and RORγt, two essential transcription factors involved in TH17 differentiation 32, whereas IL-18, in synergy with IL-12, induces IFN-γ-producing TH1 cells 33. More importantly, the differentiation of human TH17 cells requires the presence of IL-1β 34, 35. Based on recent studies suggesting that the aberrant accumulation and activation of lymphocytes in adipose tissues (including both T and B cells) impair insulin sensitivity in obesity-induced insulin resistance 36–40, it is reasonable to argue that NLRP3 inflammasome activation might lead to lymphocyte accumulation and activation in obesity and insulin resistance. Indeed, significantly decreased numbers of both CD4+ and CD8+ effector memory T cells have been observed in the adipose tissue of Nlrp3−/− mice after HFD feeding 12, suggesting that the NLRP3 inflammasome-regulated adaptive immune response may also contribute to insulin resistance.
It has been long-postulated that obesity is a strong risk factor for insulin resistance and T2D, and this is partially mediated through enhancing chronic inflammation in insulin target tissues 1. Recently it has been proposed that the inflammasome affects adipocyte differentiation and HFD-induced obesity 11, 14. Stienstra et al. demonstrated that NLRP3-dependent caspase-1 and IL-1β activation inhibits adipocyte differentiation and insulin signaling 11. This same group also found that the genetic ablation of NLRP3 inflammasome or pharmacological inhibition of caspase-1 provides a beneficial effect in HFD-induced obesity, presumably through increasing energy expenditure 14. However, the protective role played by NLRP3 inflammasome in HFD-induced obesity was not uniformly observed because two other studies have not confirmed these findings 12, 13. These studies did not observe a difference in total body weight between Nlrp3−/− mice and WT mice after HFD feeding 12, 13, although NLRP3 seems to play a role in adipocyte morphology 12. The reason for the different observations is currently unknown, but may potentially involve subtle differences such as HFD from different sources. Another important potential link to obesity is the observation that there is enhanced β-oxidation of fatty acids in Casp1−/− mice. This is likely to be due to decreased IL-1β since IL-1β limits β-oxidation and will therefore decrease adiposity. Accordingly, a caspase-1 inhibitor led to higher fat oxidation rates in obese mice 11. NLRP3 inflammasome activation might therefore promote obesity.
The inflammasome in atherosclerosis
Atherosclerosis has components of a chronic inflammatory disease characterized by the accumulation of lipid components and recruitment of immune cells in atherosclerotic lesions 41, 42. When low-density lipoprotein (LDL), a cholesterol-containing lipoprotein, is retained in the artery wall, it leads to vascular inflammation and cholesterol accumulation, partially as cholesterol crystals 41, 42. Among the inflammatory mediators, IL-1β has been shown to play a significant role in promoting the development of lipid plaques and also destabilizing the plaques in mice. In Apoe−/− mice which spontaneously develop atherosclerosis due to hypercholesterolemia, IL-1β deficiency results in an attenuated development of atherosclerotic lesions 43. The application of IL-1RA has been shown to prevent lesion development 44. Therefore, experimental animal studies support the concept that IL-1β collaborates together with other proinflammatory cytokines such as TNF to exacerbate the pathology of metabolic disorders. The role of inflammatory cytokine IL-6 in obesity and insulin resistance remains controversial. Although it has been proposed that IL-6 plays a detrimental role in impairing glucose homeostasis and insulin sensitivity 45, a recent study points to the beneficial effect of IL-6 by promoting insulin secretion and the maintenance of glucose homeostasis in diet-induced or genetic animal models 46.
Two recent studies showed that cholesterol crystals activate the NLRP3 inflammasome and IL-1β release in mouse and human macrophages, thus highlighting a posible role of NLRP3 inflammasome in atherosclerosis 47, 48. The accumulation of a small amount of cholesterol crystals was observed in early diet-induced atherosclerotic lesions, which was associated with the recruitment of inflammatory macrophages 47. Cholesterol crystals generated in vitro activate caspase-1 and both IL-1β and IL-18 cleavage in LPS-primed human peripheral blood mononuclear cells and mouse macrophages, which is dependent on NLRP3 and ASC 47, 48. Moreover, cholesterol crystal-induced NLRP3 inflammasome activation was sensitive to cytochalasin D and bafilomycin treatment, suggesting the requirement of phagocytosis and lysosome acidification for inflammasome activation, which is consistent with NLRP3 inflammasome activation induced by other crystals such as MSU, silica, asbestos and alum 49–52. In line with the in vitro data, hypercholesterolemic LDL receptor deficient (Ldlr−/−) mice reconstituted with bone marrow from Nlrp3−/−, Pycard−/− or Il1b−/− mice developed significantly less atherosclerotic plaques than those reconstituted with WT bone marrow 30. These findings suggest critical roles of hematopoietic cell-derived NLRP3, ASC and IL-1β in the development of atherosclerotic lesions. However, the crucial role of the NLRP3 inflammasome in atheroscleosis was challenged by another study using a double-mutant crossing Apoe−/− mice with Nlrp3−/−, Pycard−/− or Casp1−/− mice 53. This study failed to find any differences in atherosclerosis progression, infiltration of plaques by macrophages, or plaque stability in the presence or absence of the NLPR3 inflammasome. The obvious difference between these two studies is the investigation of hematopoietic-derived versus whole body-derived NLRP3 inflammasome in the development of atherosclerosis. It is quite clear that the NLRP3 inflammasome may also function in the stromal compartment to regulate disease progression in addition to the hematopoietic compartment, especially in the colitis and colitis-associated cancer 54, 55. Therefore, further investigations are required to clarify these differences.
The inflammasome in gout
Gout is historically known as a disease of Kings since it is believed to result from a rich diet and in particular those high in purines. It is associated with elevated levels of uric acid (hyperuricemia) in the blood which forms crystals and are then deposited in joints. Uric acid can be released from dying cells which activates cells as a danger signal 56. It is generally accepted that IL-1β plays an important role in the promotion of inflammatory responses in joints, supported by several recent clinical trials where the treatment of gout with anakinra or other drugs that inhibit IL-1β showed amelioration of symptoms 57–60.
The mechanisms by which urate crystals cause inflammatory arthritis and IL-1β is generated remain largely unknown until the NLRP3 inflammasome was identified as the link between urate crystals and gout 49. MSU and calcium pyrophosphate dihydrate (CPPD) induce the cleavage of caspase-1 and IL-1β or IL-18 in LPS-primed macrophages that is dependent on NLRP3 and ASC. In vivo, NLRP3 inflammasome-mediated IL-1β generation promotes neutrophil recruitment and peritoneal inflammation induced by MSU. A question that remains is the mechanism whereby MSU or other crystal structure activates the NLRP3 inflammasome. Unlike other germline-encoded pathogen recognition receptors, there is no evidence to suggest a direct recognition of danger signals by NLRP3. Therefore it is reasonable to argue that the NLRP3 inflammasome detects some forms of alterations in cellular homeostasis induced by danger signals. One such proposed signal has been increased ROS generation 8. One study suggests that the thioredoxin-interacting protein (TXNIP), a protein upregulated by glucose and linked to insulin resistance, interacts with NLRP3, leading to IL-1β release 10. Inflammasome activators induce the transfer of TXNIP from thioredoxin to NLRP3 in a process involving ROS. However, this finding has been challenged by a recent study, particularly in macrophages 17. A very recent study identified caspase-1 interacting proteins including cellular inhibitors of apoptosis protein 1 (cIAP1), cIAP2 and an adaptor protein TRAF2, which are required for spontaneous and agonist-induced caspase-1 cleavage 61. The cIAP E3 ligase activity mediates lysine 63-linked polyubiquitination of caspase-1, although its functional relevance to caspase-1 maturation remains to be explored. Other potential mechanisms of the inflammasome activation involve the participation of additional effector molecules such as other NLR molecules or pathogen recognition receptors (PRR) such as Toll-like receptor (TLR) and RIG-I-like receptor (RLR). For example, NAIP (NLR family, apoptosis inhibitory protein) proteins mediate the NLRC4 inflammasome activation through a direct ligand-binding mechanism 62. Prokaryotic messenger RNA promotes the NLRP3 inflammasome activation through a TRIF (TIR domain-containing adaptor protein inducing IFN-β)-dependent manner, which is an adaptor protein downstream of TLR3 and TLR4 63. RNA virus-induced activation of RIG-I signaling leads to inflammasome activation 64. However, earlier studies have noted the transcriptional induction of NLRP3 by microbes 65, 66, while a recent study suggests that ROS induces the expression of NLRP3 67. Hence the transcriptional effects on components of the inflammasome in addition to post-translational effects have to be taken into consideration.
Although it has been well documented that crystal structure such as MSU, silica, asbestos and alum induce NLRP3 inflammasome-dependent caspase-1 and IL-1β or IL-18 cleavage, particularly in vitro 49–52, recent studies have observed NLRP3 inflammasome-independent effects of uric acid, silica and alum in the development of TH2 immune response and TH2-associated IgE antibody response 68, 69. One study shows that uric acid is released in the airways of asthmatic patients and allergen-challenged mice and promotes TH2 immune response by activating dendritic cells via spleen tyrosine kinase (Syk) and PI3-kinase δ signaling pathways 54. Another study reports that silica and alum promote prostaglandin E2 (PGE2) production in macrophages through the Syk-p38 pathway, which enhances TH2 response-associated IgE production 68, 69. Both of these studies found that NLRP3 had no effect. Therefore, crystals including alum adjuvant are found to induce both NLRP3 inflammasome-dependent and -independent immune effects. Further investigation is warranted to clarify the role of the inflamamsome in these responses in physiologic settings.
Metabolic changes in inflammatory signaling
Another interesting aspect regarding IL-1β concerns the role of glucose metabolism in IL-1β gene transcription. As mentioned above, in pancreatic β cells, glucose was shown to boost production of IL-1β, although no mechanism was provided 19. This is less evident in macrophages, where although glucose is required, high levels (mM) do not boost the response. However, treating macrophages with 2-deoxyglucose (2-DG) was found to inhibit transcription of IL-1β induced by LPS, but had no effect on TNF gene transcription 17. 2-DG inhibits glycolysis by acting as a competitive substrate for hexokinase, thereby limiting glucose metabolism. Why would this affect IL-1β gene transcription?
LPS has been shown to have a profound effect on the metabolic profile of target cells. In dendritic cells LPS promotes aerobic glycolysis – a process termed the Warburg effect 70, 71. Warburg had originally defined this process in tumor cells, whereby respiration and the Kreb’s cycle (also known as the tricarboxylic acid (TCA) cycle) in mitochondria is limited, and glycolysis is enhanced. Several reasons for this metabolic shift have been proposed, notably increased ATP production to meet the energy demands of the tumor cells. Glycolysis, although less able to generate ATP, can be greatly enhanced via induction of the enzymes involved 72. A second reason is for biosynthesis since intermediates for biosynthesis of amino acids, lipids and nucleotides are made from glycolysis 73. This could in fact be a mechanism for increased production of uric acid in inflammation, in the case of the pentose phosphate pathway. The mechanism of the Warburg effect in tumors has recently been shown to involve induction of the embryonic pyruvate kinase M2 (PKM2) isoform 74, which strongly promotes hypoxia-inducible factor (HIF)-1α induction 75. PKM2 occurs in a complex with HIF-1α and prolyl hydroxylase 3 (PHD3) on the HIF-1α promoter. HIF-1α induces glycolytic enzymes, promoting the glycolytic flux 76. This same process could be happening in response to LPS, since LPS stabilizes HIF-1α via an as yet ill-defined mechanism, and HIF-1a-deficient (Hif1a−/−) macrophages are defective in their responses to LPS 77. LPS has also been shown to induce the ubiquitous isoform of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (uPFK2), which strongly promotes glycolysis 78. Attenuation of uPFK2 thus limits macrophage responses to LPS 78. Another mechanism might involve decreased expression of PHD2 and PHD3 which would stabilize HIF-1α 79. 2-DG will block the flux through glycolysis and has been shown to inhibit various LPS responses, including induction of IL-1β transcription and other gene products such as CD40, CD80 and CD86 70.
The signal coming from glycolysis for these responses is not known but could also involve a signal from mitochondria, since LPS also affects mitochondrial metabolism. LPS can limit mitochondrial metabolism and oxidative phosphorylation although again the mechanism is unknown 80. There is a decrease in the expression of genes encoding multiple proteins involved in mitochondrial function 27. This could lead to a build-up of intermediates such as succinate, which is a known inhibitor of PHD2, the enzyme that hydroxylates HIF-1α leading to its degradation 81. Succinate would therefore increase HIF-1α levels. Also, a build-up of ROS in response to LPS can play a role in the induction of HIF-1α 82. 2-DG has been shown to lower ROS and succinate production providing a possible explanation for how it blocks induction of IL-1β by LPS 83. It has also been shown that the ATP produced from glycolysis is required for maintenance of the mitochondrial membrane potential in the face of mitochondrial shut-down 84.
In essence therefore, LPS signaling via TLR4 involves an alteration in intracellular metabolism which is determining for LPS responses. The shift to aerobic glycolysis is required for induction of IL-1β mRNA. The inflammasome is activated by hyperlipidemia leading to increased IL-1β production. The IL-1β then causes insulin resistance and decreased fatty acid oxidation in the mitochondria. These events promote obesity, producing a vicious cycle which is further enhanced by the increased IAPP production in the pancreas, IAPP being synthesized in concert with insulin, and having the biochemical trait of forming amyloid. IAPP promotes further IL-1β production, which ultimately is toxic to β cells in the pancreas. It should be noted however that a causal link between IAPP and the pathogenesis of T2D, although intriguing as an hypothesis, has yet to be proven. A similar scenario could be occurring in atherosclerosis, where cholesterol crystals exacerbate the inflammatory process via NLRP3, with this process promoting plaque formation. TLR4 could be a key driver in these events since it responds to fatty acids and also minimally oxidized LDL, providing for pro-IL-1β and priming the inflammasome. The shift to the Warburg effect could therefore be determining for inflammation (events summarized in Fig 2).
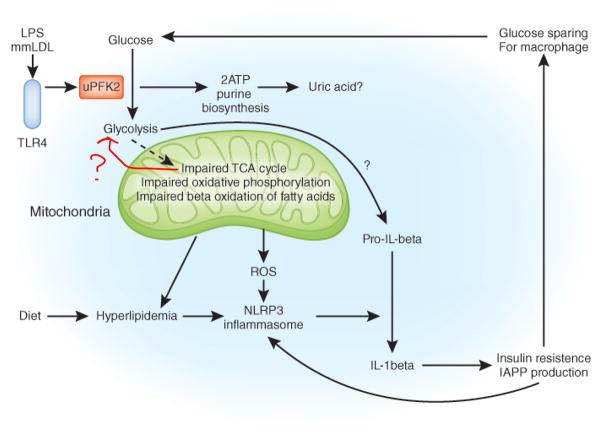
Fig 2.
Metabolic fluxes, NLRP3 and IL-1β. Hyperlipidemia in the form of saturated fatty acids such as palmitate, has been shown to activate the NLRP3 inflammasome leading to caspase-1 activation and the processing of pro- IL-1β. Impaired mitochondrial metabolism, including decreased β-oxidation of fatty acids, could promote this process, as will production of reactive oxygen species. Activation of TLR4 by LPS or minimally modified oxidized LDL promotes glycolysis via induction of enzymes such as uPFK2 leading to enhanced ATP production and nucleotide biosynthesis via the pentose phosphate pathway. Purines including uric acid, could be over-produced via this process leading to NLRP3 inflammasome activation. IL-1β will give rise to insulin resistance, causing enhanced IAPP production, which in turn will further activate NLRP3. Insulin resistance in liver and muscle could spare glucose for macrophages. See text for details.
Another example of the Warburg effect being important for inflammation was highlighted by a report on TH17 cells. HIF-1α enhances TH17 development via activation of RORγt and p300 recruitment to the IL-17 promoter 85. HIF-1α also attenuates regulatory T (Treg) cell development by binding to their lineage-specifying transcription factor Foxp3 and targeting it for proteosomal degradation. Hif1a−/− mice could not generate TH17 cells and were resistant to EAE 85. Similar results were obtained in another study and even more strikingly, it was shown that HIF-1α dependent glycolysis was acting as a metabolic checkpoint for the differentiation of TH17 cells 86. Importantly, 2-DG could convert a TH17 cell into a Treg cell 86. Since TH17 are the more pro-inflammatory cell type, this further emphasizes how the Warburg effect is determining for inflammation, being important for both inflammatory cytokine production from macrophages and dendritic cells and the generation of TH17 cells.
Why would IL-1β cause insulin resistance? There are two possible options. Insulin resistance mainly occurs in liver and smooth muscle cells, and is though to involve induction of SOCS2 and SOCS3 87, as well as activation of Jun N-terminal kinase and IκB kinase 88, to limit insulin signaling. This could spare glucose for macrophages, which require the glucose for energy demands and biosynthesis, both of these involve the switch to aerobic glycolysis, probably via HIF-1α (Fig 2). A second possibility is to limit glucose uptake in a negative feedback loop, since this could impair further IL-1β transcription and would also limit production of ROS from mitochondria. In T2D however, the on-going induction of IL-1β in response to the various stimuli including IAPP suggests any negative feedback effect is over-whelmed, and IL-1β then becomes pathogenic.
Conclusions
A wealth of evidence therefore points to an intimate relationship between IL-1β, the NLRP3 inflammasome and lipid and carbohydrate metabolism. This occurs at the level of enhanced NLRP3 inflammasome activation and IL-1β processing to the mature cytokine in response to saturated fatty acids, and also glucose metabolism via glycolysis being required for induction of mRNA encoding IL-1β. Glycolysis and HIF-1α have also been shown to be key for TH17 cell differentiation. The pathogenic role played by IL-1β in plaque formation in atherosclerosis, and in insulin resistance and β cell loss in T2D attests to the importance of these processes in these metabolic diseases. The exacerbation of NLRP3 inflammasome activation by cholesterol crystals in atherosclerosis, and IAPP in T2D provides positive feedback loops to promote disease pathogenesis. Uric acid crystals, which are another consequence of metabolic disorder, are also NLRP3 inflammasome activators which lead to IL-1β production in gout. The hope is that the recent insights into the molecular basis of these diseases will help in the design of new therapies.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Feve B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 3.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 4.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 5.Stanley TL, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Llorca J. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci. 2010;1193:153–159. doi: 10.1111/j.1749-6632.2009.05287.x. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. Jama. 2011;305:2525–2531. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 8.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Barker BR, Taxman DJ, Ting JP. Cross-regulation between the IL-1beta/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol. 2011;23:591–597. doi: 10.1016/j.coi.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 11.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stienstra R, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:158–166. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- 17.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 19.Maedler K, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genome-wide association study of 14, 000 cases of seven common diseases and 3, 000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampe J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 24.Cadwell K, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 26.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan DF, et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson D, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 34.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 35.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 36.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 38.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winer DA, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binder CJ, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 42.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 43.Kirii H, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 44.Elhage R, et al. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 45.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellingsgaard H, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajamaki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 50.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menu P, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terkeltaub R, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68:1613–1617. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.So A, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064–3076. doi: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 60.Schlesinger N, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011;70:1264–1271. doi: 10.1136/ard.2010.144063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labbe K, McIntire CR, Doiron K, Leblanc PM, Saleh M. Cellular Inhibitors of Apoptosis Proteins cIAP1 and cIAP2 Are Required for Efficient Caspase-1 Activation by the Inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 62.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sander LE, et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–389. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poeck H, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 65.Conti BJ, et al. CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J Biol Chem. 2005;280:18375–18385. doi: 10.1074/jbc.M413169200. [DOI] [PubMed] [Google Scholar]
- 66.Allen IC, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bauernfeind F, et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kool M, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 69.Kuroda E, et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011;34:514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 72.Tannahill GM, O’Neill LA. The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS Lett. 2011;585:1568–1572. doi: 10.1016/j.febslet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 75.Luo W, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 77.Cramer T, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez-Prados JC, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 79.Peyssonnaux C, et al. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 80.Calvano SE, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 81.Selak MA, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 82.Nishi K, et al. LPS induces hypoxia-inducible factor 1 activation in macrophage-differentiated cells in a reactive oxygen species-dependent manner. Antioxid Redox Signal. 2008;10:983–995. doi: 10.1089/ars.2007.1825. [DOI] [PubMed] [Google Scholar]
- 83.Pistollato F, et al. Hypoxia and succinate antagonize 2-deoxyglucose effects on glioblastoma. Biochem Pharmacol. 2010;80:1517–1527. doi: 10.1016/j.bcp.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 84.Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010;17:1540–1550. doi: 10.1038/cdd.2010.27. [DOI] [PubMed] [Google Scholar]
- 85.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Emanuelli B, Glondu M, Filloux C, Peraldi P, Van Obberghen E. The potential role of SOCS-3 in the interleukin-1beta-induced desensitization of insulin signaling in pancreatic beta-cells. Diabetes. 2004;53 (Suppl 3):S97–S103. doi: 10.2337/diabetes.53.suppl_3.s97. [DOI] [PubMed] [Google Scholar]
- 88.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]