Abstract
Dispersal dynamics have significant consequences for ecological and evolutionary processes. Previous work has demonstrated that dispersal can be context-dependent. However, factors affecting dispersal are typically considered in isolation, despite the probability that individuals make dispersal decisions in response to multiple, possibly interacting factors. We examined whether two ecological factors, predation risk and intraspecific competition, have interactive effects on dispersal dynamics. We performed a factorial experiment in mesocosms using backswimmers (Notonecta undulata), flight-capable, semi-aquatic insects. Emigration rates increased with density, and increased with predation risk at intermediate densities; however, predation had minimal effects on emigration at high and low densities. Our results indicate that factorial experiments may be required to understand dispersal dynamics under realistic ecological conditions.
Keywords: dispersal, predator–prey, Notonecta, context-dependent dispersal
1. Introduction
Dispersal plays a central role in many ecological and evolutionary processes [1]. Theory suggests that dispersal evolves to balance the costs of dispersal versus philopatry across a range of social and environmental contexts [2,3]. Empirical studies have demonstrated a dependence of dispersal on a number of local ecological factors, including predation risk, competition and habitat quality (reviews in [2,4,5]).
Despite evidence suggesting that organisms use multiple cues to make dispersal decisions (e.g. [6]), most previous studies have manipulated single factors. However, any one factor may interact with either internal or external cues in their effects on dispersal. For example, the presence of predators can eliminate the effects of personality on dispersal propensity in mosquitofish [7]. Context-dependent dispersal may also be modified by interactions in an animal's response to multiple external cues. If these multiple ecological factors have interactive, rather than additive effects, then understanding dispersal responses to environmental cues requires a factorial design. Moreover, contradictory results from single factor studies may be the result of unrecognized interactions. For example, the measured effect of competition on dispersal has been inconsistent in magnitude and even sign. As predicted by theory [2], observed dispersal rates generally exhibit positive density dependence [8,9], yet other studies report no density dependence [10], or even negative density dependence [11–13]. Negative density dependence could result from Allee effects in some cases [2,5], but not in species where individuals are solitary (e.g. [12]). An alternative hypothesis for the inconsistency in the magnitude and sign of density-dependent dispersal is that other factors interact with density to influence dispersal [2,14,15].
Predator-induced dispersal has been demonstrated in a wide variety of taxa [16,17]. Although the response to predators is usually an increase in dispersal rates, these effects may depend on prey density through predator-induced behavioural responses, including decreased foraging rates and increased refuge use [18]. Reduced foraging may ameliorate competition for food [19], while increased refuge use may intensify competition within refuges [20]. Moreover, in the absence of predator numerical responses, predation risk will depend on prey density, and predator functional responses often result in nonlinear relationships between prey density and predation risk. Thus, an interactive effect of density and predation on dispersal may be common.
Here, we tested the hypothesis that competition and predation risk interact in their effect on emigration rates in the flight-capable, semi-aquatic insect, Notonecta undulata (Heteroptera: Notonectidae). We conducted a factorial experiment in pond mesocosms in which we manipulated density and predation risk. We predicted that the relationship between density and emigration rate would depend on the level of predation risk.
2. Material and methods
Notonecta spp. are semi-aquatic insects that complete their entire life cycle in the aquatic environment, but can disperse by flight among ponds as adults. Notonecta undulata is generally associated with fishless ponds but can coexist with fishes, including the pumpkinseed sunfish, Lepomis gibbosus, which we used as the predator in our experiment. Lepomis gibbosus readily consumes N. undulata adults in the laboratory [21].
We tested whether N. undulata exhibits density-dependent dispersal, and whether the sign or strength of density dependence varied among different levels of predation risk. We performed a mesocosm experiment at the Koffler Scientific Reserve (KSR) in Ontario, Canada in July–August 2012 that manipulated predation risk and notonectid density to assess whether either of these factors plus their interaction influenced emigration rates.
We filled 30 cattle tanks (378 l; 1.35 × 0.79 × 0.64 m) with water, a standard volume of leaves, artificial vegetation to provide structure and a standard inoculation of zooplankton as food for notonectids. All tanks had fish cages consisting of a 5 l plastic basket with a Styrofoam lid, covered in 1 mm mesh screening. In predator treatments, these cages allow notonectids to receive visual and olfactory cues signalling the presence of a predator without being consumed. In predator absent treatments, empty cages controlled for the presence of this structure. A piece of 70% shade cloth covered approximately one-third of each tank to keep the water cool, while allowing notonectids to disperse.
We collected adult notonectids from a fishless pond at KSR and kept them in covered holding tanks at densities of approximately 100 insects per 378 l tank for 1–9 days (see the electronic supplementary material, Appendix). We collected adult fish (standard length = 17.3 ± 2.0 cm) from a different KSR pond and caged fish individually in half of the experimental tanks. After 24 h, we randomly assigned notonectids to experimental tanks. We crossed notonectid density with the presence of fish in a 3 × 2 factorial design. Tanks received 19, 38 or 60 notonectids to produce tanks with low, medium or high notonectid densities. These densities fall within the natural range for this species ([22]; see the electronic supplementary material, Appendix). Each density × fish treatment was replicated five times. We fed fish one cube of frozen bloodworms and one live notonectid per day.
Tanks were left uncovered for 12 days to allow notonectids to disperse. We estimated emigration rates by counting the number of notonectids remaining in each tank. Because counts could not be made on all 30 tanks in 1 day, we divided tanks into three time blocks, and counted the notonectids within these blocks on three consecutive days. Time blocks 1, 2 and 3 contained 12, 12 and 6 tanks, respectively, with each treatment equally represented in each block. We counted all remaining notonectids on two separate occasions: on the first 3 days of the experiment (first round) and again on the last 3 days (last round).
In order to avoid conflating mortality with emigration, we recorded the number of dead notonectids and removed them from the analysis. Even deaths owing to cannibalism could be accounted for in this way because notonectids consume only the insides of their prey, leaving the exoskeletons intact.
Immigration of experimental or wild notonectids into cattle tanks was possible but probably uncommon because in similar experiments on the same species, only a small proportion of experimental (3–5%) or wild (less than 0.01% of the experimental population size) notonectids did so [17].
(a). Statistical analysis
We used a generalized linear model with a quasi-binomial error distribution and a logit link (i.e. logistic regression with overdispersion) to model the effects of fish presence, notonectid density, block and all possible interactions on dispersal status (emigrated or philopatric). Since there was no replication of treatments in time block 3, we excluded it from the analysis in order to evaluate the effect of time block. We found no significant effect of block; we therefore dropped block from all future analyses.
Using the entire dataset, we then analysed the effects of fish presence and notonectid density on dispersal status using logistic regression with overdispersion, followed by independent contrasts. Notably, the results did not differ qualitatively whether block 3 was included or not. All analyses were performed in JMP v. 11.0.0.
3. Results
We only present the results from the last round here, but results for the first were similar (see the electronic supplementary material, Appendix).
The probability of emigration increased significantly with density (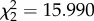 , p = 0.0003), but there was no significant main effect of predation risk (
, p = 0.0003), but there was no significant main effect of predation risk (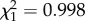 , p = 0.318). However, there was a strong and significant interaction between fish presence and notonectid density on the probability of emigration (
, p = 0.318). However, there was a strong and significant interaction between fish presence and notonectid density on the probability of emigration (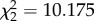 , p = 0.006; figure 1).
, p = 0.006; figure 1).
Figure 1.
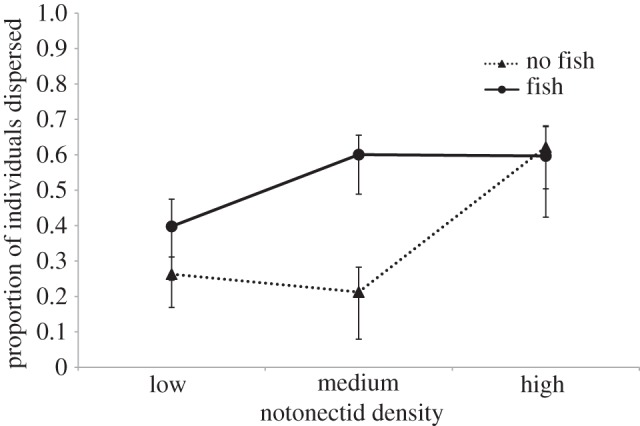
Mean proportion of individuals that emigrated from the tanks by the end of the experiment ±s.e. Low, medium, and high density tanks contained 19, 38 and 60 notonectids, respectively.
Independent contrasts demonstrate that the presence of a predator increased emigration significantly only at medium notonectid density (medium density/fish versus medium density/no fish: 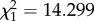 , p = 0.0002), which accounts for the interaction between density and predation risk.
, p = 0.0002), which accounts for the interaction between density and predation risk.
4. Discussion
Our results demonstrate that emigration of N. undulata depended upon density and the interaction between density and predation (figure 1). Predation risk, which was previously shown to affect emigration in this species [17], only increased emigration at intermediate densities. Overall, emigration rates exhibited positive density dependence, which is consistent with the hypothesis that individuals disperse to avoid competition. However, the relationship between density and dispersal was nonlinear (see also [13,23]). The shape of this relationship suggests a density threshold for dispersal in this species. Density thresholds represent the density at which individuals switch from being philopatric to dispersive. Here, we provide evidence for a density threshold that is dependent on predation risk. In fish tanks, the relationship between density and dispersal is shifted to the left, so that lower densities are required to induce dispersal (figure 1). This is consistent with previous theoretical work showing that when predation risk is temporally autocorrelated, as it is in the Notonecta system, patches with predators should evolve lower density thresholds for dispersal [24]. These results suggest that we cannot understand the effects of density or predation risk on dispersal without considering their interactive effects.
We focused on one stage of dispersal, emigration; however, other stages of dispersal (transience and immigration) may be differentially affected by these factors and their interaction. While predation risk generally increases emigration rates in adults, juvenile exposure to predators may reduce foraging rates or cause utilization of suboptimal foraging habitat, reducing adult condition [20,25]. Adults in poor condition may have a reduced probability of successful transience and immigration [26], further complicating our estimation of the effects of ecological factors on dispersal dynamics. We propose that future research should use multi-factor experiments to investigate dispersal dynamics in multiple ecological contexts and through the entire dispersal process.
Our results suggest that multifaceted behavioural responses to local conditions have the potential to shape patterns of connectivity at regional scales. Understanding the effects of ecological factors at these scales requires an understanding of the roles that interactions between these ecological factors play in dispersal.
Supplementary Material
Acknowledgements
We thank Zoryana Gorin for help in the field, and two anonymous reviewers for helpful comments that improved this manuscript.
The experiment was approved by the University of Toronto's Bioscience Local Animal Care Committee.
Data accessibility
Data from the Dryad Digital Repository: doi:10.5061/dryad.j8b02.
Funding statement
Funding was provided by a University of Toronto Excellence Award to CBB and grants to LR from the Canada Research Chairs Program and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Dieckmann U, O'Hara B, Weisser W. 1999. The evolutionary ecology of dispersal. Trends Ecol. Evol. 14, 88–90. ( 10.1016/s0169-5347(98)01571-7) [DOI] [Google Scholar]
- 2.Bowler DE, Benton TG. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225. ( 10.1017/s1464793104006645) [DOI] [PubMed] [Google Scholar]
- 3.Starrfelt J, Kokko H. 2012. The theory of dispersal under multiple influences. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM.), pp. 19–26. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M. 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209. ( 10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- 5.Bonte D, et al. 2012. Costs of dispersal. Biol. Rev. 87, 290–312. ( 10.1111/j.1469-185X.2011.00201.x) [DOI] [PubMed] [Google Scholar]
- 6.Mehrparvar M, Zytynska SE, Weisser WW. 2013. Multiple cues for winged morph production in an aphid metacommunity. PLoS ONE 8, e58323 ( 10.1371/journal.pone.0058323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cote J, Fogarty S, Tymen B, Sih A, Brodin T. 2013. Personality-dependent dispersal cancelled under predation risk. Proc. R. Soc. B 280, 20132349 ( 10.1098/rspb.2013.2349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson B. 1965. Wing polymorphism in aphids. II. Interaction between aphids. Ent. Exp. Appl. 8, 49–64. ( 10.1111/j.1570-7458.1965.tb02342.x) [DOI] [Google Scholar]
- 9.De Meester N, Bonte D. 2010. Information use and density-dependent emigration in an agrobiont spider. Behav. Ecol. 21, 992–998. ( 10.1093/beheco/arq088) [DOI] [Google Scholar]
- 10.Lecomte J, Boudjemadi K, Sarrasin F, Cally K, Clobert J. 2004. Connectivity and homogenisation of population sizes: an experimental approach in Lacerta vivipara. J. Anim. Ecol. 73, 179–189. ( 10.1111/j.1365-2656.2004.00796.x) [DOI] [Google Scholar]
- 11.Kuussaari M, Nieminen M, Hanski I. 1996. An experimental study of migration in the Glanville fritillary butterfly Melitaea cinxia. J. Anim. Ecol. 65, 791–801. ( 10.2307/5677) [DOI] [Google Scholar]
- 12.Roland J, Keyghobadi N, Fownes S. 2000. Alpine Parnassus butterfly dispersal: effects of landscape and population size. Ecology 81, 1642–1653. ( 10.2307/177313) [DOI] [Google Scholar]
- 13.Ims RA, Andreassen HP. 2005. Density-dependent dispersal and spatial population dynamics. Proc. R. Soc. B 272, 913–918. ( 10.1098/rspb.2004.3025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ims RA, Hjermann DO. 2001. Condition-dependent dispersal. In Dispersal (eds Clobert J, Danchin E, Dhondt AA, Nichols JD.), pp. 203–216. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Galliard JFL, Ferriere R, Clobert J. 2003. Mother–offspring interactions affect natal dispersal in a lizard. Proc. R. Soc. Lond. B 270, 1163–1169. ( 10.1098/rspb.2003.2360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronin JT, Haynes KJ, Dillemuth F. 2004. Spider effects on planthopper mortality, dispersal, and spatial population dynamics. Ecology 85, 2134–2143. ( 10.1890/03-0591) [DOI] [Google Scholar]
- 17.McCauley SJ, Rowe L. 2010. Notonecta exhibit threat-sensitive, predator-induced dispersal. Biol. Lett. 6, 449–452. ( 10.1098/rsbl.2009.1082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 19.Peacor SD, Werner EE. 2001. The contribution of trait-mediated indirect effects to the net effects of a predator. Proc. Natl Acad. Sci. USA 98, 3904–3908. ( 10.1073/pnas.071061998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner EE, Gilliam JF, Hall DJ, Mittelbach GG. 1983. An experimental test of the effects of predation risk on habitat use in fish. Ecology 64, 1540–1548. ( 10.2307/1937508) [DOI] [Google Scholar]
- 21.Cook WL, Streams FA. 1984. Fish predation on Notonecta (Hemiptera): relationship between prey risk and habitat utilization. Oecologia 64, 177–183. ( 10.1007/BF00376868) [DOI] [PubMed] [Google Scholar]
- 22.Bennett DV, Streams FA. 1986. Effects of vegetation on Notonecta (Hemiptera) distribution in ponds with and without fish. OIKOS 46, 62–69. ( 10.2307/3565381) [DOI] [Google Scholar]
- 23.Gates CC, Larter NC. 1990. Growth and dispersal of an erupting large herbivore population in Northern Canada: the Mackenzie Wood bison (Bison bison athabascae). Arctic 43, 231–238. ( 10.14430/arctic1616) [DOI] [Google Scholar]
- 24.Poethke HJ, Weisser WW, Hovestadt T. 2010. Predator-induced dispersal and the evolution of conditional dispersal in correlated environments. Am. Nat. 175, 577–586. ( 10.1086/651595) [DOI] [PubMed] [Google Scholar]
- 25.Johansson F, Stoks R, Rowe L, De Block M. 2001. Life history plasticity in a damselfly: effects of combined time and biotic constraints. Ecology 82, 1857–1869. ( 10.1890/0012-9658(2001)082[1857:LHPIAD]2.0.CO;2) [DOI] [Google Scholar]
- 26.Stamps JA. 2006. The silver spoon effect and habitat selection by natal dispersers. Ecol. Lett. 9, 1179–1185. ( 10.1111/j.1461-0248.2006.00972.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Dryad Digital Repository: doi:10.5061/dryad.j8b02.


