Abstract
Despite considerable study, mystery surrounds the use of signals that initiate cooperative hunting in animals. Using a labyrinth test chamber, we examined whether a lionfish, Dendrochirus zebra, would initiate cooperative hunts with piscine partners. We found that D. zebra uses a stereotyped flared fin display to alert conspecific and heterospecific lionfish species Pterois antennata to the presence of prey. Per capita success rate was significantly higher for cooperative hunters when compared with solitary ones, with hunt responders assisting hunt initiators in cornering the prey using their large extended pectoral fins. The initiators would most often take the first strike at the group of prey, but both hunters would then alternate striking at the remaining prey. Results suggest that the cooperative communication signal may be characteristic to the lionfish family, as interspecific hunters were equally coordinated and successful as intraspecific hunters. Our findings emphasize the complexity of collaborative foraging behaviours in lionfish; the turn-taking in strikes suggests that individuals do not solely try to maximize their own hunting success: instead they equally share the resources between themselves. Communicative group hunting has enabled Pteroine fish to function as highly efficient predators.
Keywords: collaborative foraging, animal communication, predator–prey interactions, Pteroine fish
1. Introduction
The ability to communicate effectively with other individuals plays a critical role in the lives of all social animals and the subject has long been of considerable interest to evolutionary biologists [1]. In animals, communication is often studied in relation to foraging activities where individuals communicate through an extraordinary variety of auditory, visual or olfactory signals [2]. For example, colonial swallows use a squeak call to alert conspecifics that food has been found [3], while honeybee foragers display a waggle dance to share the location of nectar patches with colony members [4]. Pioneer ants, on the other hand, leave behind pheromone trails that lead foraging ants back and forth between areas rich in food and their nest [5]. Such displays provide animals with information needed to initiate group feeding. There are other examples of more complex signals used to communicate information when multiple individuals are required in prey capture. Taï chimpanzees, for example, work together in communicative, cooperative groups when hunting for prey [6].
Long thought to be limited to more complex animals such as primates and other large-brained mammals, cooperative hunting behaviours have been found in species of fish, birds and insects [1,7]. Cooperative hunting evolves when foraging in pairs or larger groups yields higher payoffs than solitary foraging, with the catch often shared hierarchically among those involved in the hunt [8]. However, in some instances, only one of the participants will benefit from a particular interaction. In cooperatively hunting groupers and moray eels, prey are not shared, but rather are immediately swallowed whole by the individual that catches them first [9]. The potential for monopolizing prey may limit predators from displaying a predisposition to engage in cooperative hunting. Despite the long-standing interest in animal communication, we know little of the signals involved in cooperative hunting, with few examples of the cues used to instigate foraging bouts (but see [10]).
Recent field observations have suggested that predatory lionfish (Scorpaenidae: Pteroinae) hunt cooperatively both intra- and inter-specifically [11–13]. However, how cooperative hunts are initiated and whether they improve catch rates is unknown. Although some lionfish species (i.e. Pterois volitans) are highly successful predators [14], with the ability to alter prey community composition in regions where they invade [15], we know very little about the social organization or biology of any of the Scorpaenid fishes. If it holds true that the success rate of group hunting is higher than hunts by solitary individuals [8], it could help explain the exceptionally high prey capture rates of lionfish predators. In this study, we investigated whether the zebra lionfish (Dendrochirus zebra; figure 1a) would initiate cooperative hunting with conspecific and heterospecific lionfish. Furthermore, we examined whether fish involved in collaborative hunts were more successful than solitary individuals.
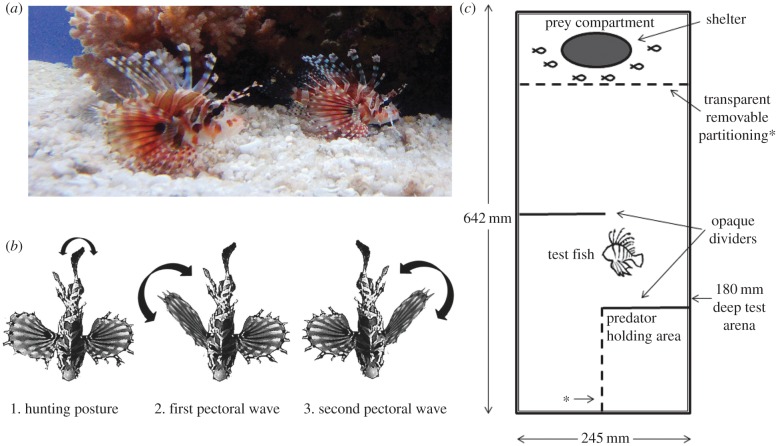
Figure 1.
(a) The study species D. zebra. (b) The flared fin display sequence by initiator predators. (c) Experimental labyrinth chamber. (Photo credit: Oona Lönnstedt.) (Online version in colour.)
2. Material and methods
(a). Preliminary observations of lionfish hunting behaviour
Over the past 2 years, we have been studying the predator ecology of lionfish in the laboratory and reefs surrounding Lizard Island (14°40′ S, 145°28′ E), on the northern Great Barrier Reef (Australia) [12,14]. In both field and laboratory, we have observed lionfish feeding together in groups of up to four individuals. They corner schools of small prey fish with their large pectoral fins and, in what appears to be a highly coordinated effort, individuals take turns striking at prey. We observed a very distinctive flared fin display that individuals perform prior to cooperative hunting (figure 1b; electronic supplementary material, Movie S1). One individual will approach another head on. With its head down and pectoral fins flared, it rapidly undulates its caudal fin for 3–9 s. This is followed by a slow and separate waving of the pectorals. Almost always the partner responds by undulating their fins, and then the pair moves off to hunt. If the partner does not follow the hunt initiator, then the initiator returns and repeats the display. Despite hundreds of hours of observations in the laboratory and the field, we have observed this behaviour in only one context: just prior to group hunting. Hence, we hypothesized that it was a display used to initiate cooperative hunting.
(b). Cooperative hunting and its effect on capture success
We conducted a controlled laboratory experiment to test whether lionfish use this fin display to attempt to initiate group foraging with conspecifics and heterospecific lionfish, but not other predator species. We used a series of dividers to design a labyrinth test arena that had a sealed prey compartment at one end and a sealed predator compartment at the other (figure 1c). The focal lionfish (hunt initiator) in the middle had to swim around a barrier to locate a hunting partner. We quantified the amount of time that focal lionfish spent near the prey compartment and near the predator compartment before and after six small prey fish (Apogon doerderlini; 1.26 ± 0.13 cm standard length SL ± s.e.) were added to the prey compartment. We also quantified any fin displays. The predator compartment held either (i) a conspecific lionfish (D. zebra; 9.46 ± 0.29 cm SL), (ii) a heterospecific lionfish often found in the vicinity of D. zebra individuals (Pterois antennata; 9.53 ± 0.52 cm SL), (iii) a freckled grouper (Cephalophalis microprion; 9.48 ± 0.41 cm SL) or (iv) an empty compartment (N = 10). We also conducted foraging trials to determine the hunting success of solitary versus paired lionfish (N = 6–10). Detailed information about the design and statistical analysis are provided in the electronic supplementary material.
3. Results
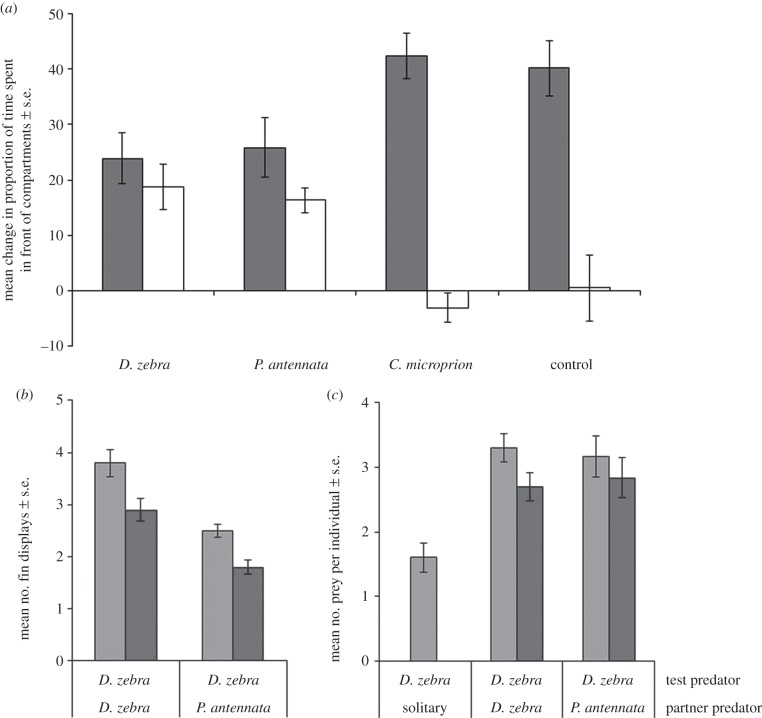
The overall behavioural change of D. zebra before and after prey were released differed significantly among the four treatments (MANOVA, F3,34 = 2.47, p = 0.005). In all trials, we observed that there was a significant increase in time that focal lionfish spent in the vicinity of the prey's compartment after the prey were added (ANOVA, F3,36 = 3.96, p = 0.015; figure 2a). In trials where there was a conspecific or heterospecific lionfish in the predator compartment, we also observed a significant increase in the time spent in front of the predator compartment (ANOVA, F3,36 = 7.51, p = 0.0005; figure 2a). The same pattern was not seen when the partner was a grouper or when there was an empty tank. The fact that lionfish repeatedly left the area in front of the prey compartment to swim to the predator compartment provides a strong indication that the focal lionfish was trying to recruit a hunting companion. More important, we observed a significant number of flared fin displays by focal fish only when there was prey present and only in front of the predator compartment in trials with lionfish partners (ANOVA, F3,36 = 13.88, p < 0.0001; figure 2b). Fin displays were never observed in trials with groupers or in the empty tank control.
Figure 2.
(a) Mean change in proportion of time D. zebra initiators spent in front of prey compartment (grey bars) and predator holding area (white bars) before and after prey were released into chamber. (b) Number of times initiators (grey bars) and responders (dark grey bars) displayed fin-signals after prey had been added. (c) Mean number of prey caught by solitary D. zebra, two D. zebra or a D. zebra and P. antennata hunting together during a 10-min hunting trial.
When examining the foraging success of paired versus solitary hunters, we found that capture success was higher for paired hunters (ANOVA, F2,23 = 13.1, p = 0.00016; figure 2c), although time to first capture was not different among groups (ANOVA, F2,23 = 1.26, p = 0.3). When partner predators were released, they followed initiators into the prey compartment area. Then, using their extended pectoral fins, the two lionfish would cooperatively herd prey into a smaller area thereby preventing their escape. When examining foraging patterns, we found that the number of switches in the order of foraging (‘taking turns’) was different from random, with fish averaging 4.2 (conspecific partners, p < 0.001) and 4.0 (heterospecific partners, p = 0.002) turns, on a scale ranging from 0 (no sharing) to 5 (fish diligently taking turns). We found no differences between the foraging metrics recorded between conspecific and heterospecific hunters (time to initiate cooperation, time to first attack, time to first capture and latency to capture all prey; see the electronic supplementary material for details), indicating that the signals and cooperative hunting strategies could be idiosyncratic to the Pteroine family.
4. Discussion
Our study provides the first experimental demonstration of mutually beneficial cooperative hunting between pairs of predators. Zebra lionfish use a highly stereotyped flared fin display to signal for hunting support to members of the same and a different species of lionfish, and hunting partners actively respond to initiators with a fin display. When hunting cooperatively, the predators used their large extended pectoral fins to herd prey into a corner and then took turns to strike and feed on the prey.
Unlike previously reported cases of cooperative hunting in animals [6–10], lionfish do not merely pursue self-interests when hunting together, but instead take turns striking at groups of prey, the consequence of which was to share the catch between hunt members. There was a relatively equal number of prey consumed by the two individuals, but the first strike was most often carried out by the initiator (8/10 times). This pattern is unlikely to be due to foraging constraints owing to handling time. Lionfish swallow their prey whole and can swallow four or five in rapid succession when fed in their holding tanks. The high level of coordination and sharing between the lionfish makes our study of collaborative foraging different from other studies, which often find that cooperative hunting is merely the simultaneous execution of hunts by two or more individuals within the same group and with the kill seldom shared equally [8]. Indeed, the foraging strategy of Pteroine fish appears to resemble a tit-for-tat-like role alteration [16] or equal prey sharing [6]. Taking turns striking at prey makes most sense if the groups are typically composed of kin or of individuals that have long-time associations with each other. In such cases, cooperation should be highly selected.
We can only speculate as to what information is conveyed by the flared fin display, but as per the ‘waggle dance’ in honeybees it may indicate that food has been found, and possibly its location [4]. Some form of communication signal is necessary for cooperative tasks to be successfully undertaken between individuals, but such gestures have rarely been studied in animals. Our findings reveal that Pteroine fish display highly complex hunting strategies that significantly increase capture success rates. This complex foraging behaviour could in part explain the high feeding rates of invasive lionfish in the Caribbean, a region where high densities of lionfish predators are threatening the stability of the whole ecosystem by the over-consumption of small prey species [15]. Communicative group hunting has enabled lionfish to function as efficient predators and highlight that some fish display highly advanced social behaviours.
This work was conducted with the approval of James Cook University ethics guidelines (permit no.: A1593).
Data accessibility
The raw data are provided as the electronic supplementary material.
Funding statement
We thank G. Lienart, M. McCormick, J. Rizzari and the staff at LIRS for logistic support. Fieldwork and research was funded by the ARC Centre of Excellence and the LIRS Fellowship program.
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1.Anderson C, Franks NR. 2001. Teams in animal societies. Behav. Ecol. 12, 534–540. ( 10.1093/beheco/12.5.534) [DOI] [Google Scholar]
- 2.Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 3.Brown CR, Brown MB, Shaffer ML. 1991. Food-sharing signals among socially foraging cliff swallows. Anim. Behav. 42, 551–564. ( 10.1016/S0003-3472(05)80239-8) [DOI] [Google Scholar]
- 4.Von Frisch K. 1967. The dance language and orientation of bees. Cambridge, MA: Harvard University Press. [Google Scholar]
- 5.Beckers R, Deneubourg JL, Goss S. 1992. Trails and U-turns in the selection of a path by the ant Lasius niger . J. Theor. Biol. 159, 397–415. ( 10.1016/S0022-5193(05)80686-1) [DOI] [Google Scholar]
- 6.Boesch C, Boesch H. 1989. Hunting behavior of wild chimpanzees in the Tai National Park. Am. J. Phys. Anthropol. 78, 547–573. ( 10.1002/ajpa.1330780410) [DOI] [PubMed] [Google Scholar]
- 7.Bertram BCR. 1978. Living in groups: predators and prey. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 64–96. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 8.Packer C, Ruttan L. 1988. The evolution of cooperative hunting. Am. Nat. 132, 159–198. ( 10.1086/284844) [DOI] [Google Scholar]
- 9.Bshary R, Hohner A, Ait-el-Djoudi K, Fricke H. 2006. Interspecific communicative and coordinated hunting between groupers and giant moray eels in the Red Sea. PLoS Biol. 4, e431 ( 10.1371/journal.pbio.0040431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vail AL, Manica A, Bshary R. 2013. Referential gestures in fish collaborative hunting. Nat. Comm. 4, 1765 ( 10.1038/ncomms2781) [DOI] [PubMed] [Google Scholar]
- 11.Naumann MS, Wild C. 2013. Foraging association of lionfish and moray eels in a Red Sea seagrass meadow. Coral Reefs 32, 1111 ( 10.1007/s00338-013-1079-0) [DOI] [Google Scholar]
- 12.Rizzari JR, Lönnstedt OM. 2014. Cooperative hunting and gregarious behaviour in the zebra lionfish, Dendrochirus zebra. Mar. Biodivers. 3, 1–2. ( 10.1007/s12526-014-0215-6) [DOI] [Google Scholar]
- 13.Kendall JJ., Jr 1990. Further evidence of cooperative foraging by the turkeyfish (Pterois miles) in the Gulf of Aqaba, Red Sea. Proc. Am. Acad. Underwater Sci. Scientific Diving Symp. 10, 209–223. [Google Scholar]
- 14.Lönnstedt OM, McCormick MI. 2013. Ultimate predators: lionfish have evolved to circumvent prey risk assessment abilities. PLoS ONE 8, e75781 ( 10.1371/journal.pone.0075781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green SJ, Dulvy NK, Brooks AL, Akins JL, Cooper AB, Miller S, Côté IM. 2014. Linking removal targets to the ecological effects of invaders: a predictive model and field test. Ecol. Appl. ( 10.1890/13-0979.1) [DOI] [PubMed] [Google Scholar]
- 16.Axelrod R, Hamilton WD. 1981. The evolution of cooperation . Science 211, 1390–1396. ( 10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data are provided as the electronic supplementary material.