Abstract
Myoglobin (Mb) is the classic vertebrate oxygen-binding protein present in aerobic striated muscles. It functions principally in oxygen delivery and provides muscle with its characteristic red colour. Members of the Antarctic icefish family (Channichthyidae) are widely thought to be extraordinary for lacking cardiac Mb expression, a fact that has been attributed to their low metabolic rate and unusual evolutionary history. Here, we report that cardiac Mb deficit, associated with pale heart colour, has evolved repeatedly during teleost evolution. This trait affects both gill- and air-breathing species from temperate to tropical habitats across a full range of salinities. Cardiac Mb deficit results from total pseudogenization in three-spined stickleback and is associated with a massive reduction in mRNA level in two species that evidently retain functional Mb. The results suggest that near or complete absence of Mb-assisted oxygen delivery to heart muscle is a common facet of teleost biodiversity, even affecting lineages with notable oxygen demands. We suggest that Mb deficit may affect how different teleost species deal with increased tissue oxygen demands arising under climate change.
Keywords: myoglobin, oxygen supply, fish evolution, climate change
1. Introduction
Myoglobin (Mb) is an oxygen-binding haemprotein of the globin family, typically expressed at high levels in aerobic striated muscle [1,2]. ‘Classic’ functions include the storage of oxygen in the intracellular compartment and the enhancement of oxygen diffusion from blood to mitochondria [1,2]. More recently characterized functions in a range of cell-types include the regulation of intracellular nitric oxide and reactive oxygen species (reviewed in [2,3]). High Mb is positively associated with lifestyles or environments that demand efficient oxygen delivery. For example, high levels of Mb are present in the muscles of diving mammals and birds, supporting active foraging behaviour while breath-holding [4].
Conversely, selection on high Mb levels may be relaxed when demands for oxygen delivery are low. An extreme example is provided by three icefish lineages that independently lost Mb expression in striated muscle, following the earlier loss of haemoglobin (Hb) in their common ancestor (reviewed in [5]). All icefishes have low oxygen demands and evolved in habitats where oxygen has been constantly saturated [5]. Such features, by relaxing the need for efficient oxygen transport, were proposed to explain how these losses were sub-lethal [5]. Nevertheless, there is evidence that Mb and Hb deficit is maladaptive, leading to the suggestion that a major lack of competition in icefish habitats was central to the evolutionary persistence of these apparently exceptional traits [5].
One little-cited study suggested that cardiac Mb deficit extends to members of four further teleost families found in temperate latitudes and that are also relatively inactive [6]. Accordingly, we hypothesized that Mb deficit may be more common than widely realized in teleost fishes. We thus characterized the evolution of cardiac Mb expression in species spanning the teleost phylogeny, occupying a broad range of environments and lifestyles.
2. Material and methods
Complete material and methods are provided in the electronic supplementary material. Heart phenotypes were established in 22 Actinopterygian species held under normoxia. Total RNA was extracted from 16 species and used as a template for first-strand cDNA synthesis (electronic supplementary material, table S1). cDNAs for each species were used in PCR reactions employing two degenerate primer pairs, the first highly conserved across Teleostei and the second highly conserved across Acanthopterygii (electronic supplementary material, table S2). Mb was sequenced in nine species as described elsewhere [7] (Data accessibility section). Quantitative PCR using species-specific primers (electronic supplementary material, table S2) was used to quantify Mb mRNA level in 11 teleost species. BLAST was performed against NCBI (http://www.ncbi.nlm.nih.gov/) and Ensembl (http://www.ensembl.org) databases. Non-synonymous (dN) and synonymous substitution (dS) rates were estimated using PAML [8]. One-way ANOVA was used to compare Mb mRNA levels across species using Minitab v. 16 (Minitab Inc.).
3. Results
(a). Mb deficit has evolved repeatedly in teleosts
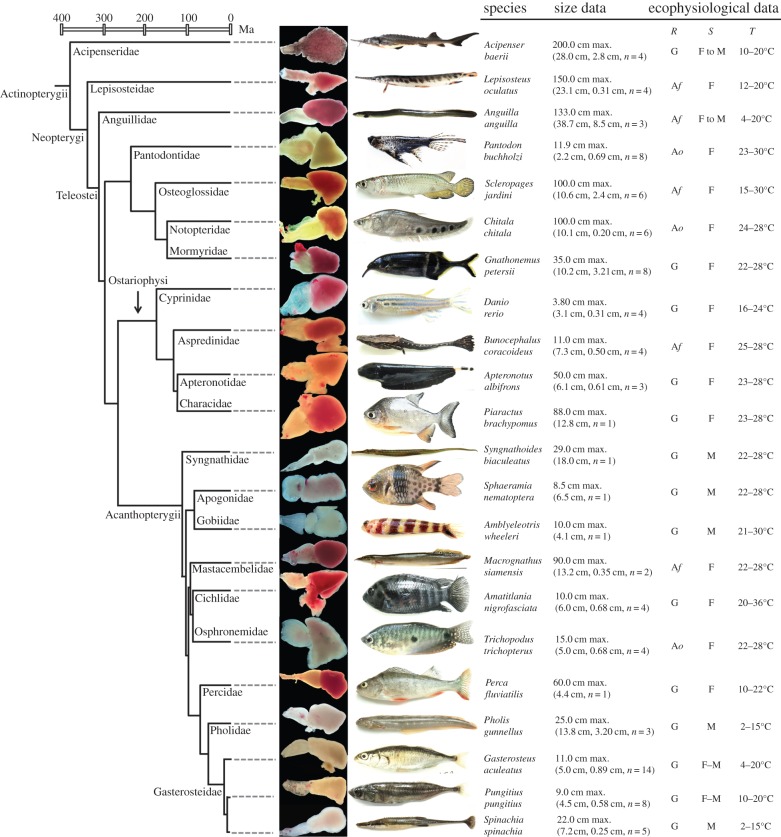
We mapped heart phenotypes from 22 Actinopterygians onto a robust phylogeny (figure 1). Red pigmentation, indicative of Mb, was present in two species branching before teleosts (figure 1). In teleosts, heart colour ranged from red to pale white/yellow, the latter indicative of Mb deficit [5,6]. Pale hearts have arisen in Osteoglossiformes and on independent occasions in Acanthopterygii, being present in temperate and tropical species from fresh and saltwater (figure 1). All tested species of Gasterosteidae were pale-hearted, as was a closely related species from the Pholidae family (figure 1). Pale-hearted Syngnathoides biacufeatus is more closely related to Scombridae [9] than other species in figure 1. As scombrids are active migratory fishes with high Mb, this suggests the existence of a further independent origin for Mb deficit. Pale hearts were present both in species that respire primarily through the gills and via accessory air-breathing organs (figure 1).
Figure 1.
Diversity of cardiac phenotypes in the ray-finned fishes studied, mapped onto a robust phylogeny and timescale [9]. For each species, size data are provided including maximum reported body length (source: FishBase, http://www.fishbase.org/) and body length for the sampled individuals (in parentheses: mean, s.d. and n). This latter data show that the range of body sizes sampled was largely randomized across species with respect to heart colour phenotypes. We also provide ecophysiological data on primary respiration phenotype (R) (G, gill breather; Af, facultative air-breather; Ao, obligate air-breather), habitat salinity (S) (F, freshwater; M, marine; F–M, diadromy possible) and habitat thermal range (T). Habitat data were sourced from FishBase and data on air-breathing were acquired from the literature [10].
(b). Mb PCRs
PCRs targeting cardiac Mb cDNAs with two degenerate primer pairs (within the coding region) were successful in nine tested red-hearted species but only two of seven tested pale-hearted species (Pantodon buchholzi and Trichopodus trichopterus) (electronic supplementary material, table S1). PCR was successful in species occupying basal clades that are less related to the species that informed primer design than the five pale-hearted Acanthopterygii species [9], where both PCR assays failed. This suggests that Mb mRNA may be absent or extremely low in these pale-hearted Acanthopterygii members. Alternatively, normally conserved regions of Mb where the primers are binding in other species may not exist, which would be expected if selective pressure to maintain the Mb protein-coding sequence had been relaxed during evolution.
(c). Maintenance of Mb function in pale-hearted species
We established dN/dS ratios comparing the Mb-coding region of species pairs within Osteoglossiformes and Acanthopterygii. When dN/dS is less than 1, there is evidence that purifying selection has been a predominant force during evolution, acting to remove changes in amino acid sequence and maintain protein function. Comparison of pale-hearted P. buchholzi with three red-hearted osteoglossiform species returned dN/dS ratios of 0.08, 0.18 and 0.24. Comparing solely the red-hearted osteoglossiform species returned similar values (dN/dS = 0.01, 0.17 and 0.22). Comparisons involving four Acanthopterygii species returned dN/dS ratios of 0.09, 0.08 and 0.10 when including pale-hearted T. trichopterus (and 0.10, 0.17 and 0.09 excluding this species). These invariant low dN/dS ratios suggest that Mb functions have been maintained as strongly during the evolution of the two pale-hearted species as for their red-hearted relatives.
(d). Mb pseudogenization in stickleback Gasterosteus aculeatus
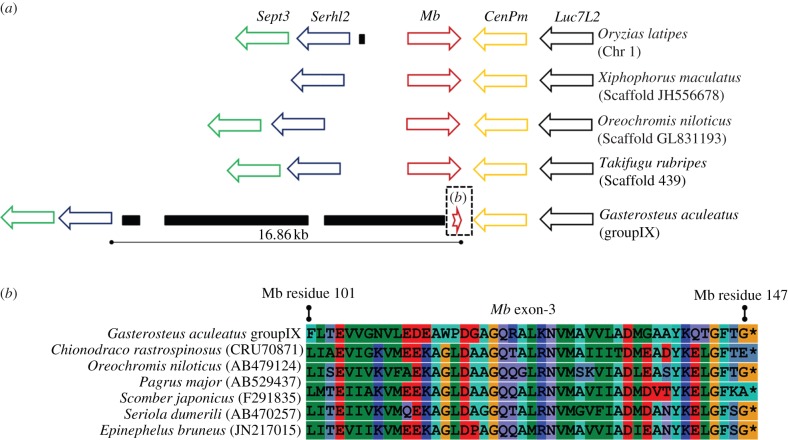
It was previously noted that an Mb gene was absent from the three-spined stickleback genome assembly [11]. We expanded this observation to better understand the pale stickleback heart. BLAST searches of Mb proteins against the G. aculeatus genome produced significant hits on a syntenic region of groupIX containing Mb in other Acanthopterygians (figure 2a). One of the G. aculeatus hits is an open reading frame (ORF) having around 50% protein-level identity to the complete exon-3 of other Acanthoptergians, which includes the Mb stop codon (figure 2b).
Figure 2.
Evidence of Mb pseudogenization in three-spined stickleback. (a) Genomic neighbourhood surrounding Mb of Acanthopterygii members. Orthologous genes are shown as arrows of the same colour. The distance separating Mb and Serhl2 is to scale. Black rectangles show repetitive elements widely distributed in the genome. (b) Alignment of Acanthopterygian Mb proteins coded in exon-3 including the stickleback pseudogene ORF. NCBI accession numbers are provided.
For most Acanthopterygians, 4.1–5 kb separates Mb exon-3 from the last exon of the upstream Serhl2 gene (figure 2a), a region containing Mb exon-1 and -2, intron-1 and -2 and the proximal promoter. In stickleback, this region is 16.7 kb long (figure 2a) and highly repetitive in the genome: the top 100 BLAST hits (all 0e + 0) for this region are located across 16 additional chromosomes and range in length from 1.3 to 6.3 kb, sharing an average of 98.6% nucleotide identity with the groupIX sequence. The equivalent region in other tested Acanthopterygii species was completely or near-completely non-repetitive within each genome. BLAST searches of assembled G. aculeatus traces (NCBI Archive) demonstrated that the groupIX region is covered by multiple overlapping traces, including single traces linking exon-3 to repetitive sequences. The stickleback groupIX region also contains large ORFs that code for conserved proteins with retrovirus domains (respective top BLASTp hits: 1 × 10−54 and 0.0). These data suggest that repetitive elements have invaded the region containing the stickleback Mb gene, consistent with its pseudogenization.
(e). Cardiac Mb mRNA levels in a range of teleosts
There was extensive variation in cardiac Mb mRNA levels across teleost species (table 1; F = 84.1, p < 0.0001: one-way ANOVA) with red hearts having higher levels than pale hearts. Pantodon buchholzi had approximately 250–700 times less mean cardiac Mb mRNA than Osteoglossiformes relatives, whereas T. trichopterus had approximately 40 times less than its cichlid relative Amatitlania nigrofasciata (table 1). These differences were highly statistically relevant (Tukey's test). A cardiac mRNA was transcribed from exon-3 of the G. aculeatus Mb pseudogene. However, its significance is contextualized by the lack of potential for translation of a functional Mb protein and its approximate 300- to 500 000-fold lower mean abundance versus the other species (table 1).
Table 1.
Cardiac Mb mRNA levels in 11 teleost species. (Phylogenetic relationships are shown in figure 1.)
| species | family | order | heart colour | Mb mRNA (mean) | Mb mRNA (s.d.) | n |
|---|---|---|---|---|---|---|
| Anguilla anguilla | Anguillidae | Anguilliformes | red | 33.30 | 11.39 | 3 |
| Gnathonemus petersii | Mormyridae | Osteoglossiformes | red | 13.58 | 2.25 | 4 |
| Chitala chitala | Notopteridae | Osteoglossiformes | red | 4.75 | 1.07 | 4 |
| Pantodon buchholzi | Pantodontidae | Osteoglossiformes | palea | 0.02 | 0.01 | 4 |
| Danio rerio | Cyprinidae | Cypriniformes | red | 10.45 | 1.16 | 3 |
| Bunocephalus coracoideus | Aspredinidae | Siluriformes | red | 18.02 | 0.59 | 3 |
| Apteronotus albifrons | Apteronotidae | Gymnotiformes | orange | 17.05 | 5.97 | 3 |
| Salmo salar | Salmonidae | Salmoniformes | red | 5.16 | 1.43 | 4 |
| Amatitlania nigrofasciata | Cichlidae | Perciformes | red | 16.10 | 2.58 | 4 |
| Trichopodus trichopterus | Osphronemidae | Perciformes | palea | 0.41 | 0.39 | 4 |
| Gasterosteus aculeatus | Gasterosteidae | Perciformes | paleb | 6.46 × 10−5 | 4.33 × 10−5 | 4 |
aRetains a functional Mb protein.
bMb pseudogene.
4. Discussion
It is often stated that Mb expression is essential for aerobic function in vertebrate striated muscle [1]. However, even before this study, exceptions to this ‘rule’ were suspected, including the entire class Amphibia and a few teleost species [2,5,6]. Nevertheless, such cases have been deemed extraordinary [2,5]. Surprisingly, we revealed that cardiac Mb deficit has evolved repeatedly in teleosts under diverse ecological settings. The pale hearts observed are unlikely to have high compensatory levels of other globin proteins, as posited for amphibians [2], as this would produce red pigmentation.
As classic Mb functions require high expression in myocytes [1], we conclude that oxygen diffusion is happening with little or no assistance from Mb in pale teleost hearts. This is noteworthy because tropical fishes typically require six times more oxygen at rest than polar species such as icefishes [12]. The total loss of Mb function in three-spined stickleback is also paradoxical, as this species can migrate long distances [13]. Under the current paradigm, such ability for aerobic performance should have favoured maintenance of Mb.
Our data also raise the possibility that Mb deficit is common in teleosts as a group. In fact, there is evidence suggesting that Mb deficit may be an ancestral character shared by five Acanthopterygii families containing hundreds of species. All species tested to date from Gasterosteidae, Pholidae, Anarhichadidae, Cyclopteridae and Zoarcidae have pale hearts (this work, [6]) and are more closely related to one another than to the next red-hearted species [9] (Perca fluviatilis in figure 1). The massive invasion of repetitive elements into the Mb gene region of stickleback (Gasterosteidae) is consistent with an ancient origin for pseudogenization, which provides a hypothesis to explain the pale-heartedness shared by these Acanthopterygii families.
The diversity of species with cardiac Mb deficit implies that a spectrum of biological settings exist where selective pressure on Mb-assisted oxygen supply into heart is relaxed. However, the associated physiological and ecological factors remain uncharacterized. Our data offer limited clues in the way of explanation. First, all identified tropical and temperate species with pale hearts have relatively small adult body size (figure 1), suggesting that some aspect of allometry may affect constraints on Mb-assisted oxygen-transport into hearts. However, as other small species have high Mb expression e.g. Danio (figure 1 and table 1), additional factors must also be at play. Second, two small obligate air-breathing species have cardiac Mb deficit. This is notable as air-breathing may enhance oxygen supply to the heart [10], perhaps relaxing the need for Mb-assisted oxygen-transport in certain settings, for example in combination with small body size. Overall, the circumstances under which Mb deficit is tolerated are likely to involve many interacting factors—this is an area that demands further attention.
It will also be important to establish whether Mb deficit has potential to negatively affect fitness, as proposed in icefish [5], especially in the context of contemporary climate change. The temperature of the Earth's habitats will rise significantly in the near future [14], increasing the routine oxygen demands of most ectotherms [15]. The ability to meet cellular oxygen requirements dictates the extent of higher physiological functions possible, e.g. behaviour/reproduction [15]. It is hypothesized that when temperature surpasses an ectotherm's optimum range, the capacity to supply enough oxygen to tissues is exceeded, leading to greatly reduced fitness [15,16]. Evidence favouring this model exists for the eelpout Zoarces viviparous, where populations may already be suffering under contemporary global warming [16]. Considering Mb's role in supporting cardiac performance, it is plausible that Mb deficit will affect the ability to meet tissue oxygen demands in warmer future habitats. Intriguingly, Z. viviparous is part of the aforementioned Acanthopterygii group that may share Mb deficit.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The paper was greatly improved by the constructive comments of anonymous reviewers. D.J.M. is grateful to Drs Michael Berenbrink (University of Liverpool) and Graham Scott (University of McMaster) for discussing and sharing ideas about this research.
All experiments were approved by the University of St Andrews Animal Ethics and Welfare Committee.
Data accessibility
Accession numbers for new Mb cDNAs: KJ561849–KJ561856. More details in the electronic supplementary material.
Funding statement
The study was supported by the Marine Alliance for Science and Technology for Scotland (Scottish Funding Council grant no. HR09011).
References
- 1.Ordway KM, Mueller IA. 2004. Myoglobin: an essential hemoprotein in striated muscle. J. Exp. Biol. 207, 3441–3446. ( 10.1242/jeb.01172) [DOI] [PubMed] [Google Scholar]
- 2.Helbo S, Weber RE, Fago A. 2013. Expression patterns and adaptive functional diversity of vertebrate myoglobins. Biochim. Biophys. Acta 1834, 1832–1839. ( 10.1016/j.bbapap.2013.01.037) [DOI] [PubMed] [Google Scholar]
- 3.Cossins AR, Berenbrink M. 2008. Physiology: myoglobin's new clothes. Nature 454, 416–417. ( 10.1038/454416a) [DOI] [PubMed] [Google Scholar]
- 4.Kooyman GL, Ponganis PJ. 1998. The physiological basis of diving to depth: birds and mammals. Annu. Rev. Physiol. 60, 19–32. ( 10.1146/annurev.physiol.60.1.19) [DOI] [PubMed] [Google Scholar]
- 5.Sidell BD, O'Brien KM. 2006. When bad things happen to good fish: the loss of haemoglobin and myoglobin expression in Antarctic icefishes. J. Exp. Biol. 209, 1791–1802. ( 10.1242/jeb.02091) [DOI] [PubMed] [Google Scholar]
- 6.Grove TJ, Sidell BD. 2002. Myoglobin deficiency in the hearts of phylogenetically diverse temperate-zone fish species. Can. J. Zool. 80, 893–901. ( 10.1139/z02-071) [DOI] [Google Scholar]
- 7.Macqueen DJ, Garcia de la Serrana D, Johnston IA. 2013. Evolution of ancient functions in the vertebrate insulin-like growth factor system uncovered by study of duplicated salmonid fish genomes. Mol. Biol. Evol. 30, 1060–1076. ( 10.1093/molbev/mst017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591. ( 10.1093/molbev/msm088) [DOI] [PubMed] [Google Scholar]
- 9.Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL. 2012. Resolution of ray-finned fish phylogeny and timing of diversification. Proc. Natl Acad. Sci. USA 109, 13 698–13 703. ( 10.1073/pnas.1206625109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grahams J. 1997. Air-breathing fishes: evolution, diversity, and adaptation. New York, NY: Academic Press. [Google Scholar]
- 11.Hoffmann FG, Opazo JC, Storz JF. 2011. Differential loss and retention of cytoglobin, myoglobin, and globin-E during the radiation of vertebrates. Genome Biol. Evol. 3, 588–600. ( 10.1093/gbe/evr055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke A, Johnston NM. 1999. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 68, 893–905. ( 10.1046/j.1365-2656.1999.00337.x) [DOI] [PubMed] [Google Scholar]
- 13.Dalziel AC, Schulte PM. 2012. Correlates of prolonged swimming performance in F2 hybrids of migratory and non-migratory threespine stickleback. J. Exp. Biol. 215, 3587–3596. ( 10.1242/jeb.071951) [DOI] [PubMed] [Google Scholar]
- 14.Collins MR, et al. 2013. Long-term climate change: projections, commitments and irreversibility. In Climate change 2013: the physical science basis. Contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change (eds Stocker TF, et al.), pp. 1029–1136. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Pörtner HO. 2010. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881–893. ( 10.1242/jeb.037523) [DOI] [PubMed] [Google Scholar]
- 16.Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97. ( 10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Accession numbers for new Mb cDNAs: KJ561849–KJ561856. More details in the electronic supplementary material.