Abstract
Batesian mimicry evolves when individuals of a palatable species gain the selective advantage of reduced predation because they resemble a toxic species that predators avoid. Here, we evaluated whether—and in which direction—Batesian mimicry has evolved in a natural population of mimics following extirpation of their model. We specifically asked whether the precision of coral snake mimicry has evolved among kingsnakes from a region where coral snakes recently (1960) went locally extinct. We found that these kingsnakes have evolved more precise mimicry; by contrast, no such change occurred in a sympatric non-mimetic species or in conspecifics from a region where coral snakes remain abundant. Presumably, more precise mimicry has continued to evolve after model extirpation, because relatively few predator generations have passed, and the fitness costs incurred by predators that mistook a deadly coral snake for a kingsnake were historically much greater than those incurred by predators that mistook a kingsnake for a coral snake. Indeed, these results are consistent with prior theoretical and empirical studies, which revealed that only the most precise mimics are favoured as their model becomes increasingly rare. Thus, highly noxious models can generate an ‘evolutionary momentum’ that drives the further evolution of more precise mimicry—even after models go extinct.
Keywords: Batesian mimicry, convergent evolution, evolutionary momentum, predation, rapid evolution
1. Introduction
When selection is strong, evolutionary change can occur in natural populations rapidly enough to observe [1]. Because selection to avoid being eaten is typically strong [2], a context in which rapid evolution may readily arise is Batesian mimicry. Batesian mimicry occurs when an edible species (the ‘mimic’) evolves to resemble a conspicuous, noxious species (the ‘model’), thereby gaining protection from predation [3–5]. The degree of resemblance between mimics and their models is generally sensitive to changes in model abundance [5,6]; mimetic fidelity can decrease or increase, depending on whether the model becomes relatively more or less abundant, respectively [7,8].
How phenotypic resemblance between mimics and their models changes immediately following model extirpation is unclear, however. Three outcomes are possible. First, mimics may remain unchanged. Such an outcome might arise if, for instance, there has not been enough time for mimics to respond to changes in model abundance. Second, less precise mimicry may evolve [9,10]. Mimicry may break down following model extirpation, because local predators would no longer experience selection to recognize mimics as dangerous [6,11,12]. Third, more precise mimicry may evolve. Greater mimetic precision may evolve after model extirpation if alternative prey are abundant, and if the fitness costs associated with mistaking a model for a mimic were historically greater than those associated with mistaking a mimic for a model (as might be the case with highly noxious models). Indeed, theoretical [13–15] and empirical studies [7,8] have shown that only the most precise mimics receive protection from predation when the model becomes increasingly rare (as would be expected to occur when model extirpation is imminent); thus, selection may continue to favour the evolution of more refined mimicry, even after the model is gone.
Here, we focus on a well-studied mimicry complex to evaluate whether and how Batesian mimicry evolved following extirpation of the model.
2. Material and methods
(a). Study system
Non-venomous scarlet kingsnakes (Lampropeltis elapsoides) resemble venomous coral snakes (Micrurus fulvius; figure 1a,b). Although both species co-occur in the southeastern US, L. elapsoides also occurs further north (figure 1c). Field experiments have found that natural predators avoid Plasticine replicas of L. elapsoides in sympatry with M. fulvius but not in these northern allopatric regions [6], verifying that L. elapsoides are Batesian mimics of M. fulvius. Moreover, even naive sympatric predators avoid coral snake patterns [16].
Figure 1.
(a) Non-venomous scarlet kingsnakes, Lampropeltis elapsoides, are Batesian mimics of (b) highly venomous eastern coral snakes, Micrurus fulvius. (c) Historically, M. fulvius and L. elapsoides co-occurred in the North Carolina Sandhills (as shown here). Around 1960, however, M. fulvius was apparently extirpated from this region, but not from the Florida panhandle. (Online version in colour.)
Historically, M. fulvius reached its northernmost limit in the North Carolina Sandhills [17], a 3900 square kilometres area of gently rolling, sand-covered hills characterized by longleaf pine savannah (figure 1c). Local predators include black bears (Ursus americanus), bobcats (Lynx rufus), coyotes (Canis latrans), foxes (Vulpes and Urocyon sp.), raccoons (Procyon lotor), hawks (Buteo sp.), kestrels (Falco sparverius) and loggerhead shrikes (Lanius ludovicianus).
Micrurus fulvius has always been considered rare in the Sandhills (only five specimens exist in museums; see the electronic supplementary material), and no recent records exist [17]. Indeed, no specimens have been collected in the Sandhills since 1960 (see the electronic supplementary material), despite extensive activity there by herpetologists [18]. Thus, although the causes are unknown, M. fulvius has apparently been extirpated from the Sandhills (or, at the very least, they are so rare that they are functionally extirpated). By contrast, L. elapsoides are common in the Sandhills [17].
Interestingly, the L. elapsoides that most closely resemble Micrurus occur in sympatric populations near the sympatry/allopatry border (i.e. ‘edge sympatry’) [7]. Field experiments have shown that selection for mimicry is strongest in edge sympatry [8]. Because the model is rare in edge sympatry (see above), the probability of mistakenly attacking it is low, and predators are therefore more willing to risk attacking imprecise mimics. Consequently, only precise mimics are favoured in such edge sympatric regions as the Sandhills [7].
(b). Data collection and analysis
To determine whether and how mimicry changed over time, we compared five pre-extirpation M. fulvius to 27 post-extirpation L. elapsoides from the Sandhills (too few pre-extirpation L. elapsoides were available for analysis). These L. elapsoides were collected in the 1970s (n = 5 individuals), 1980s (n = 5), 1990s (n = 3), 2000s (n = 11) and 2010s (n = 3; the electronic supplementary material). Specimens were photographed using a digital camera (Canon PowerShot SX110; Canon zoom lens, 6.0–60.0 mm, 1 : 2.8–4.3); the width of each ring was measured from digital images using ImageJ v. 1.46 [19]. We then calculated the proportions of red and black on the mid-dorsum of each snake from its snout to its cloaca. Previous work showed that these characteristics changed the most as the mimetic pattern breaks down in allopatry [10,11] and that these characteristics are targets of predator-mediated selection [7,8].
We combined the mean proportion of dorsum red and black on mimics and models into a common principal component score. We then subtracted the mean PC1 score for M. fulvius from the PC1 score for each individual mimic to calculate a mimic–model dissimilarity score (where a score of zero indicates that L. elapsoides and M. fulvius were identical in proportion of red and black; for example calculation, see the electronic supplementary material). Using JMP v. 10.0.1, we regressed the dissimilarity score of each L. elapsoides against the year it was sampled to determine whether resemblance between L. elapsoides and M. fulvius changed over time (one outlier was omitted from analysis).
Next, we sought to control for the possibility that any change in L. elapsoides colour pattern might reflect not predator-mediated selection favouring mimicry, but some other agent of selection (e.g. a change in light environment following recent anthropogenic changes in habitat). We did so in two ways. First, we assessed whether phenotypic changes similar to those observed among L. elapsoides from the Sandhills were observed among L. elapsoides from the Florida panhandle, where M. fulvius remains abundant (figure 1c). This region is similar to the Sandhills in habitat; moreover, the assemblage of predators is similar across regions. Using the methods above, we compared 23 M. fulvius and 23 L. elapsoides from the Florida panhandle. The L. elapsoides were collected in the 1970s (n = 13 individuals), 1980s (n = 1), 1990s (n = 2), 2000s (n = 7; electronic supplementary material). Second, we assessed whether similar phenotypic changes occurred in corn snakes, Pantherophis guttatus, a non-mimetic species found in the Sandhills. Like L. elapsoides, P. guttatus has red and black on its dorsum, but its pattern is characterized by blotches, not rings. Using the methods above, we sampled 82 P. guttatus that were collected in the 1970s (n = 5 individuals), 1980s (n = 14), 1990s (n = 18), 2000s (n = 41) and 2010s (n = 4; electronic supplementary material); these specimens were compared with the five M. fulvius from the Sandhills (see above).
3. Results
In 50 years following the apparent extirpation of M. fulvius, L. elapsoides from the North Carolina Sandhills became more similar to the former in colour pattern (F1,26 = 6.997; p = 0.014; figure 2a). Moreover, these L. elapsoides became less variable in colour pattern (Spearman correlation between coefficient of variation in dissimilarity score and decade sampled = −0.8; n = 5 decades; p = 0.05 (one-tailed test)). By contrast, L. elapsoides from Florida did not change significantly in mimic–model dissimilarity (F1,22 = 1.417; p = 0.247; figure 2b), nor did P. guttatus, a non-mimetic species from the North Carolina Sandhills (F1,81 = 1.028; p = 0.314; figure 2c).
Figure 2.
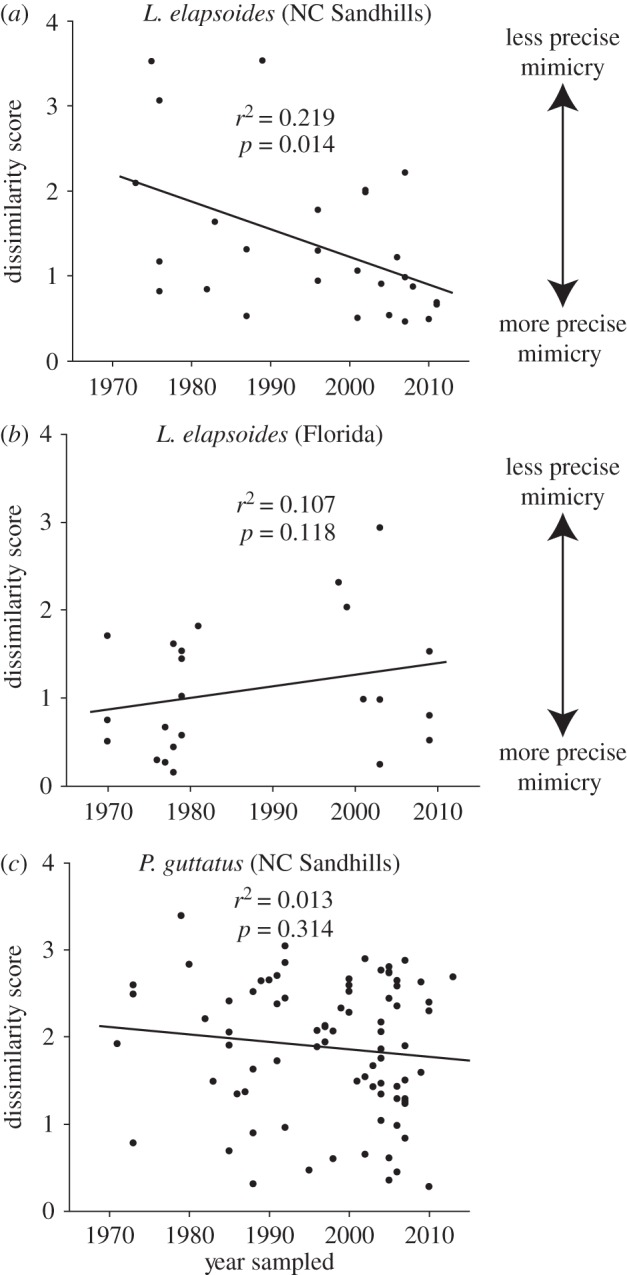
(a) Over the past four decades, L. elapsoides from the Sandhills (where M. fulvius became extirpated around 1960) have become more similar to M. fulvius. By contrast, no such trend was found among (b) L. elapsoides from the Florida panhandle (where M. fulvius remains abundant) or (c) P. guttatus (a non-mimetic species) from the Sandhills.
4. Discussion
Theory predicts that mimicry should break down in the absence of models [5]. Indeed, mimicry in L. elapsoides breaks down where it occurs in allopatry with its model [10]. However, instead of observing an erosion of mimicry following extirpation of M. fulvius from the North Carolina Sandhills, we observed rapid evolution of more precise mimicry (figure 2a). No such pattern was detected among L. elapsoides from Florida, where the model has not been extirpated (figure 2b), nor among a non-mimetic species from the Sandhills (figure 2c).
Two lines of evidence suggest that precise mimicry has evolved in the Sandhills. First, snakes were sampled over a 38 year interval, twice the maximum lifespan of L. elapsoides [20]. Thus, changes occurred across generations. Second, these changes are unlikely to reflect phenotypic plasticity: there is no evidence of plasticity in L. elapsoides coloration [21]. Thus, our data (figure 2a) appear to reflect evolutionary change.
This rapid evolution of precise mimicry is consistent with theoretical and empirical studies. Theory predicts that selection for mimetic precision should increase as models become scarcer [13–15], as would probably have occurred in the Sandhills. Additionally, field experiments recently conducted in this population revealed that free-ranging predators only avoid precise (but not imprecise) L. elapsoides mimics [7,8]. Thus, predators in the Sandhills continue to exert strong selection for more precise mimicry.
Presumably the generalist predators in the Sandhills [17] are likely to pay a low cost of passing up a palatable meal by mistaking a mimic for a model. By contrast, because M. fulvius are highly venomous [22], prior to 1960 (when M. fulvius were extirpated), predators were likely to have paid a high cost for mistaking a model for a mimic. This asymmetry in fitness costs explains the strong selection to avoid the model and its lookalikes.
Eventually, however, mimicry should break down. How rapidly it does so depends on such factors as the generation times of predators and mimics, the standing variation in coloration among mimics, gene flow between mimics in sympatry versus allopatry [10], and the intensity of selection against mimics.
In sum, our data suggest that, paradoxically, selection imposed on mimics by predators can generate an ‘evolutionary momentum’ towards more precise mimicry—even after models go extinct.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Karin Pfennig, Bryan Stuart and two anonymous referees for comments, Cody Porter for help collecting data, the museums listed in the electronic supplementary material for access to their specimens, and the NSF for support.
References
- 1.Hendry AP, Kinnison MT. 1999. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution 53, 1637–1653. ( 10.2307/2640428) [DOI] [PubMed] [Google Scholar]
- 2.Mallet J, Barton NH. 1989. Strong natural selection in a warning color hybrid zone. Evolution 43, 421–431. ( 10.2307/2409217) [DOI] [PubMed] [Google Scholar]
- 3.Bates HW. 1862. Contributions to an insect fauna of the Amazon valley (Lepidoptera: Heliconidae). Trans. Linn. Soc. Lond. 23, 495–556. ( 10.1111/j.1096-3642.1860.tb00146.x) [DOI] [Google Scholar]
- 4.Forbes P. 2009. Dazzled and deceived: mimicry and camouflage. New Haven, CT: Yale University Press. [Google Scholar]
- 5.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals & mimicry. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Pfennig DW, Harcombe WR, Pfennig KS. 2001. Frequency-dependent Batesian mimicry. Nature 410, 323 ( 10.1038/35066628) [DOI] [PubMed] [Google Scholar]
- 7.Harper GR, Jr, Pfennig DW. 2007. Mimicry on the edge: why do mimics vary in resemblance to their model is different parts of their geographical range? Proc. R. Soc. B 274, 1955–1961. ( 10.1098/rspb.2007.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi DW, Pfennig DW. 2010. High-model abundance may permit the gradual evolution of Batesian mimicry: an experimental test. Proc. R. Soc. B 277, 1041–1048. ( 10.1098/rspb.2009.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brower JV. 1960. Experimental studies in mimicry. IV. The reactions of starlings to different proportions of models and mimics. Am. Nat. 94, 271–282. ( 10.1086/282128) [DOI] [Google Scholar]
- 10.Harper GR, Pfennig DW. 2008. Selection overrides gene flow to break down maladaptive mimicry. Nature 451, 1103–1106. ( 10.1038/nature06532) [DOI] [PubMed] [Google Scholar]
- 11.Pfennig DW, Harper GR, Jr, Brumo AF, Harcombe WR, Pfennig KS. 2007. Population differences in predation on Batesian mimics in allopatry with their model: selection against mimics is strongest when they are common. Behav. Ecol. Sociobiol. 61, 505–511. ( 10.1007/s00265-006-0278-x) [DOI] [Google Scholar]
- 12.Pfennig DW, Mullen SP. 2010. Mimics without models: causes and consequences of allopatry in Batesian mimicry complexes. Proc. R. Soc. B 277, 2577–2585. ( 10.1098/rspb.2010.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan CJ, Sheppard PM. 1963. Continuous and quantal theories of sensory discrimination. Proc. R. Soc. Lond. B 158, 343–363. ( 10.1098/rspb.1963.0052) [DOI] [PubMed] [Google Scholar]
- 14.Oaten A, Pearce CE, Smyth ME. 1975. Batesian mimicry and signal detection theory. Bull. Math. Biol. 37, 367–387. ( 10.1007/BF02459520) [DOI] [PubMed] [Google Scholar]
- 15.Sherratt TN. 2002. The evolution of imperfect mimicry. Behav. Ecol. 13, 821–826. ( 10.1093/beheco/13.6.821) [DOI] [Google Scholar]
- 16.Smith SM. 1975. Innate recognition of coral snake pattern by a possible avian predator. Science 187, 759–760. ( 10.1126/science.187.4178.759) [DOI] [PubMed] [Google Scholar]
- 17.Palmer WM, Braswell AL. 1995. Reptiles of North Carolina. Chapel Hill, NC: University of North Carolina Press. [Google Scholar]
- 18.Beane JC, Graham SP, Thorp TJ, Pusser LT. 2014. Natural history of the southern hognose snake (Heterodon simus) in North Carolina, USA. Copeia 2014, 168–174. ( 10.1643/CH-13-044) [DOI] [Google Scholar]
- 19.Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophoton. Int. 11, 36–42. [Google Scholar]
- 20.Isberg T. 2002. Lampropeltis triangulum. Animal Diversity Web See http://animaldiversity.ummz.umich.edu/accounts/Lampropeltis_triangulum/ (accessed 30 October 2013).
- 21.Kikuchi DW, Pfennig DW. 2012. A Batesian mimic and its model share color production mechanisms. Curr. Zool. 58, 657–666. [Google Scholar]
- 22.Roze JA. 1996. Coral snakes of the Americas: biology, identification, and venoms. Malabar, FL: Krieger Publishing Company. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



