Abstract
Processes driving and maintaining disjunct genetic populations in marine systems are poorly understood, owing to a lack of evidence of hard barriers that could have shaped patterns of extant population structure. Here, we map two genetically divergent lineages of an obligate rocky shore fish, Clinus cottoides, and model sea-level change during the last 110 000 years to provide the first evidence of a vicariant event along the southern coastline of Africa. Results reveal that lowered sea levels during glacial periods drastically reduced rocky intertidal habitat, which may have isolated populations in two refugia for at least 40 000 years. Contemporary coastal dynamics and oceanography explain secondary contact between lineages. This scenario provides an explanation for the origin of population genetic breaks despite a lack of obvious present-day geographical barriers and highlights the need for including palaeo-oceanography in unravelling extant population patterns.
Keywords: vicariance, southern Africa, sea-level change
1. Introduction
Phylogeographic breaks, identifiable through high levels of genetic structure resulting from intraspecific genetic divergences, can provide a starting point for speciation [1–3]. However, in marine systems the processes that have shaped biodiversity and genetic patterns are complex, ambiguous and difficult to elucidate, varying considerably among species [4]. Several studies suggest that glacial to interglacial climate oscillations of the Pleistocene, which caused drastic alterations in sea level and other oceanographic parameters, had major influences on patterns of marine biodiversity [5]. The most common pattern is that of allopatric divergence, where populations became separated because of vicariant events. Several studies have demonstrated this for marine taxa [6–8], yet for some regions, such as linear coastlines that lack distinct physical features such as landbridges or headlands, evidence for vicariance is deficient.
Situated in the transition zone of the cold Benguela Upwelling System and the warm Agulhas Current, the South African marine fauna encompasses a wide range of biota, rich in biodiversity [9]. In contrast to many other examples globally, no prominent barriers are apparent along this coastline, yet numerous marine species show some degree of genetic structure [10,11]. Hypotheses that include isolation by physical barriers, changes in oceanography and diversifying selection over ecological barriers have been put forward in order to explain at least some of the observed patterns [12], yet evidence for specific processes is lacking.
Using population genetic data from an endemic, obligate rocky shore fish, the clinid Clinus cottoides, and models of sea-level change and associated changes in the ratios of rocky and sandy shores along the South African coastline, we test whether vicariance associated with changes in habitat can explain extant patterns of population structuring. Southern African clinids are live-bearers and probably have limited dispersal abilities due to extremely abbreviated larval phases [13]. This makes them ideal organisms to study the effects of past climatic changes, as historical changes in their habitat should be reflected in their genetic structure. Phylogeographic studies have uncovered two divergent lineages within C. cottoides (lineage A and lineage B, figure 1), which are separated by one fixed nucleotide difference in the mitochondrial DNA (mtDNA) control region [13]. A large interval between sampling sites on the south coast (figure 1) prevented the identification of a potential break or overlap in lineages, although molecular dating analyses suggest they diverged approximately 68 000 years ago [13].
Figure 1.
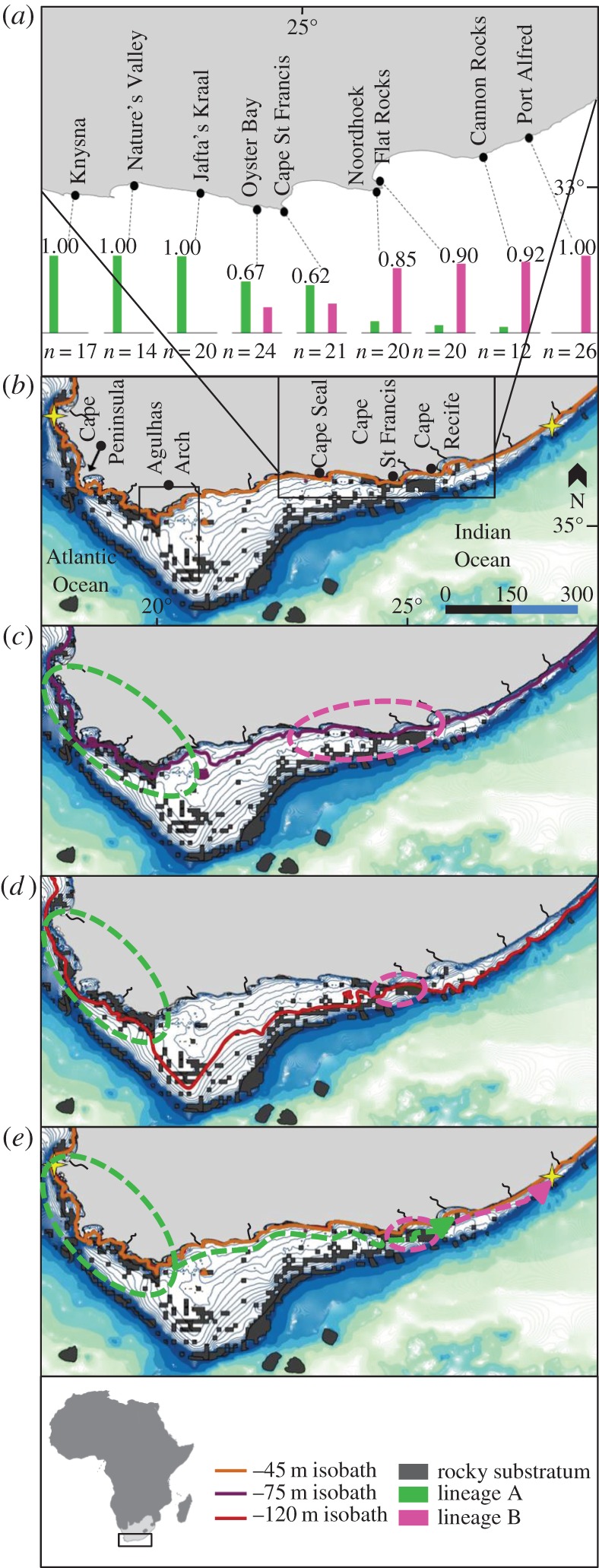
(a) Sampling localities with columns indicating the proportions of each lineage recovered at each site; n = sample size; squares demarcate the sampling gap between Knysna and Port Alfred from [13]. (b–e) Map of bathymetry around South Africa [16] overlaid with rocky substrate habitat types [17] and palaeo-shoreline positions. From 110–75 000 years ago sea level ranged between (a), (b) and (c); from 75–14 000 years ago sea level varied between (b), (c) and (d); and from 14 000 years ago to present between (b) and (a). (e) Migration paths since 14 000 years ago and the present-day range limit of C. cottoides (stars). Dashed lines indicate areas of suitable refuge habitat for C. cottoides. Stars = present-day range limit of C. cottoides. (Online version in colour.)
Using fine-scale sampling of C. cottoides populations along the unexplored region between the different lineages and a restriction enzyme approach as a means of lineage separation, we test whether vicariance shaped extant patterns of population structure. Specifically, we use GIS modelling to reconstruct the South African coastline over the last 110 000 years to investigate changes between sandy and rocky shore habitats and use these to place the contemporary distribution of C. cottoides into a historical context.
Results suggest vicariance as the major causal mechanism producing the distinct lineages observed today. Additionally, using previous studies [13], we show that contemporary coastal oceanographic patterns played a major role in promoting the geographical expansion of both lineages.
2. Material and methods
(a). Molecular analyses
Sampling was carried out at nine localities along 440 km of the South African coastline, with 12–26 individuals collected from each site (figure 1a) and stored in 95% ethanol.
Approximately 25 mg of muscle tissue was cut from each specimen. Total genomic DNA was extracted using a CTAB-DNA extraction protocol [14]. The mtDNA control region (420 bp) was amplified using primers CR-A and CR-E following von der Heyden et al. [13]. PCR products were purified using GeneJET PCR purification kits (Thermo Fisher Scientific). Purified products were incubated with the restriction endonuclease DraI (Thermo Fisher Scientific) at 37°C for 2–3 h. DraI cleaves DNA at its nuclease-specific recognition site, TTT^AAA, only present in the control region sequences of lineage B individuals. The products were run on a 2% agarose gel adjacent to a 1 kb DNA ladder (Promega), which resulted in the visual distinction of the different genotypes (lineage A one band (420 bp) and lineage B two bands (171 bp and 249 bp)).
(b). Palaeo-oceanographic modelling
Previous analyses showed that the divergence time and time to the most recent common ancestor of the two lineages fell within the Late Pleistocene and Holocene epochs [13]. Using reconstructed sea-level data of the last 110 000 years (end of previous interglacial) (reviewed in [15]), the palaeo-oceanography relevant to C. cottoides at different glacial stages was explored.
Bathymetry around southern Africa was selected from the GEBCO digital world atlas [16] (global projection wgs: 84; datum: Hartebeeshoek). Five and 10 m depth contours were then extracted using a raster to vector conversion function, after which isobaths at −45, −75 and −120 m were highlighted in the program Quantum GIS (QGIS, v. 1.8.0, Lisboa). Habitat maps of the South African National Biodiversity Assessment [17] were overlaid onto the bathymetry map in order to reconstruct rocky substrate palaeo-shorelines.
(c). Coalescent-based analyses
Using the data from the previous study [13], we estimated the divergence time between lineages A and B using IMa2 [18] and a 3.6% Myr−1 mutation rate [13]. This approach also allowed the estimation of the female population size for both lineages. IMa2 runs were performed in Markov chain simulation mode using the following specifications: ‘-b 100000’ (burn-in, i.e. the initial number of steps that are discarded); ‘-l 10000’ (number of genealogies saved every 100 steps following burn-in); ‘-hn 100’ (number of heated chains); ‘-hfg’ (geometric heating scheme) and ‘-ha 0.99 -hb 0.75’ (terms of the geometric heating increment model). Each run was repeated three times with different random starting seeds and analysed for convergence of similar values.
3. Results
A total of 174 fishes were analysed from nine localities; there is a distinct region of overlap of lineages A and B in the vicinity of Cape St. Francis (figure 1a). Coalescent analyses date the divergence between lineages A and B to approximately 54 000 years ago (95% confidence intervals: 19 000–124 000 years ago). Notably, the estimated female population size of lineage B is significantly smaller than that of A (85 000 versus 730 000).
Coastline reconstruction during four major climate stages is shown in figure 1b–e. Below is a breakdown of these periods, with a brief explanation of the associated transformations in palaeo-shoreline position and composition. GIS models suggest that lineages A and B may have been separated for at least 40 000 years between 75 000 and 14 000 years ago, as a result of significant changes along the coastline.
From 110 000 to 75 000 years ago (figure 1a–c), sea level fluctuated between 0 and −75 m, with the coastline similar to the present [16,19,20]. From 75 000 to 14 000 years ago (figure 1b–d), sea level fluctuated between −45 and −120 m [15,16]. From 75 000 to 60 000 years ago (MIS 4: Marine Isotope Stage 4) and from 50 000 to 35 000 years ago, sea level was between −75 and −110 m [15]. During this time, the southern coastal plain (SCP) expanded southward, comprising windswept sandy beaches fed by sediments of south coast rivers [19]. The southwestern shoreline mostly comprised rocky shores [19], with rocky shores only becoming prominent again on the southeast coast towards present-day Cape St. Francis [20]. During this period, the two lineages would have started to become isolated.
From 35 000 to 14 000 years ago, sea level fluctuated between −75 and −120 m. During the Last Glacial Maximum (LGM) 26 000–18 000 years ago, sea level regressed to −120 m [15]. The distribution of shoreline habitats changed significantly during expansion of the SCP during the LGM [15,18,19]. Rocky shore habitats of the southwest coast and southeast coast were separated by approximately 500 km of predominantly sandy beaches, isolating the two populations [20]. Notably, the rocky shore refugium on the southeast coast (harbouring lineage B) was significantly smaller than that on the southwest coast (lineage A) [20]. This is mirrored by the estimates of female population size, which is an order of magnitude lower for lineage B compared with A.
From 14 000 years ago to present (figure 1a,e), following the LGM, sea level rose sharply from −120 m to reach −75 m by 14 000 and −45 m by 12 000 years ago. The existing shoreline, its habitat types and coastal dynamics were established by 9000 years ago [19,20], allowing dispersal of both lineages into newly established rocky shore areas.
4. Discussion
To date, there has been no direct evidence of vicariant processes shaping marine population genetic and biodiversity patterns in southern Africa. Processes shaping and maintaining population structure in this region are poorly understood because the shoreline lacks obvious physical barriers such as land bridges and offshore islands that elsewhere have been invoked in structuring populations and species [6–8].
Using an approach that reconstructs the palaeo-shoreline and maps the geographical distribution of two distinct lineages of C. cottoides along the southeast coast of South Africa, we provide the first compelling evidence for a phylogeographic break due to vicariance. This vicariant event is most probably linked to changes in the distribution of rocky shore habitats along the South African coastline related to past sea-level fluctuations. Rocky shore refugia on the southwest and southeast coasts were separated by predominantly sandy shores for at least 40 000 years between MIS 4 and the LGM. Isolation of C. cottoides on eastern and western rocky shore refugia during the MIS 4 glacial period low-stand between 75 000 and 60 000 years ago is consistent with molecular dating that suggests the two lineages separated about 54 000–68 000 years ago [13]. Although reduced sea surface temperatures, changes in current intensity and upwelling-driven primary productivity cannot be excluded as additional factors [12,21], the lack of rocky shore habitat was probably the prominent barrier that separated the two populations. At least five other South African marine species share this phylogeographic break [2,11–13] and analyses of historical gene flow show drastically decreased rates of migration [22]. Other prominent barriers emergent during sea-level lows have affected multiple species and have been described between Australia and Tasmania [6], in the Coral Triangle [7] and across the Mediterranean–Atlantic Ocean transition [8]. When multiple species, especially those with different life-history characteristics, show concordant breaks in gene flow, it is usually the result of long-standing historical barriers [23], such as the separation of C. cottoides lineages for at least 40 000 years.
Given the signature of overlap of lineages A and B around Cape St. Francis, it is likely that contemporary oceanography facilitated the movement of both lineages to the east, as revealed by previous gene flow analyses ([13]; figure 1a,e). In addition, older, more persistent populations tend to accumulate mutations and have greater genetic diversity. Clinus cottoides populations at the edge of the distributional range are less diverse than others, plausibly a reflection of recent (post LGM) colonization [13] and a smaller founding population size.
Our study provides the first evidence of vicariance shaping population genetic patterns along a coastline with no obvious barriers to gene flow. Future studies using a similar combination of molecular and palaeo-oceanographic analyses may help in the understanding of other marine regions exhibiting ambiguous and diverse genetic patterns.
SANParks and DAFF South Africa provided permits that enabled sample collection.
Funding statement
S.vdH. thanks the Discretionary Fund of Stellenbosch University for financial assistance, and J.A.T. thanks the Science faculty for her bursary.
References
- 1.Avise JC. 2009. Phylogeography: retrospect and prospect. J. Biogeogr. 36, 3–15. ( 10.1111/j.1365-2699.2008.02032.x) [DOI] [Google Scholar]
- 2.von der Heyden S, Bowie RCK, Prochazka K, Bloomer P, Crane NL, Bernardi G. 2011. Phylogeographic patterns and cryptic speciation across oceanographic barriers in South African intertidal fishes. J. Evol. Biol. 24, 2505–2519. ( 10.1111/j.1420-9101.2011.02382.x) [DOI] [PubMed] [Google Scholar]
- 3.Rocha LA, Craig MT, Bowen BW. 2007. Phylogeography and the conservation of coral reef fishes. Coral Reefs 26, 501–512. ( 10.1007/s00338-007-0261-7) [DOI] [Google Scholar]
- 4.Bowen BW, Rocha LA, Toonen RJ, Karl SA, ToBo Laboratory. 2013. The origins of tropical marine biodiversity. Trends Ecol. Evol. 28, 359–366. ( 10.1016/j.tree.2013.01.018) [DOI] [PubMed] [Google Scholar]
- 5.Hewitt G. 2000. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913. ( 10.1038/35016000) [DOI] [PubMed] [Google Scholar]
- 6.Waters JM. 2008. Marine biogeographical disjunction in temperate Australia: historical landbridge, contemporary currents, or both? Divers. Distrib. 14, 692–700. ( 10.1111/j.1472-4642.2008.00481.x) [DOI] [Google Scholar]
- 7.Gaither MR, Brian BW, Bordenave T, Rocha LA, Newman SJ, Gomez JA, van Herwerden L, Craig MT. 2011. Phylogeography of the reef fish Cephalopholis argus (Epinephelidae) indicates Pleistocene isolation across the indo-pacific barrier with contemporary overlap in the coral triangle. BMC Evol. Biol. 11, 189 ( 10.1186/1471-2148-11-189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wares JP, Cunningham CW. 2001. Phylogeography and historical ecology of the north Atlantic intertidal. Evolution 55, 2455–2469. ( 10.1111/j.0014-3820.2001.tb00760.x) [DOI] [PubMed] [Google Scholar]
- 9.Griffiths CL, Robinson TB, Lange L, Mead A. 2010. Marine biodiversity in South Africa: an evaluation of current states of knowledge. PLoS ONE 5, e12008 ( 10.1371/journal.pone.0012008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teske PR, von der Heyden S, McQuaid CD, Barker NP. 2011. A review of marine phylogeography in southern Africa. South Afr. J. Sci. 107, 43–53. ( 10.4102/sajs.v107i5/6.514) [DOI] [Google Scholar]
- 11.von der Heyden S. 2009. Why do we need to integrate population genetics into South African marine protected area planning? Afr. J. Mar. Sci. 31, 263–269. ( 10.2989/AJMS.2009.31.2.14.886) [DOI] [Google Scholar]
- 12.Teske PR, Zardi GI, McQuaid CD, Nicastro K. 2013. Two sides of the same coin: extinctions and originations across the Atlantic/Indian Ocean boundary as a consequence of the same climate oscillation. Front. Biogeogr. 5, 48–59. [Google Scholar]
- 13.von der Heyden S, Prochazka K, Bowie RCK. 2008. Significant population structure and asymmetric gene flow patterns amidst expanding populations of Clinus cottoides (Perciformes, Clinidae): application of molecular data to marine conservation planning in South Africa. Mol. Ecol. 17, 4812–4826. ( 10.1111/j.1365-294X.2008.03959.x) [DOI] [PubMed] [Google Scholar]
- 14.Doyle JJ, Doyle JL. 1990. A rapid total DNA preparation procedure for fresh plant tissue. Focus 12, 13–15. [Google Scholar]
- 15.Compton JS. 2011. Pleistocene sea-level fluctuations and human evolution on the southern coastal plain of South Africa. Quat. Sci. Rev. 30, 506–527. ( 10.1016/j.quascirev.2010.12.012) [DOI] [Google Scholar]
- 16.GEBCO Digital Atlas. 2003. The GEBCO One Minute Grid See http://www.gebco.net/data_and_products/gridded_bathymetry_data/.
- 17.SANBI National Biodiversity Assessment, NBA. 2011. Marine Benthic and Coastal Habitat Map See http://www.bgis.sanbi.org/nba/marine_habitattypes.asp.
- 18.Hey J. 2010. Isolation with migration models for more than two populations. Mol. Biol. Evol. 27, 905–920. ( 10.1093/molbev/msp296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dingle RV, Rogers J. 1972. Pleistocene paleogeography of the Agulhas Bank. Trans. R. Soc. South Afr. 40, 155–165. ( 10.1080/00359197209519415) [DOI] [Google Scholar]
- 20.Van Andel TH. 1989. Late Pleistocene sea levels and the human exploitation of the shore and shelf of South Africa. J. Field Archaeol. 16, 133–155. ( 10.1179/jfa.1989.16.2.133) [DOI] [Google Scholar]
- 21.Beal LM, De Ruijter WPM, Biastoch A, Zahn R. 2011. On the role of the Agulhas system in ocean circulation and climate. Nature 472, 429–436. ( 10.1038/nature09983) [DOI] [PubMed] [Google Scholar]
- 22.Neethling M, Matthee CA, Bowie RCK, von der Heyden S. 2008. Evidence for panmixia despite barriers to gene flow in the southern African endemic, Caffrogobius caffer (Teleostia: Gobiidae). BMC Evol. Biol. 8, 325 ( 10.1186/1471-2148-8-325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo CH, Avise JC. 2005. Phylogeographic breaks in low dispersal species: the emergence of concordance across gene trees. Genetica 124, 179–186. ( 10.1007/s10709-005-2095-y) [DOI] [PubMed] [Google Scholar]


