Abstract
BACKGROUND
Results from an observational study involving neonates suggested that high-frequency oscillatory ventilation (HFOV), as compared with conventional ventilation, was associated with superior small-airway function at follow-up. Data from randomized trials are needed to confirm this finding.
METHODS
We studied 319 adolescents who had been born before 29 weeks of gestation and had been enrolled in a multicenter, randomized trial that compared HFOV with conventional ventilation immediately after birth. The trial involved 797 neonates, of whom 592 survived to hospital discharge. We compared follow-up data from adolescents who had been randomly assigned to HFOV with follow-up data from those who had been randomly assigned to conventional ventilation, with respect to lung function and respiratory health, health-related quality of life, and functional status, as assessed with the use of questionnaires completed when the participants were 11 to 14 years of age. The primary outcome was forced expiratory flow at 75% of the expired vital capacity (FEF75).
RESULTS
The HFOV group had superior results on a test of small-airway function (z score for FEF75, −0.97 with HFOV vs. −1.19 with conventional therapy; adjusted difference, 0.23 [95% confidence interval, 0.02 to 0.45]). There were significant differences in favor of HFOV in several other measures of respiratory function, including forced expiratory volume in 1 second, forced vital capacity, peak expiratory flow, diffusing capacity, and impulse-oscillometric findings. As compared with the conventional-therapy group, the HFOV group had significantly higher ratings from teachers in three of eight school subjects assessed, but there were no other significant differences in functional outcomes.
CONCLUSIONS
In a randomized trial involving children who had been born extremely prematurely, those who had undergone HFOV, as compared with those who had received conventional ventilation, had superior lung function at 11 to 14 years of age, with no evidence of poorer functional outcomes. (Funded by the National Institute for Health Research Health Technology Assessment Programme and others.)
Although survival rates have improved among infants with extremely low gestational age, the proportion of surviving infants with bronchopulmonary dysplasia remained unchanged between 1995 and 2006.1 Infants born extremely prematurely usually require respiratory support. High-frequency oscillatory ventilation (HFOV) was proposed as a means of reducing the risk of bronchopulmonary dysplasia among neonates receiving ventilatory support. During HFOV, a constant pressure is applied to improve lung volume and oxygenation, while ventilation is achieved with the use of very low tidal volumes.
In an early randomized trial comparing HFOV (with the use of a low-volume strategy) with conventional ventilation, a significantly higher proportion of infants in the HFOV group had grade 3 or 4 intraventricular hemorrhage and periventricular leukomalacia.2 Systematic reviews of randomized trials3,4 did not confirm these findings, but the adverse outcomes and beneficial effects were inconsistent across the trials. One meta-analysis of randomized trials3 concluded that the use of HFOV resulted in a significant but modest reduction in the risk of bronchopulmonary dysplasia, but a meta-analysis of patient-level data4 did not show any advantage of HFOV over conventional ventilation, with respect to short-term outcomes, including broncho-pulmonary dysplasia.
Limited data are available on lung function at the time of follow-up of infants who had been enrolled in trials of HFOV. No significant differences with respect to measurements of pulmonary mechanics were observed at 9 months of corrected age between infants who had undergone HFOV (with the use of a low-volume strategy) and those who had undergone conventional ventilation in a randomized trial.5 Although small-airway function appears to decline during infancy in prematurely born infants supported with conventional ventilation,6 the results of an observational study of 36 infants born very prematurely suggested that this decline did not occur among infants initially supported by HFOV.7
The United Kingdom Oscillation Study (UKOS) was a multicenter, randomized trial, involving very premature infants, in which HFOV (with the use of a high-volume strategy) was compared with conventional ventilation, initiated within 1 hour after birth, with respect to the risk of bronchopulmonary dysplasia or death.8 Examination of a subgroup of the infants at 1 year of corrected age revealed no significant differences in the results of lung-function tests,9 but tests of small-airway function were not assessed. The current study was designed to determine the long-term outcomes in children enrolled in the UKOS to test the hypothesis that the use of HFOV during the newborn period would be associated with superior small-airway function at school age. We also assessed other respiratory and educational outcomes in these children.
METHODS
STUDY DESIGN AND OVERSIGHT
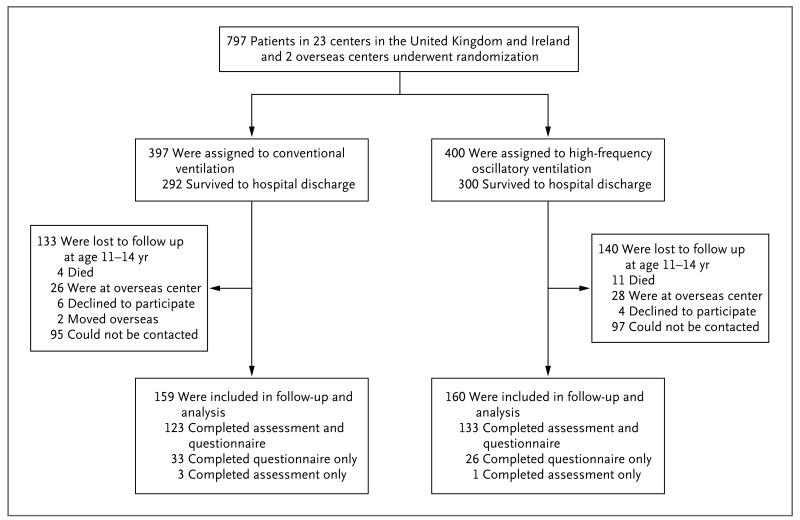
In the UKOS, we recruited 797 infants, all born before 29 weeks of gestation, at 25 centers: 22 centers in England, Scotland, and Wales and 1 each in Ireland, Singapore, and Australia. The target group for the current study included all 538 children from England, Scotland, Wales, and Ireland who had survived to hospital discharge (Fig. 1).
Figure 1. Randomization, Follow-up, and Analysis Populations.
We had kept in contact with the children involved in the UKOS since the 2-year follow-up, sending birthday cards and news updates. Families were invited to participate in the follow-up study that included children 11 to 14 years of age; invitations were sent by mail; if there was no response to the initial letter, attempts were made to contact the families by means of e-mail, telephone calls, or both.
Children whose parents provided consent were asked to undergo comprehensive lung-function assessments (performed at King’s College Hospital National Health Service Foundation Trust [KCH]). All the assessments were conducted by researchers who were unaware of the child’s assigned ventilation strategy. Children and parents were also asked to complete questionnaires regarding respiratory disorders, health-related quality of life, and functional status, and teachers were asked to complete questionnaires regarding the children’s academic achievement and behavior (described below and in the Supplementary Appendix). Parents and their children who were unable to come to KCH completed the questionnaires only.
The South West London National Research Ethics Service Committee approved the current study. Parents provided written informed consent for their child to take part in the study; formal consent was not required from the child. All the children who underwent lung-function measurements assented to the measurements. The second and last authors vouch for the integrity and completeness of the data and analyses reported and for the fidelity of the trial to the study protocol.
ASSESSMENTS
Respiratory Function
The primary outcome was small-airway function, as assessed by measurement of forced expiratory flow at 75% of the expired vital capacity (FEF75) with the use of a spirometer. All assessments were performed according to guidelines from the American Thoracic Society and the European Respiratory Society. Airway function was also assessed by means of spirometric measurement of the forced expiratory flow at 50% and 25% of expired vital capacity (FEF50 and FEF25, respectively), forced expiratory volume in 1 second (FEV1), and peak expiratory flow (PEF). Impulse oscillometry was used to assess respiratory-system resistance.10 Inhomogeneity of ventilation distribution was assessed by means of a multiple-breath technique assessing the lung-clearance index (Innocor photoacoustic gas analyzer, Innovision).11,12 Lung volumes were assessed by means of measurements of functional residual capacity with the use of a helium-dilution technique (FRCHe) and forced vital capacity (FVC) by means of spirometry.
The following assessments of lung volumes were also undertaken: functional residual capacity as assessed by means of plethysmography (FRCpleth) and plethysmographic assessments of total lung capacity and residual volume. Two measurements within 5% of each other were averaged to calculate the results.13-15 The diffusing capacity of the lung for carbon monoxide (DLCO), alveolar volume, and gas transfer per unit volume were assessed with the use of the single-breath gas-transfer technique.16 All lung-function results were expressed as the percentage predicted for height with the use of established reference ranges17-19 and were converted into z scores as appropriate. The fraction of exhaled nitric oxide (FeNO) was measured by means of a real-time method with the use of a computerized system and visual display (HypAir FeNO, Medisoft Cardio-respiratory Instrumentation).
Other Assessments
Parents were asked about a family history of asthma (in parents or siblings). Atopy was assessed by means of skin-prick testing. The allergens tested were mixed-grass pollen, Dermatophagoides pteronyssinus, D. farinae, cat dander, and dog dander.
Behavioral outcomes, health-related quality of life, and academic achievement were assessed by means of questionnaires; these included the Strengths and Difficulties Questionnaire (SDQ; completed separately by the child, the parents, and the teacher; total scores from the five sub-scales range from 0 to 40, with higher scores indicating a greater degree of difficulty), the Health Utilities Index Mark 3 (HUI3; completed separately by the parents and the child; scores range from −0.36 to 1, with lower scores indicating more severe health problems), and the Teacher Academic Attainment Scale (completed by the teachers in mainstream schools; scores range from 1 to 5 for each school subject, with higher scores indicating better performance). A questionnaire regarding respiratory health, symptoms, medicine use, hospital admissions, and neurologic illness was also completed by the parents. Parents were asked about the presence of smokers in the house, and a urine sample for the detection of cotinine was obtained from all the children at the time of assessment.
STATISTICAL ANALYSIS
The study was analyzed as a two-group, parallel study in keeping with the original trial design. We calculated that with a follow-up sample of 320 children, the study would have 90% power to show a difference in means of 0.36 SD for the FEF75 results and the other lung-function results, at the 5% significance level.
For the main analysis of outcomes, including the FEF75 z score (primary outcome), we used mixed models, with the mother or the pregnancy as the random effect to allow for clustering due to multiple births.20 Skewed lung-function FeNO outcome data were log-transformed. We adjusted for imbalances in baseline factors by including those factors as fixed effects in the models. In sensitivity analyses, each lung-function outcome was also adjusted for bronchopulmonary dysplasia, pubertal stage, and cotinine level. The primary outcome, FEF75, and 19 secondary lung-function outcomes were prespecified. Initially, we did not adjust for multiple testing, but we then undertook a sensitivity analysis using the Bonferroni adjustment. This provided a test of the composite hypothesis that the mean lung-function results would not differ between the two ventilation groups, such that if any comparison had a P value of less than 0.05÷19 (i.e., <0.0026), the composite hypothesis would be rejected.21
In the case of children who were unable to complete all the lung-function tests, multiple imputation with the use of chained equations was used to impute missing data. Nine variables plus all lung-function variables were used to impute the data. Differences in means between two groups can be difficult to interpret clinically, so we calculated the equivalent group difference as the difference in the proportion of children in each group with an FEF75 value below the 10th percentile (z score less than −1.28). We used a statistical method that was based on the normal distribution that gives the same P value as a test of the equivalent differences in means.22 All statistical analyses were performed with the use of Stata software, version 12.1 (StataCorp).
RESULTS
PARTICIPANT RECRUITMENT
Figure 1 shows the numbers of children enrolled, as well as the reasons for nonparticipation, among the 592 children at all the centers who survived to hospital discharge. A total of 319 children completed the study: 59 children completed the detailed questionnaires only, 4 completed the assessment only, and 256 completed both the questionnaires and the assessment at KCH. No child had an adverse event during assessment. As compared with children who were not recruited for the study, those who were recruited were more likely to have a mother who was white and who did not smoke during pregnancy and were less likely to live in a disadvantaged area (Table S1 in the Supplementary Appendix).
BASELINE AND FOLLOW-UP CHARACTERISTICS
As compared with children in the HFOV group, those in the conventional-ventilation group had had a higher mean weight and gestational age at birth and were more likely to have received surfactant (Table 1, and Table S2 in the Supplementary Appendix). There were no significant be tween-group differences in the characteristics of the children when they were assessed at 11 to 14 years of age (Table 1).
Table 1. Maternal, Neonatal, and Follow-up Characteristics, According to Ventilation Group.*.
| Characteristic | Conventional Ventilation (N = 159) | HFOV (N = 160) | P Value |
|---|---|---|---|
| Maternal | |||
| Race — no./total no. (%)† | 0.92 | ||
| White | 142/158 (90) | 143/160 (89) | |
| Black | 11/158 (7) | 10/160 (6) | |
| Other | 5/158 (3) | 7/160 (4) | |
| Smoking during pregnancy — no./total no. (%.) | 31/146 (21) | 38/146 (26) | 0.34 |
| Neonatal | |||
| Male sex — no. (%) | 85 (53) | 77 (48) | 0.34 |
| Birth weight — g | 923±206 | 867±209 | 0.02 |
| Birth-weight z score | 0.52 | ||
| Mean | −0.55 | −0.62 | |
| Range | −2.94 to 1.73 | −3.45 to 2.41 | |
| Gestational age at birth — wk | 27.0±1.2 | 26.7±1.5 | 0.04 |
| Multiple birth — no. (%) | 39 (25) | 37 (23) | 0.77 |
| Surfactant administered — no. (%) | 158 (99) | 152 (95) | 0.04 |
| Receipt of postnatal glucocorticoids — no./total no. (%) | 36/157 (23) | 48/157 (31) | 0.13 |
| Oxygen dependency at 36 wk of postmenstrual age — no. (%) | 95 (60) | 88 (55) | 0.39 |
| Follow-up at 11–14 yr of age | |||
| No. of participants with assessment | 121 | 127 | |
| Age — yr | 12.5±0.6 | 12.6±0.6 | 0.66 |
| Weight — kg | 0.53 | ||
| Mean | 44.4 | 44.9 | |
| Range | 23.4 to 102 | 19.0 to 86.7 | |
| Height — cm | 0.26 | ||
| Mean | 153 | 151 | |
| Range | 129 to 173 | 124 to 172 | |
| Cotinine level‡— no./total no. (%) | 0.84 | ||
| Undetectable | 85/106 (80) | 92/115 (80) | |
| Passive smoking | 4/106 (4) | 3/115 (3) | |
| Likely active smoking | 17/106 (16) | 20/115 (17) | |
| No. of parents who completed questionnaires | 150 | 154 | |
| Report of smoker in the family — no./total no. (%)§ | 44/149 (30) | 51/152 (34) | 0.45 |
| Report of doctor-diagnosed asthma — no./total no. (%)§ | 76/150 (51) | 72/154 (47) | 0.50 |
Plus–minus values are means ±SD. HFOV denotes high-frequency oscillatory ventilation.
Race was reported by the mother.
A cotinine level of less than 10 ng per milliliter was defined as undetectable, a level of 10 to 15 ng per milliliter was considered to indicate passive smoking, and a level of more than 15 ng per milliliter was considered to indicate likely active smoking.
Reports refer to members of the child’s family living in the same home (e.g., mother, father, a partner, and siblings).
LUNG-FUNCTION AND ALLERGY ASSESSMENT RESULTS
Our final follow-up sample comprised 319 children, but only 248 underwent lung-function testing; hence, the study was powered to detect a difference of 0.41 SD for the FEF75 results. The mean z score for the FEF75 was higher in the HFOV group than in the conventional-ventilation group (−0.97 vs. −1.19) (Table 2). This difference was significant in both the unadjusted model that allowed for multiple births and in the fully adjusted model. The two groups had similar distributions of FEF75 z scores, with the distribution shifted downward in the conventional-ventilation group (Fig. S1 in the Supplementary Appendix); the percentage of children with results below the 10th percentile was 37% in the HFOV group, as compared with 47% in the conventional-ventilation group (P = 0.04).
Table 2. Lung-Function and Allergy-Test Results, According to Ventilation Group.*.
| Result | No. of Participants with Result† | Conventional Ventilation (N = 121) | HFOV (N = 127) | Adjusted Difference (95% CI)‡ | P Value |
|---|---|---|---|---|---|
| FEF z score | |||||
| FEF75 | 248 | −1.19±0.80 | −0.97±0.95 | 0.23 (0.02 to 0.45) | 0.04 |
| FEF50 | 248 | −1.37±0.85 | −1.07±0.93 | 0.30 (0.09 to 0.52) | 0.006 |
| FEF25 | 248 | −1.16±0.95 | −0.84±0.90 | 0.29 (0.07 to 0.51) | 0.01 |
| FEF25–75 | 231 | −1.58±1.05 | −1.34±1.09 | 0.21 (−0.04 to 0.47) | 0.10 |
| FEV1 z score | 248 | −0.95±1.02 | −0.60±1.08 | 0.35 (0.09 to 0.60) | 0.008 |
| FVC z score | 248 | −0.44±0.89 | −0.29±1.05 | 0.13 (−0.10 to 0.37) | 0.27 |
| FEV1:FVC ratio z score | 248 | −1.75±1.78 | −1.16±1.75 | 0.58 (0.16 to 0.99) | 0.007 |
| PEF — % of predicted | 247 | 80.3±15.0 | 86.3±15.5 | 5.85 (2.21 to 9.49) | 0.002 |
| Gas transfer | |||||
| DLCO z score | 210 | −1.10±0.92 | −0.81±1.19 | 0.31 (0.04 to 0.58) | 0.02 |
| VA (liters) | 210 | 3.44±0.66 | 3.40±0.59 | −0.05 (−0.20 to 0.09) | 0.48 |
| DLCO/VA (mmol/min/kPa/liter) | 210 | 1.73±0.20 | 1.76±0.21 | 0.04 (−0.01 to 0.09) | 0.11 |
| Residual volume z score | 211 | 0.46±1.19 | 0.31±1.35 | −0.09 (−0.42 to 0.24) | 0.60 |
| Total lung capacity z score | 213 | 0.20±1.00 | 0.36±1.13 | 0.16 (−0.12 to 0.43) | 0.26 |
| FRC z score | |||||
| FRCpleth | 218 | −0.07±1.26 | −0.11±1.28 | −0.08 (−0.41 to 0.25) | 0.63 |
| FRCHe | 229 | −0.62±1.10 | −0.75±1.05 | −0.18 (−0.44 to 0.08) | 0.19 |
| Vital capacity z score | 213 | −0.50±0.88 | −0.17±1.09 | 0.31 (0.05 to 0.57) | 0.02 |
| Respiratory resistance — % of predicted | |||||
| At 5 Hz | 237 | 99.6±23.2 | 92.5±20.9 | −7.1 (−12.5 to −1.8) | 0.009 |
| At 20 Hz | 237 | 95.5±23.8 | 90.2±22.1 | −5.2 (−10.7 to 0.2) | 0.06 |
Plus–minus values are means ±SD. CI denotes confidence interval, DLCO diffusing capacity of the lung for carbon monoxide, FEF forced expiratory flow (with FEF25, FEF50, and FEF75 indicating 25%, 50%, and 75%, respectively, of the expired vital capacity), FRCHe functional residual capacity with the use of a helium-dilution technique, FRCpleth functional residual capacity as assessed by means of plethysmography, FVC forced vital capacity, PEF peak expiratory flow, and VA alveolar volume.
Lung-function values were missing for the following measures: FEF25–75 for 10 participants in the conventional-ventilation group and 7 in the HFOV group, PEF for 1 in the conventional-ventilation group, DLCO and VA for 14 in the conventional-ventilation group and 24 in the HFOV group, residual volume for 15 and 22, respectively, total lung capacity for 14 and 21, respectively, FRCpleth for 14 and 16, respectively, FRCHe for 8 and 11, respectively, vital capacity for 14 and 21, respectively, and respiratory resistance at 5 Hz and at 20 Hz for 5 and 6, respectively.
The differences in z scores are presented as HFOV group − conventional-ventilation group, with adjustment for birth weight, gestational age, and whether surfactant had been administered. The differences in percentages are presented as mean percentage points (HFOV group – conventional-ventilation group), with adjustment for birth weight, gestational age, and whether surfactant had been administered before birth.
There were significant differences between the ventilation groups — all favoring the HFOV group — with respect to the results of the following tests: FEF25, FEF50, FEV1, FEV1:FVC ratio, PEF, DLCO, vital capacity, and respiratory resistance at 5 Hz (Table 2). When adjustment was made for multiple testing, the results were essentially unchanged. The differences between the groups remained significant and materially unchanged in a model that was also adjusted for pubertal stage and cotinine levels (Table S4 in the Supplementary Appendix). Results were also similar when multiple imputation was used to address incomplete lung-function data (Table S5 in the Supplementary Appendix). Post hoc calculation of the intercorrelations among all pairs of lung-function measurement results showed correlations ranging from −0.01 to 0.92 (Table S6 in the Supplementary Appendix).
OTHER OUTCOMES
There were no significant differences between the ventilation groups with regard to reported respiratory disorders during the previous 12 months or health problems as documented by the parent-completed questionnaire (Table 3, and Table S7 in the Supplementary Appendix). There were also no significant between-group differences in the results of the HUI3 or the SDQ (Table S8 in the Supplementary Appendix). When the SDQ scores were dichotomized, the only significant difference between the two groups was in children’s reporting of emotional symptoms, which was more frequent in the HFOV group than in the conventional-ventilation group (odds ratio, 2.50; 95% confidence interval, 1.13 to 5.56) (Table S9 in the Supplementary Appendix).
Table 3. Respiratory and Other Disorders in the Previous 12 Months, as Documented in the Parent Questionnaire.
| Respiratory Disorder | Conventional Ventilation (N = 150) | HFOV (N = 154) | Adjusted Odds Ratio (95% CI)* | P Value |
|---|---|---|---|---|
| Wheezing — no. (%) | 22 (15) | 23 (15) | 1.01 (0.53–1.90) | 0.98 |
| Frequency of wheezing — no./total no. (%)† | 0.76 | |||
| Daily | 1/22 (5) | 5/22 (23) | ||
| Weekly | 1/22 (5) | 2/22 (9) | ||
| Monthly | 4/22 (18) | 4/22 (18) | ||
| Less than monthly | 16/22 (73) | 11/22 (50) | ||
| Medication for chest problems — no./total no. (%)‡§ | ||||
| Antibiotic agent | 22/150 (15) | 18/154 (12) | 0.69 (0.34–1.43) | 0.32 |
| Other medicine | 24/150 (16) | 23/152 (15) | 0.94 (0.50–1.77) | 0.85 |
| Hospital admission — no./total no. (%)¶ | 15/150 (10) | 18/152 (12) | 0.95 (0.45–1.99) | 0.89 |
| Chest problem — no.§ | 4 | 0 | ||
| Surgery — no. | 8 | 13 | ||
| Other — no. | 8 | 5 | ||
| Cerebral palsy — no.∥ | 13 | 18 | 0.38 |
The odds ratio was adjusted for birth weight, gestational age, and whether surfactant had been administered.
Data were missing for one child in the HFOV group.
Analyses were based on yes versus no responses.
Chest problems were defined as respiratory infection and asthma.
Patients may have been admitted to the hospital for multiple reasons.
The analysis assumed that participants who did not respond did not have the particular health problem. Estimates were unadjusted, owing to small numbers.
A questionnaire regarding academic achievement and special-education provision was completed by a teacher at each child’s school for 225 of the 319 children. The HFOV group was rated significantly higher in three of eight school subjects assessed: art and design, information technology, and design and technology. There were no significant differences between the two groups with regard to the percentages of children attending a mainstream school or requiring special education (Table 4, and Table S10 in the Supplementary Appendix).
Table 4. Educational-Attainment Scores and Educational Provision, According to Ventilation Group.*.
| Variable | No. of Participants with Result† | Conventional Ventilation (N = 109) | HFOV (N = 116) | Adjusted Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| Area of study — score‡ | |||||
| English or literacy | 219 | 2.81±1.04 | 2.92±0.91 | 0.12 (−0.13 to 0.37) | 0.35 |
| Mathematics | 218 | 2.76±1.03 | 2.76±1.01 | 0.04 (−0.22 to 0.31) | 0.75 |
| Art and design | 208 | 2.76±0.89 | 3.00±0.79 | 0.31 (0.09 to 0.54) | 0.006 |
| Geography | 206 | 2.79±0.91 | 2.88±0.77 | 0.11 (−0.09 to 0.32) | 0.27 |
| History | 205 | 2.81±0.89 | 2.92±0.84 | 0.18 (−0.06 to 0.41) | 0.14 |
| Information technology | 204 | 2.82±0.80 | 3.00±0.78 | 0.24 (0.03 to 0.45) | 0.02 |
| Science | 215 | 2.83±0.99 | 2.96±0.83 | 0.19 (−0.05 to 0.43) | 0.12 |
| Design and technology | 197 | 2.80±0.88 | 3.04±0.75 | 0.27 (0.05 to 0.49) | 0.02 |
| Average of all subjects | 221 | 2.79±0.79 | 2.93±0.70 | 0.16 (−0.02 to 0.35) | 0.08 |
| Educational provision — no./total no. (%) | |||||
| Mainstream school | 301 | 88/148 (59) | 85/153 (56) | 0.90 (0.54 to 1.49) | 0.69 |
| Special-education needs§ | 224 | 57/108 (53) | 60/116 (52) | 0.94 (0.54 to 1.62) | 0.83 |
Plus–minus values are means ±SD. Data for scores in areas of study and for special-education needs were obtained from teacher questionnaires (for 225 of 319 children), and data on mainstream school were obtained from parent questionnaires (for 305 of 319 children).
School-attainment scores were missing or not applicable for the following subjects: English or literacy for 2 children in the conventional-ventilation group and 4 in the HFOV group, mathematics for 4 and 3, respectively, art and design for 9 and 8, respectively, geography for 10 and 9, respectively, history for 11 and 9, respectively, information technology for 12 and 9, respectively, science for 6 and 4, respectively, design and technology for 17 and 11, respectively, and the average of all subjects for 1 and 3, respectively. Mainstream-school data were missing for 2 children in the conventionaltherapy group and 2 in the HFOV group. Special education data were missing for 1 child in the conventional-therapy group.
Teachers of children in mainstream schools rated each child’s performance in eight school subjects, according to the following scores: 1 indicated very below average, 2 below average, 3 average, 4 above average, and 5 very above average. The average subject score is the mean of all available subject scores.
Results were based on the teacher’s yes-or-no response to the question, “Does this child have any special-education needs?”
DISCUSSION
We found that among school children who had been born extremely prematurely, those who had been supported by HFOV during the neonatal period had significantly, albeit modestly, better outcomes in tests of small-airway function than those who had been supported by conventional ventilation (between-group difference in mean FEF75, 0.23 SD). The children who had been randomly assigned to HFOV also had superior outcomes in tests of large-airway function, as assessed by means of several volitional measures (FEV1, FEF50, and FEF25) and a nonvolitional test (impulse oscillometry), and they had better DLCO results than those assigned to conventional ventilation, suggesting a greater functional lung-surface area for gas exchange.
The differences in lung-function measures, although significant, were relatively small: approximately 0.3 SD, on average. When we analyzed these data to assess the proportion of children with an FEF75 below the 10th percentile (for age, height, and sex), we observed a significantly higher percentage in the conventional-ventilation group (47%, vs. 37% in the HFOV group), a difference that is likely, in our opinion, to be of clinical importance. The relatively small mean effect size and the respiratory reserve in childhood may explain the absence of a significant increase in respiratory disorders in the conventional-ventilation group, as documented by responses on the parent-completed questionnaires to questions regarding symptom status and the need for medication. Nevertheless, the poorer lung function in the conventional-ventilation group than in the HFOV group may have consequences over time — for example, by causing greater vulnerability to lung-function insults such as smoking.
A prior follow-up study involving 69 children at approximately 6 years of age who had been enrolled as neonates in another randomized trial of HFOV with the use of a lung-recruitment strategy versus conventional ventilation23 also showed no significant between-group differences in the frequency of respiratory disorders but did show superior lung function in the HFOV group.24 The conventional-ventilation group had decreased PEF, increased residual volume, and greater maldistribution of ventilation, as compared with the HFOV group.24
We planned that 320 children would undergo full assessment. Our total recruitment was on target (319 children), and most children completed questionnaires; however, only 248 children underwent full assessment, including lung-function measurements. Nevertheless, our study was adequately powered to detect a small difference in the means of the lung-function results with the use of a mixed-effects model.
We compared the results of the lung-function testing with reference ranges that did not correct for ethnic group. Reference ranges that apply to multiple ethnic groups are now available, but for spirometric results only.25 Our study assessed a wide range of lung-function tests, and we considered it to be important to use the same reference ranges for as many as possible of our lung-function measurements for consistency. Furthermore, 90% of the participants in our study population were white, rendering the need for adjustment for ethnic group less important.
We were concerned that any respiratory benefit associated with use of HFOV might have been associated with adverse neurodevelopmental outcomes, because, in some trials, HFOV has been associated with an increased risk of neonatal brain injury.2,26 There were no significant differences between our study groups with regard to health-related quality of life or behavior, other than a higher proportion of children in the HFOV group reporting emotional symptoms. Multiple comparisons were performed, however, and this finding may be explained by chance. In contrast, the HFOV group had significantly higher mean ratings by teachers with respect to art and design, information technology, and design and technology, suggesting the possibility that visuospatial skills were better in that group than in the conventional-ventilation group. A limitation of our study is the absence of formal testing of neurocognitive function, but our findings provide no evidence of worse functional outcomes in the HFOV group than in the conventional-ventilation group.
In the original trial,8 we had specified initial ventilator settings (inflation rate and duration) for the conventional-ventilation group; further adjustments to bring blood gases into the target ranges were made at the discretion of the individual clinician. This design represents actual practice in many newborn intensive care units.27
New triggered modes and volume-targeted ventilation are now being used, in addition to other conventional-ventilation modes that were used in the UKOS. In a systematic review, volume-targeted ventilation was associated with a reduction in the combined outcome of death or bronchopulmonary dysplasia,28 but there are no data to inform whether this strategy or the new triggered modes will influence long-term pulmonary outcomes. The volumes used during HFOV are less than half those used during volume-targeted ventilation29; we speculate that this may be the mechanism for the protective effect of HFOV on small-airway function.
In conclusion, our results suggest that the use of HFOV, as compared with conventional ventilation, immediately after birth in very prematurely born infants was associated with modest improvements in lung function and with no evidence of a poorer functional outcome when the children were 11 to 14 years of age.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and not necessarily those of the National Health Service (NHS), the National Institute for Health Research (NIHR), or the Department of Health.
Supported by a grant (08/116/10) from the NIHR Health Technology Assessment Programme, the South London Comprehensive Local Research Network, the Department of Health NIHR Biomedical Research Centre funding scheme at University College London Hospitals and University College London, and the NIHR Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The HIFI Study Group High-frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. N Engl J Med. 1989;320:88–93. doi: 10.1056/NEJM198901123200204. [DOI] [PubMed] [Google Scholar]
- 3.Cools F, Henderson-Smart DJ, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2009;3:CD000104. doi: 10.1002/14651858.CD000104.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Cools F, Askie L, Offriga M, et al. Elective high-frequency oscillatory ventilation versus conventional ventilation in pre-term infants: a meta-analysis of individual patients’ data. Lancet. 2010;375:2082–9. doi: 10.1016/S0140-6736(10)60278-4. [Erratum: Lancet. 2011;377:1572] [DOI] [PubMed] [Google Scholar]
- 5.HIFI Study Group High-frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants: assessment of pulmonary function at 9 months of corrected age. HiFi Study Group. J Pediatr. 1990;116:933–41. doi: 10.1016/s0022-3476(05)80657-2. [DOI] [PubMed] [Google Scholar]
- 6.Hoo AF, Dezateux C, Henschen M, Costeloe K, Stocks J. Development of airway function in infancy after preterm delivery. J Pediatr. 2002;141:652–8. doi: 10.1067/mpd.2002.128114. [DOI] [PubMed] [Google Scholar]
- 7.Hofhuis W, Huysman MW, van der Wiel EC, et al. Worsening of V’maxFRC in infants with chronic lung disease in the first year of life: a more favorable outcome after high-frequency oscillation ventilation. Am J Respir Crit Care Med. 2002;166:1539–43. doi: 10.1164/rccm.2202046. [DOI] [PubMed] [Google Scholar]
- 8.Johnson AH, Peacock JL, Greenough A, et al. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med. 2002;347:633–42. doi: 10.1056/NEJMoa020432. [DOI] [PubMed] [Google Scholar]
- 9.Thomas MR, Rafferty GF, Limb ES, et al. Pulmonary function at follow-up of very preterm infants from the United Kingdom Oscillation Study. Am J Respir Crit Care Med. 2004;169:868–72. doi: 10.1164/rccm.200310-1425OC. [DOI] [PubMed] [Google Scholar]
- 10.Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. Eur Respir Mon. 2005;31:72–105. [Google Scholar]
- 11.Horsley A. Lung clearance index in the assessment of airways disease. Respir Med. 2009;103:793–9. doi: 10.1016/j.rmed.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Latzin P, Thamrin C, Kraemer R. Ventilation inhomogeneities assessed by the multibreath washout (MBW) technique. Thorax. 2008;63:98–9. doi: 10.1136/thx.2007.085332. [DOI] [PubMed] [Google Scholar]
- 13.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 14.Coates AL, Peslin R, Rodenstein D, Stocks J. Measurement of lung volumes by plethysmography. Eur Respir J. 1997;10:1415–27. doi: 10.1183/09031936.97.10061415. [DOI] [PubMed] [Google Scholar]
- 15.Clausen JL, Wanger JS. [12 Nov 03]; (ATS/NHLBI consensus document).Consensus statement on measurement of lung volumes in humans. http://www.thoracic.org/adobe/lungvolume.pdf.
- 16.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal M, Bain SH, Cramer D, et al. Lung function in white children aged 4 to 19 years: I — spirometry. Thorax. 1993;48:794–802. doi: 10.1136/thx.48.8.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: II — single breath analysis and plethysmography. Thorax. 1993;48:803–8. doi: 10.1136/thx.48.8.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowowiejska B, Tomalak W, Radliński J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3-18 years. Pediatr Pulmonol. 2008;43:1193–7. doi: 10.1002/ppul.20926. [DOI] [PubMed] [Google Scholar]
- 20.Sauzet O, Wright KC, Marston L, Brocklehurst P, Peacock JL. Modelling the hierarchical structure in datasets with very small clusters: a simulation study to explore the effect of the proportion of clusters when the outcome is continuous. Stat Med. 2013;32:1429–38. doi: 10.1002/sim.5638. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peacock JL, Sauzet O, Ewings SM, Kerry SM. Dichotomising continuous data while retaining statistical power using a distributional approach. Stat Med. 2012;31:3089–103. doi: 10.1002/sim.5354. [DOI] [PubMed] [Google Scholar]
- 23.Gerstmann DR, Minton SD, Stoddard RA, et al. The Provo multicenter early high-frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics. 1996;98:1044–57. [PubMed] [Google Scholar]
- 24.Gerstmann DR, Wood K, Miller A, et al. Childhood outcome after early high-frequency oscillatory ventilation for neonatal respiratory distress syndrome. Pediatrics. 2001;108:617–23. doi: 10.1542/peds.108.3.617. [DOI] [PubMed] [Google Scholar]
- 25.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriette G, Paris-Llado J, Walti H, et al. Prospective randomized multicenter comparison of high-frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics. 2001;107:363–72. doi: 10.1542/peds.107.2.363. [DOI] [PubMed] [Google Scholar]
- 27.Stark AR. High-frequency oscillatory ventilation to prevent bronchopulmonary dysplasia — are we there yet? N Engl J Med. 2002;347:682–4. doi: 10.1056/NEJMe020080. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler K, Klingenberg C, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in the neonate. Cochrane Database Syst Rev. 2010;11:CD003666. doi: 10.1002/14651858.CD003666.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Greenough A, Milner AD, Murthy V. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst Rev. 2012;1:CD000456. doi: 10.1002/14651858.CD000456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.