Abstract
In species where females mate promiscuously, competition between ejaculates from different males to fertilize the ova is an important selective force shaping many aspects of male reproductive traits, such as sperm number, sperm length and sperm–sperm interactions. In eusocial Hymenoptera (bees, wasps and ants), males die shortly after mating and their reproductive success is ultimately limited by the amount of sperm stored in the queen's spermatheca. Multiple mating by queens is expected to impose intense selective pressure on males to optimize the transfer of sperm to the storage organ. Here, we report a remarkable case of cooperation between spermatozoa in the desert ant Cataglyphis savignyi. Males ejaculate bundles of 50–100 spermatozoa. Sperm bundles swim on average 51% faster than solitary sperm cells. Team swimming is expected to increase the amount of sperm stored in the queen spermatheca and, ultimately, enhance male posthumous fitness.
Keywords: sexual selection, sperm cooperation, ants
1. Introduction
Sperm competition is recognized as a main determinant of male fitness and has led to a number of evolutionary adaptations in sperm phenotype, such as increased sperm production, higher sperm viability or improved sperm motility [1–3]. In eusocial bees, wasps and ants, reproductive activity is typically concentrated into a brief mating flight early at sexual maturation [4]. Males die soon after mating but sperm can survive for long periods (up to 20–30 years) when stored in the queen storage organ, the spermatheca. Polyandry, i.e. females mating with more than one male, has evolved in a number of species [5] and opportunities for postcopulatory sexual conflict through sperm competition have repeatedly emerged [6–8].
In Cataglyphis desert ants, queens from most species mate with high promiscuity [9]. They copulate with different males in quick succession and store their sperm jointly in the spermatheca. Sperm competition for access to the storage organ is expected to be particularly intense in the highly polyandrous species Cataglyphis savignyi, where queens can mate with up to 14 males (average number of matings = 9.25 ± 0.99 [10]). While investigating the effect of sperm competition on sperm traits in the ant C. savignyi, we found that males ejaculate highly motile sperm bundles.
2. Material and methods
(a). Sperm sampling and staining
Colonies of C. savignyi were excavated from the Arad desert (Israel) and reared under laboratory conditions. Males were marked upon emergence to control for age and reared until sexual maturation (28 days). Mature males were killed by chloroform, and ejaculation was provoked by squeezing the abdomen until semen appeared at the tip of the endophallus.
Ejaculates (2 µl) were diluted in 100 µl Ham's F-10 (Sigma-Aldrich) and spread onto microscope slides. Slides were air-dried for 10 min, fixed for 15 min in absolute methanol at −20°C and rinsed twice in PBS before incubation with Pisum sativum agglutinin (PSA)-FITC (Sigma-Aldrich) 30 µg ml−1 in PBS, for 30 min at room temperature. Slides were rinsed in distilled water for 10 min and mounted with ProLong Gold antifade with DAPI (Life Technologies). They were examined with a Nikon Eclipse 50i microscope equipped with an epifluorescence device.
For electron microscopy, sperm samples were fixed overnight at 4°C in 2.5% glutaraldehyde, 0.1 M cacodylate buffer (pH 7.2) and postfixed in 2% OsO4 in the same buffer. After serial dehydration in ethanol, the samples were critical-point dried and coated with platinum. Observations were made in a Tecnai FEG ESEM Quanta 200 (FEI), and images were processed with AnalySIS software.
(b). Number of sperm cells per bundle
The number of sperm cells per bundle was estimated by multiplying sperm density by the area at the base of the cap (where sperm heads are agglutinated; figure 1b). The number of sperm was calculated for 130 aggregates of different sizes as
where W is the basal width of the sperm bundle cap and d is the average sperm density per square micrometre. Both W and d (45.12 sperm µm−2) were measured from electron microscopy images.
Figure 1.
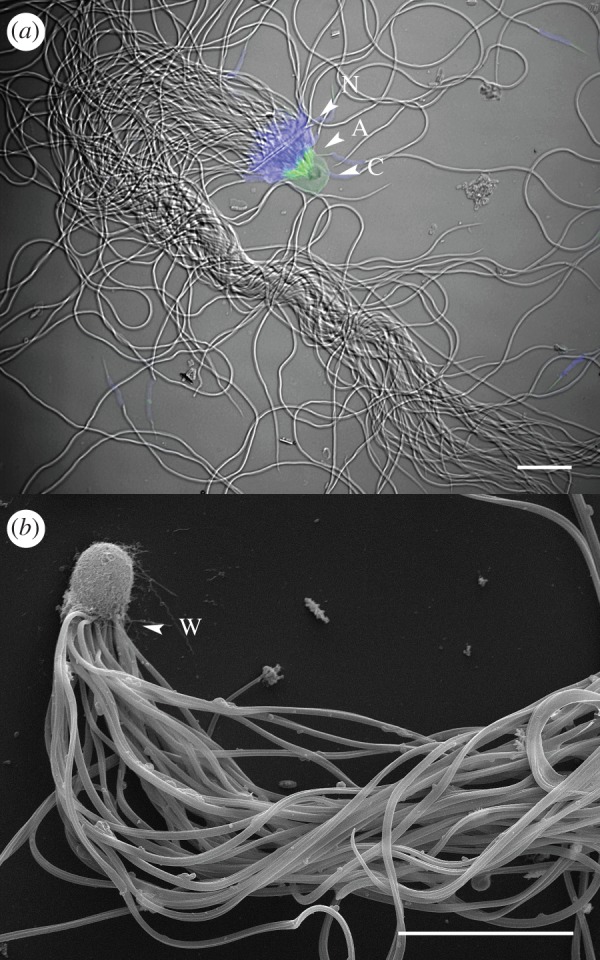
Sperm bundles ejaculated by mature males of C. savignyi. (a) PSA-FITC staining of sperm bundles. Sperm nuclei (N) are stained in blue with DAPI, while sperm acrosome (A) and bundle cap (C) stain with PSA-FITC (green) (Picture: L. Twyffels). Scale bar, 10 µm. (b) Scanning electron micrograph of a sperm bundle showing the cap and the sperm flagella. The white arrow (W) designates the base of the cap (Picture: D. Monteyne and D. Perez-Morga). Scale bar, 10 µm.
(c). Sperm velocity
To measure sperm velocity, 2 µl semen was diluted in 50 µl Ham's F-10 with pH (9.2) and temperature (35°C) controlled, at four different viscosity levels (0.0, 0.5, 1.5 and 4.0% polyvinyl pyrrolidone (PVP)). Males (n = 34) were assigned to different experimental groups independently of their nest of origin and date of emergence. Six microlitres of diluted ejaculate was injected in a calibrated counting chamber (sc20–01-C, Leja). Sperm movement was recorded with a Nikon Eclipse 50i microscope equipped with a digital camera. The linear distance travelled by single sperm and sperm bundles in 10 s was measured with ImageJ (http://imagej.nih.gov/ij/). Velocity measurements were realized blindly with regard to treatment (i.e. viscosity level).
All comparisons were performed with non-parametric Kruskal–Wallis tests with p-values given by a χ2 probability distribution at (k − 1) degrees of freedom [11].
3. Results
Epifluorescence microscopy revealed that most sperm cells from the ejaculate of C. savignyi males are organized in bundles. In these aggregates, several dozens of sperm cells (n = 73.2 ± 20.0, N = 130 bundles; electronic supplementary material, figure S1) are oriented in the same direction and bound together with their acrosome stuck in an agglutinative cap of extracellular material (figure 1a). Glycoproteic composition of the cap is confirmed by staining with Pisum sativum agglutinin (PSA)-FITC, Masson's trichrome and periodic acid–Schiff (electronic supplementary material, figure S2a,b). Flagella remain completely free (figure 1b). Their synchronous movements propel bundles forward in a very characteristic helical pattern [12] (see the electronic supplementary material, movie S1).
Speed measurements showed that sperm bundles swim significantly faster than solitary sperm cells (n = 658, p < 0.001; figure 2a). The difference was particularly marked at high viscosity, where bundles move, on average, two times faster than solitary cells (table 1; complete dataset available in the electronic supplementary material). For isolated spermatozoa, velocity first increased at 0.5% PVP, then declined significantly with increasing viscosity. Microscope observations suggested that the lack of friction in aqueous medium (0% PVP) lowered the efficiency of sperm flagellum's helical beating. This explanation has indeed been proposed to account for the higher velocity displayed by bacteria in viscous media [13]. By contrast, motility of sperm bundles was not affected by viscosity. Overall, bundles moved 51% faster than isolated spermatozoa of the same individual (figure 2b).
Figure 2.
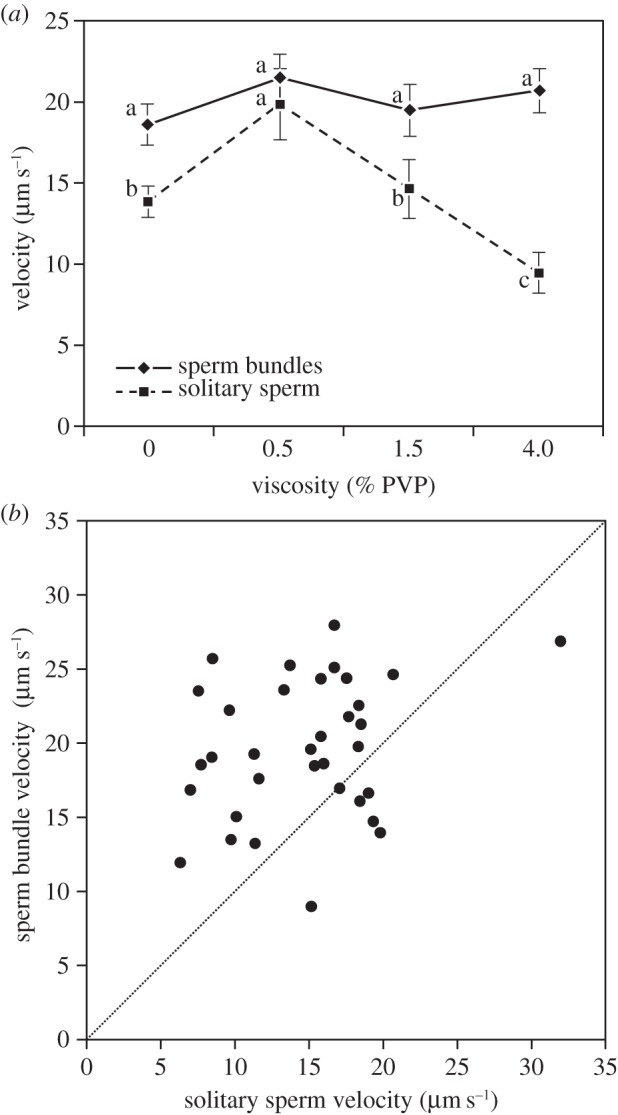
Motility of sperm bundles and solitary spermatozoa in C. savignyi. (a) Average motility of sperm bundles (solid line) and solitary spermatozoa (dotted line) with increasing viscosity of medium (PVP addition, in per cent). Sperm bundles display significantly greater average velocity than solitary spermatozoa (Kruskal–Wallis χ2 = 114.13, d.f. = 1, p < 0.001). Treatments with different lower case letters differed significantly (after Bonferroni correction, Kruskal–Wallis χ2). See table 1 for numeric values and sample sizes. (b) Mean sperm bundle velocity versus solitary sperm velocity for each male. Dotted line indicates a similar velocity for bundles and solitary spermatozoa. Sperm bundles display higher velocity for 27 males (above the line) out of 34.
Table 1.
Sperm bundle and solitary sperm velocity. Mean velocity ± s.e. (µm s−1) of sperm bundles and solitary spermatozoa at four viscosity levels (n, number of males sampled; the total number of sperm bundles or solitary sperm analysed is given in parentheses).
| 0.0% PVP n = 17 |
0.5% PVP n = 6 |
1.5% PVP n = 6 |
4.0% PVP n = 5 |
|
|---|---|---|---|---|
| sperm bundle | 18.75 ± 1.26 (137) | 21.66 ± 1.46 (41) | 19.60 ± 1.60 (67) | 20.72 ± 1.26 (40) |
| solitary sperm | 14.08 ± 0.96 (188) | 20.06 ± 2.19 (46) | 14.89 ± 1.81 (80) | 9.67 ± 1.36 (55) |
Histological examinations revealed no bundles in the sperm storage organ of mature queens (N = 5). This indicates that sperm bundles disband in or before entering the spermatheca.
4. Discussion
Sperm swimming speed has been recognized as a major determinant of fertilization success in various internal and external fertilizing taxa (e.g. mammals [14], birds [15] and fishes [16]). Similarly, enhanced swimming ability conferred by sperm cooperation potentially represents a selective advantage for C. savignyi males in the context of competition for access to the female sperm storage organ. In line with this, a recent study in ants has shown that fertilization success is proportional to the respective contribution of each male [17]. Estimating the fitness benefits of ejaculating sperm in bundles in terms of the proportion of offspring sired by C. savignyi males awaits further study.
Evidence for increased sperm velocity induced by cooperation between spermatozoa remains rare across vertebrates and invertebrates. In the grey short-tailed opossum, Monodelphis domestica, most sperm cells mature in pairs, which display greater velocity than solitary sperm [18]. In the promiscuous wood mouse, Apodemus sylvaticus, the falciform head of each spermatozoa allows them to intertwine to other spermatozoa, forming motile ‘trains’ of several hundred cells. These ‘trains’ exhibit increased swimming velocity and thrusting power compared with individual sperm in highly viscous media [19]. In the fishfly Parachauliodes japonicus, hundreds of sperm cells are bound together in very large bundles whose swimming speed is correlated with the number of spermatozoa [20].
Sperm bundles, also referred to as ‘spermatodesmata’, have been documented in testes and seminal vesicles of immature males of various Hymenoptera, including sawflies [21], bees [22,23] and ants [24]. Nevertheless, aggregates in these taxa are extremely short lived: they usually break up before sexual maturation, so that only free spermatozoa are found in seminal vesicles at the time of mating. This contrasts with our observations of mature males of C. savignyi showing that sperm bundles are still present and highly motile at the mating flight period. Interestingly, sperm bundles were recently reported in seminal vesicles of mature males of the ant Lasius pallitarsis [25], suggesting that sperm cooperation might also occur in other ants.
A convergent adaptive response to sperm competition may exist in other eusocial Hymenoptera that have evolved from single to multiple queen-mating. Males of Hymenoptera being haploid, their germinal cells do not undergo meiosis and all spermatozoids of a given father are genetically identical. Compared to sibling sperm from diploid organisms, which share half of their genes, the fitness interest of clonal spermatozoa is perfectly aligned. This close genetic relatedness predisposes cooperation between a male's sperm cells to gain an advantage when sperm competition is intense [26]. In C. savignyi, mating promiscuity by queens and the absence of re-mating opportunity for males must impose severe selection on males to maximize the amount of their own sperm reaching and being stored in the queen spermatheca. By ejaculating sperm in bundles, males potentially benefit from increased velocity conferred by team swimming and, ultimately, enhance their posthumous fitness.
Supplementary Material
Supplementary Material
Acknowledgments
We thank G. Vechs, D. Perez-Morga, D. Monteyne and L. Twyffels for providing microscopy images, and G. Parker, B. Baer, B. Gassner and R. Gadagkar for their comments on the manuscript.
Funding statement
This work was supported by the Belgian FRS-FNRS grant no. 1.5.088.10F (S.A.) and 1.B211.11F (M.P.) and the ULB grant no. ARC 2010–2015 #5 (S.A.).
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. London, UK: Academic Press. [Google Scholar]
- 3.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Wilson EO. 1971. The insect societies. Cambridge, UK: Belknap Press. [Google Scholar]
- 5.Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. 2008. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320, 1213–1216. ( 10.1126/science.1156108) [DOI] [PubMed] [Google Scholar]
- 6.Boomsma JJ, Baer B, Heinze J. 2005. The evolution of male traits in social insects. Annu. Rev. Entomol. 50, 395–420. ( 10.1146/annurev.ento.50.071803) [DOI] [PubMed] [Google Scholar]
- 7.Den Boer SPA, Baer B, Boomsma JJ. 2010. Seminal fluid mediates ejaculate competition in social insects. Science 327, 1506–1509. ( 10.1126/science.1184709) [DOI] [PubMed] [Google Scholar]
- 8.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 9.Lenoir A, Aron S, Cerdá X, Hefetz A. 2009. Cataglyphis desert ants: a good model for evolutionary biology in Darwin's anniversary year: a review. Israel J. Entomol. 39, 1–32. ( 10.1007/bf02223652) [DOI] [Google Scholar]
- 10.Leniaud L, Heftez A, Grumiau L, Aron S. 2011. Multiple mating and supercoloniality in Cataglyphis desert ants. Biol. J. Linn. Soc. 104, 866–876. ( 10.1111/j.1095-8312.2011.01772.x) [DOI] [Google Scholar]
- 11.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 12.Werner M, Simmons LW. 2008. Insect sperm motility. Biol. Rev. 83, 191–208. ( 10.1111/j.1469-185X.2008.00039.x) [DOI] [PubMed] [Google Scholar]
- 13.Magariyama Y, Kudo S. 2002. A mathematical explanation of an increase in bacterial swimming speed with viscosity in linear-polymer solutions. Biophys. J. 83, 733–739. ( 10.1016/S0006-3495(02)75204-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firman RC, Simmons LW. 2011. Experimental evolution of sperm competitiveness in a mammal. BMC Evol. Biol. 11, 19 ( 10.1186/1471-2148-11-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birkhead TR, Martinez JG, Burke T, Froman DP. 1999. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. Lond. B 266, 1759–1764. ( 10.1098/rspb.1999.0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. 2004. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47. ( 10.1016/j.cub.2003.12.028) [DOI] [PubMed] [Google Scholar]
- 17.Holman L, Sturup M, Trontti K, Boomsma JJ. 2011. Random sperm use and genetic effects on worker caste fate in Atta colombica leaf-cutting ants. Mol. Ecol. 20, 5092–5102. ( 10.1111/j.1365-294X.2011.05338.x) [DOI] [PubMed] [Google Scholar]
- 18.Moore HDM, Taggart DA. 1995. Sperm pairing in the opossum increases the efficiency of sperm movement in a viscous environment. Biol. Reprod. 52, 947–953. ( 10.1095/biolreprod60.6.1353) [DOI] [PubMed] [Google Scholar]
- 19.Moore H, Dvoráková K, Jenkins N, Breed W. 2002. Exceptional sperm cooperation in the wood mouse. Nature 418, 174–177. ( 10.1038/nature00832) [DOI] [PubMed] [Google Scholar]
- 20.Hayashi F. 1998. Sperm co-operation in the fishfly, Parachauliodes japonicus. Funct. Ecol. 12, 347–350. ( 10.1046/j.1365-2435.1998.00205.x) [DOI] [Google Scholar]
- 21.Quicke DJL, Ingram SN, Baillie HS, Gaitens PV. 1992. Sperm structure and ultrastructure in the Hymenoptera (Insecta). Zool. Scr. 21, 381–400. ( 10.1111/j.1463-6409.1992.tb00339.x) [DOI] [Google Scholar]
- 22.Bawa SR, Marwaha RK. 1975. The sperm bundles of honeybee Apis cerana indica Fabr. Experientia 31, 684–686. ( 10.1007/BF01944627) [DOI] [Google Scholar]
- 23.Zama U, Lino-Neto J, Dolder H. 2004. Structure and ultrastructure of spermatozoa in Meliponini (stingless bees) (Hymenoptera: Apidae). Tissue Cell 36, 29–41. ( 10.1016/j.tice.2003.08.003) [DOI] [PubMed] [Google Scholar]
- 24.Moreira J, Zama U, Lino-Neto J. 2004. Release, behavior and phylogenetic significance of spermatozoa in bundles in the seminal vesicle during sexual maturation in Aculeata (Hymenoptera). Braz. J. Morphol. Sci. 21, 185–189. [Google Scholar]
- 25.Burnett WE, Heinze J. 2014. Sperm bundles in the seminal vesicles of sexually mature Lasius ant males. PLoS ONE 9, e93383 ( 10.1371/journal.pone.0093383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher HS, Hoekstra HE. 2010. Competition drives cooperation among closely related sperm of deer mice. Nature 463, 801–803. ( 10.1038/nature08736) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


