Graphical abstract
Keywords: Carboxyfluorescein diacetate, Rhodamine B, N-Hydroxysuccinimide ester, 5(6)-Carboxyfluorescein
Abstract
Diacetate protection of 5 and 6-carboxyfluorescein followed by synthesis of the N-hydroxysuccinimide esters allowed ready separation of the two isomers on a multi-gram scale. The 5 and 6-carboxyrhodamine B N-hydroxysuccinimide esters were also readily synthesised and separated.
Fluorescein and its derivatives represent one of the most popular families of fluorescent labelling agents for various biomolecules,1 including labelling of actin,2 myosin,3,4 haemoglobin,5 histones,6 DNA,7 RNA,8 and antibodies.1,9 Peptides are also routinely tagged with carboxyfluorescein, as demonstrated by Nguyen who reported a carboxyfluorescein conjugated peptide that labels nerves in human tissues, with potential to aid surgery and prevent accidental transection.10 The monitoring of enzymatic activities using fluorescein-based probes is wide spread. For example, Tanaka designed a quenched fluorescein phosphate-polymer that in the presence of alkaline phosphatase liberated fluorescein, while Bradley has developed a quenched multi-branched scaffold liberating fluorescein in the presence of human neutrophil elastase.11,12 Furthermore, fluorescein has been incorporated into numerous chemical sensors that have been used to detect reactive oxygen species,13 hydrogen peroxide,13 nitric oxide,13 or measure pH (e.g., pH sensing in living cells).14,15
Widely used derivatives of fluorescein are the N-hydroxysuccinimide esters of 5 and 6-carboxyfluorescein diacetate (often referred as CFSE), which have been extensively used to monitor cellular division,16,17 with over 226 reports in 2013 alone.18 Here the two acetate groups render the molecule membrane permeant, while once inside cells, the active ester labels intracellular proteins, while esterases remove the acetate groups restoring the fluorescein’s fluorescence.19
Fluorescein is commonly used as a mixture, namely 5(6)-carboxyfluorescein, and the synthesis of fluorescein-labelled probes results in a mixture of isomers. This complicates their purification and analysis of the resulting fluorescein-tagged probes since labelling will result in two probes with slightly differing properties. Kvach studied the properties of 5 and 6-carboxyfluorescein conjugated to an oligonucleotide and demonstrated that, although they had similar absorbance and fluorescence quantum yields, the emission band from the 6-carboxyfluorescein–oligonucleotide was substantially sharper than that of the 5-carboxyfluorescein analogue, making it the optimal isomer for multiplex detection.20 When proteins are labelled at multiple sites the situation is even more complex.
The separation of the 5 and 6-isomers of carboxyfluorescein by chromatography21,22 or crystallisation23,24 has been reported but the latter method, in our hands, was inconsistent and not easily reproduced. A recent review supports the view that a more efficient method of separation of the isomers is necessary.25 Another fluorophore that is also used as a mixture is (5)6-carboxyrhodamine B. Rhodamine dyes are highly fluorescent and have good photostability,26 and therefore have broad applications, such as a fluorescence standard for quantum yield determinations,27 detection of reactive oxygen species,13 ion sensors in living cells,28 DNA and protein labelling29,30 to name but a few. The efficient synthesis of 5 or 6-carboxytetraethylrhodamine N-hydroxysuccinimide ester is not well established.
Herein, a simple two-step process for the synthesis and subsequent separation of the two isomers of the N-hydroxysuccinimide esters of 5 and 6-carboxyfluorescein diacetate and 5 and 6-carboxytetraethylrhodamine is reported. The proposed routes have multiple advantages over existing methods in terms of scale, speed and ease of separation of the two isomers.
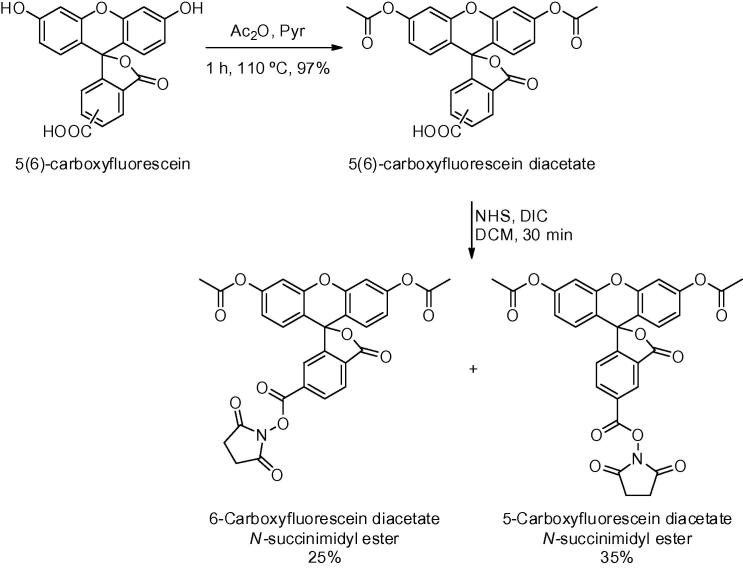
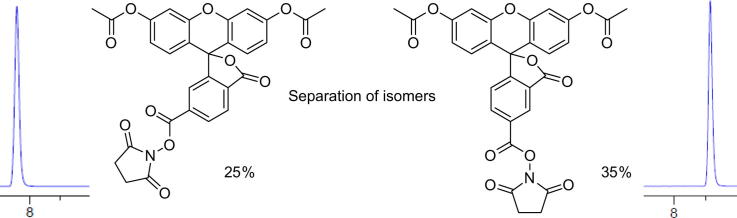
Synthesis began with acetylation of the phenol moieties of fluorescein, modifying the procedure reported by Tour31 using acetic anhydride and pyridine (>15 g scale, >95% yield), with a mild acid wash being the only work-up necessary (Scheme 1).32 Carboxylic acid activation used N,N′-diisopropylcarbodiimide (DIC) and N-hydroxysuccinimide (NHS) in dichloromethane. The two carboxyfluorescein diacetate N-hydroxysuccinimide esters were readily purified on a plug of silica gel (7 × 15 cm) using an optimised solvent system of EtOAc/Toluene (20:80) to give a 35% yield of 5-isomer and 25% yield of 6-isomer.33
Scheme 1.
N-Hydroxysuccinimide ester formation and isomer separation of carboxyfluorescein diacetate.
Fung reported the synthesis of 5 and 6-carboxytetraethylrhodamine N-hydroxysuccinimide esters using N,N′-disuccinimidyl carbonate (DSC) and DMAP,34 but in our hands, this gave a mixture of starting material and the di-ester product (Fig. 1).
Figure 1.
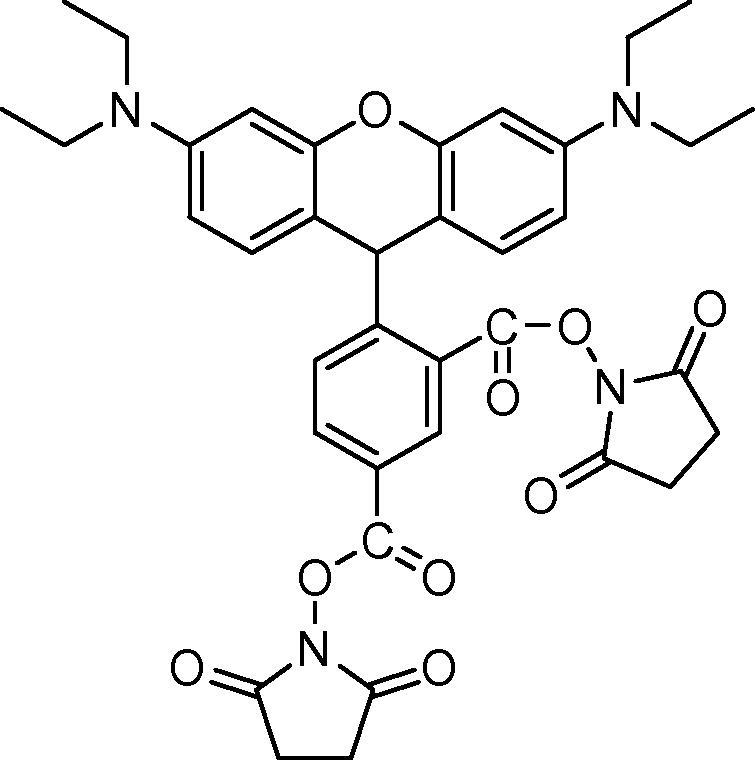
Di-ester obtained when treating carboxytetraethylrhodamine with DSC and DMAP.
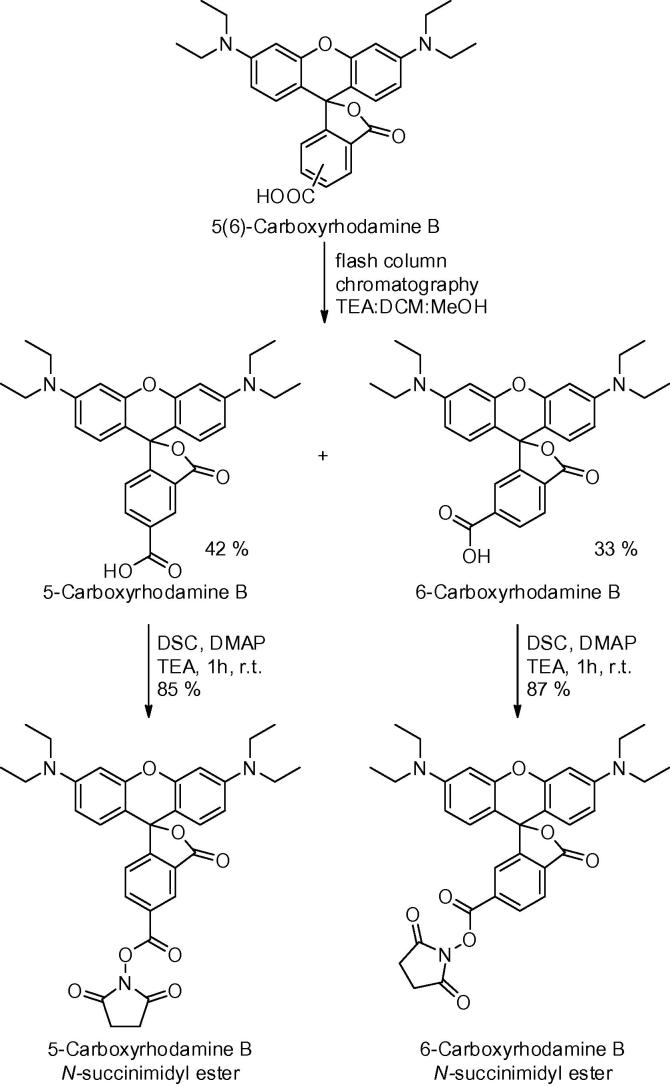
To achieve 5 or 6-carboxylic acid regioselectivity over the 3-carboxylic acid, rhodamine must react in its closed lactone form; however, unlike fluorescein, rhodamine B is in the lactone form under basic conditions, and in the open form under acidic conditions.35 Therefore it was reasoned that the active ester of 5 and 6-carboxytetraethylrhodamine would be generated using a combination of DMAP and DSC with 5 equiv of triethylamine to give the desired regioselectivity (Scheme 2).36 Larger quantities of base interfered with the efficiency of the reaction. The separation of the isomers of 5 and 6-carboxytetraethylrhodamine by column chromatography was straightforward using a gradient of TEA/DCM/MeOH (5:95:0–5:75:20).37
Scheme 2.
Isomer separation and active ester formation of rhodamine B.
In conclusion, methods have been developed for the formation and separation of the active esters of 5 and 6-isomers of carboxyfluorescein and carboxyrhodamine B. These methods are robust and reliable, and make single isomers of these two widely used fluorophores readily available.
Acknowledgments
Financial support from the MRC and University of Edinburgh is gratefully acknowledged. Dr Annamaria Lilienkampf is acknowledged for help with the manuscript preparation.
References and notes
- 1.Hermanson G.T. Academic Press; New York: 2008. Bioconjugate Techniques. [Google Scholar]
- 2.Konno K., Morales M.F. Proc. Nat. Acad. Sci. 1985;82:7904. doi: 10.1073/pnas.82.23.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando T. Biochemistry. 1984;23:375. doi: 10.1021/bi00297a029. [DOI] [PubMed] [Google Scholar]
- 4.Aguirre R., Gonsoulin F., Cheung H.C. Biochemistry. 1986;25:6827. doi: 10.1021/bi00370a015. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch R.E., Zukin R.S., Nagel R.L. Biochem. Biophys. Res. Commun. 1986;138:489. doi: 10.1016/0006-291x(86)90307-4. [DOI] [PubMed] [Google Scholar]
- 6.Cocco L., Martelli A.M., Billi A.M., Matteucci A., Vitale M., Neri L.M., Manzoli F.A. Exp. Cell Res. 1986;166:465. doi: 10.1016/0014-4827(86)90491-x. [DOI] [PubMed] [Google Scholar]
- 7.Kumke M.U., Li G., McGown L.B., Walker G.T., Linn C.P. Anal. Chem. 1995;67:3945. doi: 10.1021/ac00117a020. [DOI] [PubMed] [Google Scholar]
- 8.Pagano J.M., Farley B.M., McCoig L.M., Ryder S.P. J. Biol. Chem. 2007;282:8883. doi: 10.1074/jbc.M700079200. [DOI] [PubMed] [Google Scholar]
- 9.Talian J.C., Olmsted J.B., Goldman R.D. J. Cell Biol. 1983;97:1277. doi: 10.1083/jcb.97.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitney M.A., Crisp J.L., Nguyen L.T., Friedman B., Gross L.A., Steinbach P., Tsien R.Y., Nguyen Q.T. Nat. Biotechnol. 2011;29:352. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka K., Kitamura N., Chujo Y. Macromolecules. 2010;43:6180. [Google Scholar]
- 12.Avlonitis N., Debunne M., Aslam T., McDonald N., Haslett C., Dhaliwal K., Bradley M. Org. Biomol. Chem. 2013;11:4414. doi: 10.1039/c3ob40212f. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y., Zhao Q., Feng W., Li F. Chem. Rev. 2013;113:192. doi: 10.1021/cr2004103. [DOI] [PubMed] [Google Scholar]
- 14.Unciti-Broceta A., Yusop R.M., Richardson P.R., Walton J., Bradley M. Tetrahedron Lett. 2009;50:3713. [Google Scholar]
- 15.Bradley M., Alexander L., Duncan K., Chennaoui M., Jones A.C., Sánchez-Martín R.M. Bioorg. Med. Chem. Lett. 2008;18:313. doi: 10.1016/j.bmcl.2007.10.075. [DOI] [PubMed] [Google Scholar]
- 16.Quah B.J.C., Warren H.S., Parish C.R. Nat. Protoc. 2007;2:2049. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 17.Thaunat O., Granja A.G., Barral P., Filby A., Montaner B., Collinson L., Martinez-Martin N., Harwood N.E., Bruckbauer A., Batista F.D. Science. 2012;335:475. doi: 10.1126/science.1214100. [DOI] [PubMed] [Google Scholar]
- 18.Web of Knowledge, Search Topic ‘CFSE’ for year 2013; 227 hits; Accessed on the 14th February 2014.
- 19.Parish C.R. Immunol. Cell Biol. 1999;77:499. doi: 10.1046/j.1440-1711.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- 20.Kvach M.V., Tsybulsky D.A., Ustinov A.V., Stepanova I.A., Bondarev S.L., Gontarev S.V., Korshun V.A., Shmanai V.V. Bioconjug. Chem. 2007;18:1691. doi: 10.1021/bc7001874. [DOI] [PubMed] [Google Scholar]
- 21.Adamczyk M., Fishpaugh J.R., Heuser K.J. Bioconjug. Chem. 1997;8:253. doi: 10.1021/bc9600877. [DOI] [PubMed] [Google Scholar]
- 22.Adamczyk M., Chan C.M., Fino J.R., Mattingly P.G. J. Org. Chem. 2000;65:596. doi: 10.1021/jo991449h. [DOI] [PubMed] [Google Scholar]
- 23.Ueno Y., Jiao G.-S., Burgess K. Synthesis. 2004:2591. [Google Scholar]
- 24.Rossi F.M., Kao J.P.Y. Bioconjug. Chem. 1997;8:495. doi: 10.1021/bc970078d. [DOI] [PubMed] [Google Scholar]
- 25.Duan Y., Liu M., Sun W., Wang M., Liu S., Li Q.X. Mini-Rev. Org. Chem. 2009;6:35. [Google Scholar]
- 26.Demchenko A.P., Goncalves T., Callis P.R., Sameiro M., editors. Advanced Fluorescence Reporters in Chemistry and Biology I: Fundamentals and Molecular Design. Springer; 2010. p. 49. [Google Scholar]
- 27.Crosby G.A., Demas J.N. J. Phys. Chem. 1971;75:991. [Google Scholar]
- 28.Chen X., Pradhan T., Wang F., Kim J.S., Yoon J. Chem. Rev. 1910;2011:112. doi: 10.1021/cr200201z. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Brunner A.M., Meilan R., Strauss S.H. Tree Physiol. 2009;29:299. doi: 10.1093/treephys/tpn028. [DOI] [PubMed] [Google Scholar]
- 30.Mayasari D.S., Emoto N., Yagi K., Vignon-Zellweger N., Nakayama K., Miyoshi T., Miyata O., Hirata K. Kobe J. Med. Sci. 2013;59:E54. [PubMed] [Google Scholar]
- 31.Tour O., Adams S.R., Kerr R.A., Meijer R.M., Sejnowski T.J., Tsien R.W., Tsien R.Y. Nat. Chem. Biol. 2007;3:423. doi: 10.1038/nchembio.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.5(6)-Carboxyfluorescein diacetate: To a solution of 5(6)-carboxyfluorescein (15.0 g, 39.9 mmol) in Ac2O (180 mL) was added pyridine (18 mL, 22.3 mmol) and the reaction was stirred for 30 min at 110 °C. The clear solution was concentrated in vacuo. The crude mixture was dissolved in EtOAc (300 mL) and washed with aqueous KHSO4 (1 M, 2 × 300 mL) and brine (300 mL). The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure to give the desired compound as a light yellow solid (18.1 g, 98%). HPLC tR 7.5 and 7.6 min.Analytical HPLC was performed by using a diode array detector and Supelco Discovery® C18 column (4.6 × 50 mm), eluting from 5% ACN–H2O to 95% ACN–H2O (with 0.1% formic acid) over 10 min, followed by 4 min isocratic (flow rate 1 min/mL). The summed absorbance between 220 and 495 nm was used for purity and homogeneity assessment.
- 33.5 and 6-Carboxyfluorescein diacetate N-hydroxysuccinimide ester: 5(6)-Carboxyfluorescein diacetate (15.0 g, 32.6 mmol) was dissolved in DCM (225 mL) and N-hydroxysuccinimide (4.5 g, 39.1 mmol) was added and stirred until dissolved. DIC (6.06 mL, 39.1 mmol) was added and the reaction was stirred for 30 min. The organic layer was washed with water (2 × 50 mL), brine (50 mL), and dried over Na2SO4. The crude mixture was dissolved in toluene (50 mL), loaded onto an equilibrated column (7 × 15 cm bed of silica) and eluted with 20% EtOAc in toluene to afford the pure individual isomers (5-isomer 4.5 g, 25%; 6-isomer 6.4 g, 35%). 5-Carboxyfluorescein diacetate N-hydroxysuccinimide ester: Rf 0.37 (50:50 EtOAc/Toluene), HPLC tR 8.6 min (<1% of 6-isomer). 6-Carboxyfluorescein diacetate N-hydroxysuccinimide ester: Rf 0.53 (50:50 EtOAc/Toluene), HPLC tR 7.8 min (<1% of 5-isomer).
- 34.Fung, S.; Menchen, S. M. EP0272007 A2; 1988.
- 35.Adamczyk M., Grote J. Bioorg. Med. Chem. Lett. 2003;13:2327. doi: 10.1016/s0960-894x(03)00411-6. [DOI] [PubMed] [Google Scholar]
- 36.5-Carboxyrhodamine B N-hydroxysuccinimide ester: 5-Carboxytetraethylrhodamine (195 mg, 0.40 mmol), DMAP (244 mg, 2.0 mmol) and TEA (278 μL, 2.0 mmol) were dissolved in dry DCM (20 mL). DSC (205 mg, 0.80 mmol) was added and the reaction stirred for 1 h. The reaction was quenched with AcOH (229 μL, 4.0 mmol) and the solution was directly loaded onto an equilibrated flash chromatography column and eluted with 1% AcOH in acetone to afford the pure compound (198 mg, 85%). Rf 0.27 (9:89:2 MeOH/DCM/AcOH), HPLC tR 6.4 min. 6-Carboxyrhodamine B N-hydroxysuccinimide ester was prepared as described above (87% yield). Rf 0.31 (9:89:2 MeOH/DCM/AcOH), HPLC tR 6.5 min.
- 37.5 and 6-Carboxyrhodamine B: 5(6)-Carboxytetra-ethylrhodamine (2.0 g, 4.11 mmol) was purified by flash column chromatography (gradient of TEA/DCM/MeOH from 5:95:0 to 5:75:20). The two separated isomers were obtained as a TEA salt. Solvents were removed under reduced pressure. The individual isomers (as their salts) were dissolved in EtOAc (80 mL) and washed with 1 M KHSO4 (3 × 50 mL), brine (50 mL), dried over Na2SO4 and filtered, to afford the pure individual isomers 5-carboxytetraethylrhodamine (833 mg, 42%) and 6-carboxytetraethylrhodamine (670 mg, 33%) as dark pink solids. 5-Carboxyrhodamine B: Rf 0.11 (10:89.5:0.5 MeOH/DCM/TEA), HPLC tR 7.5 min. 6-Carboxyrhodamine B: Rf 0.22 (10:89.5:0.5 MeOH/DCM/TEA), HPLC tR 7.0 min.