Abstract
In spite of the approaches that have been proposed to reduce postoperative peritoneal adhesions, they remain a major clinical problem because of the associated intestinal obstruction, chronic pelvic pain, female infertility and difficulties at the time of reoperation. The pathogenesis of the process have been focused almost exclusively on the local events induced by the surgical trauma, and the strategies for adhesion prevention thus focused on barriers to separate surgically denuded areas. The important role of the peritoneal cavity environment only recently became apparent and is not yet incorporated in adhesion reducing strategies. Recent data demonstrate that, in the presence of a direct surgical trauma, the entire peritoneal environment is quantitatively the most important factor in adhesion formation and hence adhesion prevention after both open and laparoscopic surgery. Indeed mesothelial hypoxia (CO2 pneumoperitoneum) or hyperoxia (open surgery), desiccation and surgical manipulation have been identified as factors cumulatively enhancing adhesions. The clinical implication is especially relevant for laparoscopic surgery because the pneumoperitoneum, being a closed environment, can be easily conditioned. Although human studies are lacking, animal data indicate that peritoneal adhesions can be reduced by over 80% with a good surgical technique, with adequate pneumoperitoneum conditioning as adding 3-4% of oxygen to the CO2 pneumoperitoneum, prevention of desiccation and slight cooling. Adhesion prevention barriers remain additionally effective, although quantitatively less important. The relevance of all these strategies for adhesion prevention still have to be confirmed in humans, but since it seems that the peritoneal environment is quantitatively much more important than the surgical trauma, adhesion prevention research and strategies should be directed more to conditioning the peritoneal cavity than to the use of agents.
Keywords: laparoscopy, pneumoperitoneum, pneumoperitoneum, adhesion formation, adhesion prevention
Aetiology and types of adhesions
Adhesions are pathological fibrous connections between surfaces within body cavities (e.g. peritoneal, pericardial, pleural, uterine, joint cavities). Peritoneal adhesions can be congenital or acquired, which can be postinflammatory or postoperative (Ellis, 1997). Abdominal surgery is the most common cause of adhesions, 70-85% of all adhesions being attributed to previous surgery (Weibel and Majno, 1973). On the other hand, surgery has been documented as causing adhesions in 55% to 100% of the cases (Diamond, 2000).
Different types of adhesion formation can be distinguished (Diamond and Nezhat, 1993):
Type 1 (de novo adhesion formation): adhesions formed at sites that did not have adhesions previously.
Type 1A: no previous operative procedures at the site of adhesions.
Type 1B: previous operative procedures at the site of adhesions.
Type 2 (adhesion reformation): adhesions formed at sites where adhesiolysis was performed.
Type 2A: no operative procedures at the site of adhesions besides adhesiolysis.
Type 2B: other operative procedures at the site of adhesions besides adhesiolysis.
Clinical significance of adhesions
Depending on their structure and location, peritoneal adhesions may remain silent or cause clinically important complications, such as intestinal obstruction, female infertility, chronic pelvic pain and difficulties at the time of reoperation.
Intestinal obstruction is the most serious complication of peritoneal adhesions as it can be life threatening due to strangulation. Adhesions are the leading cause of intestinal obstruction, accounting for more than 40% of all cases of intestinal obstruction and for 60-70% of those involving the small bowel (Ellis, 1997).
Chronic pelvic pain has been associated with adhesion formation. Although it has been reported that adhesions cause chronic pelvic pain in some 25% of patients (Steege, 2000), from a clinical point of view this association is unclear because does not necessarily imply a causal relationship. Indeed, it was demonstrated that a large number of infertility patients with adhesions do not experience pelvic pain (Steege, 2000). It was suggested that pelvic pain is a consequence of the restricted organ mobility imposed by adhesions and a relief of symptoms after adhesiolysis was reported (Steege, 2000).
Female infertility also has been associated with peritoneal adhesions, which are well recognised as a cause of distortion of the peritoneal factor due to the restrictions of the sweeping of the fimbria over the ovary. Periadnexal adhesions were found in some 20-30% of infertile women and marked increases in pregnancy rates were reported after adhesiolysis (Marana and Muzii, 2000).
Adhesions increases the technical difficulties for repeated surgeries (e.g., access to the abdomen and/or to the operative site, complication rates, anaesthesia, operating and recovery time, use of surgical materials and need for blood transfusion). Therefore, the magnitude of adhesions related disorders (ARD) is larger than could be anticipated and is better illustrated by the reports showing that hospital readmission for ARD rival the number of hip replacements, heart bypass or appendix surgeries, that 35% of women having open gynaecologic surgery are readmitted 1.9 times in 10 years for operation due to adhesions or complicated by adhesions, and that the estimated annual cost for ARD in the USA is 1.3 billion US$ (Ray et al., 1998).
Pathogenesis of peritoneal adhesions
The peritoneum, with a surface are of some 10000 cm2 in adults, almost equal to that of the skin, is the largest organ in humans (diZerega, 1997). It serves to minimise friction and facilitate free movement of abdominal viscera, to resist and localise infections and to store fat. It is composed of a continuous layer of mesothelial cells and a layer of loose connective tissue (diZerega, 2000). Mesothelial cells are highly differentiated in the peritoneum, as well as in the pleura and the pericardium, and their apical surface contain abundant long microvilli that increase the functional surface for absorption and secretion. Mesothelial cells secrete glycosaminoglycans, proteoglycans, and phospholipids to provide a slippery, non-adhesive glycocalyx that protects the serosal surface from abrasion, infection, and tumour dissemination. In addition, mesothelial cells can synthesize cytokines, chemokines, growth factors, and matrix components that regulate inflammation; initiate cell proliferation, differentiation, and migration; and mediate tissue repair (Yung and Chan, 2007). Mesothelial cells are connected to one another by desmosomes and very loosely attached to the underlining basement membrane. The connective tissue is composed of bundles of collagenous and elastic fibres oriented in different directions and a rich network of blood and lymphatic vessels. Interspersed among these fibres and vessels there are poorly differentiated epithelioid-like cells, fibroblasts, macrophages, mast cells and fat cells (diZerega, 2000).
The intact peritoneal cavity contains 3-50 ml of peritoneal fluid with plasma proteins, including a large amount of fibrinogen, and a variety of free-floating cells, including macrophages, lymphocytes, eosinophils, mast cells and desquamated mesothelial cells (diZerega, 2000).
Peritoneal injury, due to surgery, infection or irritation, initiates an inflammatory reaction that increases peritoneal fluid’s proteins and cells, generating a fibrinous exudate and fibrin formation (Holmdahl, 1997). This is the result of the activation of the coagulation cascade, which includes two pathways (i.e., the contact factor or intrinsic pathway and the tissue factor or extrinsic pathway). Activation of these pathways transforms prothrombin (Factor II) into thrombin (Factor IIa) via the common pathway. Thrombin then triggers the conversion of fibrinogen into monomers of fibrin, which interact with each other and polymerise. The initially soluble polymer becomes insoluble by some coagulation factors such as Factor XIIIa and is deposited on the wound surface (Holmdahl, 1997). Within this fibrinous exudate, polymorphonuclears (PMN), macrophages, fibroblasts and mesothelial cells migrate, proliferate and/or differentiate. During the first two postoperative days, a large number of PMN enter and, in the absence of infection, depart within 3-4 days. Macrophages increase in number and change their functions, becoming the most important component of the leukocyte population after day 5. They phagocyte more accurately, have greater respiratory burst activity and secrete a variety of substances including cytokines and growth factors that recruit new mesothelial cells onto the injury surface. Mesothelial cells migrate, form islands throughout the injured area and proliferate in order to cover the denuded area. This reepithelialisation process is different from that occurring in the skin because the entire surface becomes epithelialised simultaneously from the islands of mesothelial cells and not gradually from the borders. Therefore, it is irrespective of the size of the injury and is complete in 5-7 days (diZerega, 2000). The mechanism of mesothelial healing suggests the involvement of stem cells in the process, which is consistent with the fact that mesothelial stem cells can differentiate into mesothelial cells and a few other phenotypes and that mesothelial cells are themselves stem cells (Lucas, 2007).
PMN, macrophages, fibroblasts and mesothelial cells release a variety of substances including plasminogen system components, arachidonic acid metabolites, reactive oxygen species (ROS), cytokines and growth factors, which modulate the process of peritoneal healing and adhesion formation at different stages (Chegini, 2008, Holmdahl, 1997).
Although the fibrinous exudate and fibrin deposition are essential parts of normal tissue repair, a complete resolution is required to restore the preoperative peritoneal conditions. The degradation of fibrin is regulated by the plasminogen system, in which the inactive proenzyme plasminogen is converted into active plasmin by plasminogen activators (PAs), a process that is inhibited by plasminogen activator inhibitors (PAIs) (Holmdahl et al., 1997). Plasminogen is a glycoprotein synthesised in the liver that is abundant in almost all tissues. It is the inactive precursor of plasmin, a serine protease that is highly effective in the degradation of fibrin into fibrin degradation products (FDP) and that has a role in other stages of tissue repair, such as extracellular matrix (ECM) degradation (Wong et al., 1992), activation of proenzymes of the matrix metalloprotease (MMP) family (Murphy et al., 1992), and activation of growth factors (Saksela and Rifkin, 1990). The principal activator of plasminogen is the serine protease tissue-type PA (tPA), which is expressed in endothelial cells, mesothelial cells and macrophages. tPA has a high affinity for fibrin and binds to a specific receptor, which exposes a strong plasminogen-binding site on the surface of the fibrin molecule. Therefore, in the presence of fibrin the activation rate of plasminogen is strikingly enhanced, whereas in the absence of fibrin tPA is a poor activator of plasminogen (Ichinose et al., 1986; Norrman et al., 1985). This results in higher plasminogen activation on the sites where it is required, whereas systemic activation is prevented. The other activator of plasminogen is the serine protease urokinase-type PA (uPA). The properties of uPA differ from those of tPA as it lacks high-affinity binding for fibrin and thus the increased activity in the presence of fibrin. Therefore, uPA is limited in its capacity to activate plasminogen (Lu et al., 1992).
PAs can be counteracted by PAI-1 and PAI-2 through the formation of inactive complexes. The glycoprotein PAI-1 is the most potent inhibitor of tPA and uPA and is expressed in endothelial cells, mesothelial cells, macrophages, platelets and fibroblasts. The glycoprotein PAI-2 is a poorer inhibitor of tPA and uPA and is expressed in mesothelial cells, macrophages and epithelial cells. Other two PAIs have been identified (i.e., PAI-3 and protease nexin 1), but their roles in peritoneal fibrinolysis remain unknown. Plasmin can be directly inhibited by plasmin inhibitors (i.e., 2-macroglobulin, α2-antiplasmin and α1-antitrypsin), but their roles in peritoneal fibrinolysis are not well defined either (Holmdahl et al., 1997).
The balance between fibrin deposition and degradation is critical in determining normal peritoneal healing or adhesion formation. If fibrin is completely degraded, normal peritoneal healing will occur. In contrast, if fibrin is not completely degraded, it will serve as a scaffold for fibroblasts and capillary ingrowth. Indeed, fibroblast will invade the fibrin matrix and ECM will be produced and deposited. The ECM can be completely degraded by MMPs, leading to normal healing. However, if this process is inhibited by tissue inhibitors of MMPs (TIMPs), peritoneal adhesions will be formed.
Laparoscopic surgery and adhesion formation
It has been claimed that laparoscopy is less adhesiogenic than laparotomy but the data are not conclusive. Some authors reported fewer type 1B adhesions after laparoscopy than after laparotomy in rats (Schafer et al., 1998), dogs (Schippers et al., 1998), pigs (Garrard et al., 1999) and rabbits (Luciano et al., 1989), whereas others failed to show differences in rats (Filmar et al., 1987) and in rabbits (Jorgensen et al., 1995, Marana et al., 1994). It was also reported fewer type 1A and type 2A-B adhesions after laparoscopy in rabbits (Luciano et al., 1989), which was not confirmed in other studies in rabbits (Marana et al., 1994). In humans, the only RCT comparing laparotomy and laparoscopy (i.e., patients who underwent surgical treatment for ectopic pregnancy and who then underwent a second look laparoscopy) demonstrated fewer type 1A and type 2A-B adhesions in the laparoscopy group (Lundorff, 1993). Other non-randomised clinical trial also demonstrated less adhesion formation after laparoscopy (Bulletti et al., 1996; Levrant et al., 1997), whereas others reported a low incidence of type 1B adhesions, a very low incidence of type 1A adhesions, and a high incidence of type 2 A-B adhesions after laparoscopy (Diamond et al., 1987).
From all these animal and human data, no definitive conclusion can be drawn, but they strongly suggest that laparoscopy very seldom induces type 1A adhesions, that laparoscopy has some advantages for type 1B adhesions, and that laparoscopy is similar to laparotomy for type 2A-B adhesions.
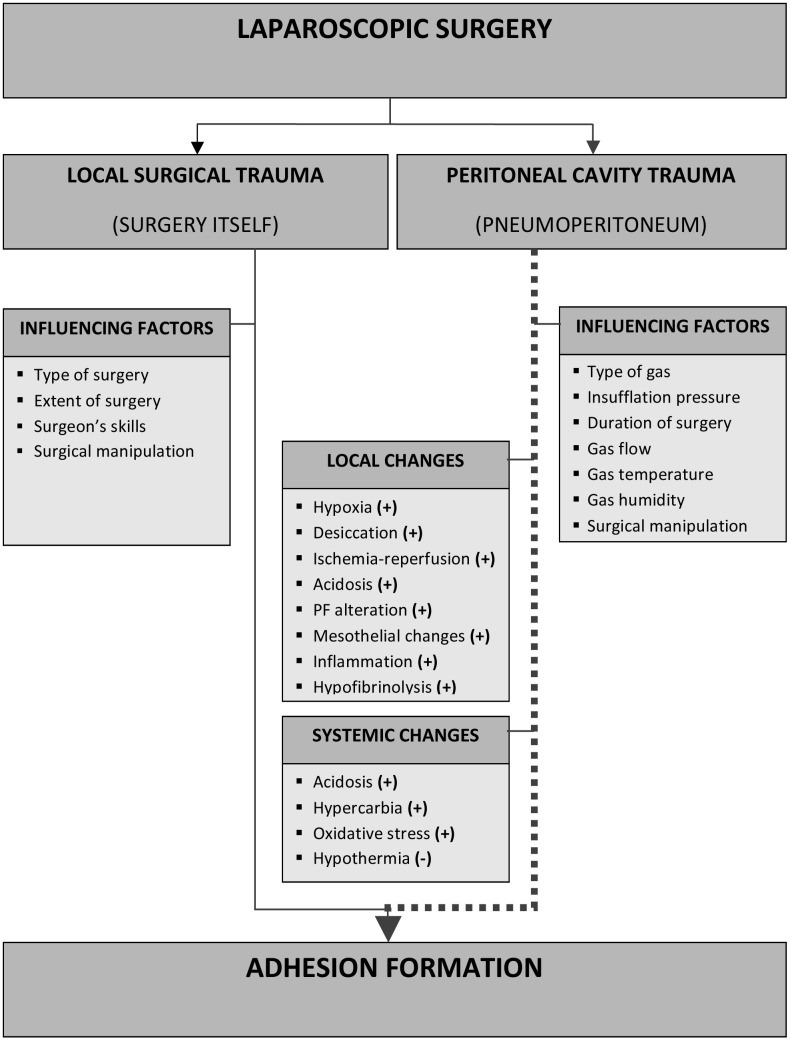
To interpret these data it is important to highlight the differences between laparoscopy and laparotomy in terms of the direct trauma induced by the surgery itself and the indirect trauma that might be induced by the peritoneal environment (Fig. 1). If performed adequately by well-trained surgeons, laparoscopy should induce less direct surgical trauma because of gentle tissue handling, meticulous haemostasis, constant irrigation, the use of microsurgical instruments and the smaller operative field, which may reduce the risk of adhesion formation. On the other hand, laparoscopy and laparotomy are performed in different gas environments, CO2 pneumoperitoneum for the former and air for the latter. Indeed, during laparoscopy pneumoperitoneum is necessary and CO2 is generally used for safety reasons (i.e., its high solubility in water and its high exchange capacity in lungs). CO2 pneumoperitoneum, however, induces some adverse systemic and local effects (Fig. 1).
CO2 pneumoperitoneum’s systemic and local effects
Systemically, CO2 pneumoperitoneum impairs venous return depending on the intraabdominal pressure (Horvath et al., 1998) and induces CO2 absorption from the abdominal cavity, causing acidosis and hypercarbia (Junghans et al., 1997; Liem et al., 1996; Volz et al., 1996), which if not compensated adequately by ventilation can negatively affects the cardiovascular and respiratory function (Neuberger et al., 1996; Volz et al., 1996). CO2 pneumoperitoneum also induces hypothermia (Hazebroek et al., 2002b; Ott, 1991a, 1991b) and decreases splanchnic perfusion with resulting oxidative stress (Sammour et al., 2009), and it is associated with postoperative pain (Helvacioglu and Weis, 1992).
Locally, CO2 pneumoperitoneum induces desiccation (Gray et al., 1999) and peritoneal acidosis (Volz et al., 1996), which may mediate suppression of peritoneal macrophage function (Neuhaus and Watson, 2004). In addition, CO2 alters the peritoneal microcirculation decreasing the ROS scavengers (Taskin et al., 1998), alters the peritoneal fluid (Ott, 2001), modulates the local immune system and the inflammatory reaction (Brokelman et al., 2010), and inhibits the peritoneal plasmin system, leading to peritoneal hypofibrinolysis. Furthermore, it has been demonstrated that the pneumoperitoneum is a cofactor in postoperative adhesion formation (Fig. 1).
1. Factors involved in adhesion formation after laparoscopic surgery.
CO2 pneumoperitoneum is a cofactor in adhesion formation
In an early study of our group evaluating the effect of training upon adhesion formation in rabbits it become accidentally evident that longer laparoscopic surgeries were associated with more adhesions, and that both duration of surgery and adhesion formation decreased with training (Ordonez et al., 1997). Based on these observations it was assumed that the surgical trauma was more severe during the longer surgeries reported at the beginning of the study, and remained undetermined the specific contribution of each factor (i.e., surgical trauma and duration of surgery/pneumoperitoneum) to adhesion formation.
To evaluate the effect of duration of surgery, and more specifically of the pneumoperitoneum, laparoscopic rabbit and mouse models were developed and surgeries were performed by well trained surgeons in order ascertain standardised lesions to induce adhesions. The first variable evaluated was the duration of the CO2 pneumoperitoneum. Indeed, the lesions were performed in some 3-5 minutes but the pneumoperitoneum was maintained for different time periods. Since adhesion formation clearly increased with the duration of the pneumoperitoneum the concept that pneumoperitoneum is a cofactor in adhesion formation was born (Molinas et al., 2001; Molinas and Koninckx, 2000; Yesildaglar et al., 1999). Subsequently, a series of experiments were carried out to assess a variety of pneumoperitoneum-related parameters and thus to elucidate the potential mechanisms involved (e.g., peritoneal hypoxia, peritoneal acidosis/hypercarbia, desiccation, hypothermia, etc.).
Pneumoperitoneum-induced hypoxia as a driving mechanism
It was hypothesised that the pneumoperitoneum compresses the capillary flow in the superficial peritoneal layers inducing ischemia, which might trigger a hypoxic peritoneal response eventually leading to adhesion formation. This hypothesis was tested by evaluating the effect of duration of pneumoperitoneum, insufflation pressure and insufflation gas.
In a series of rabbit and mouse studies using CO2 and helium pneumoperitoneum it was demonstrated that adhesion formation increases with the duration of the pneumoperitoneum and with the insufflation pressure, without differences between both insufflation gases. Furthermore, it was reported that adhesion formation decreases with the addition of 2-4% of oxygen to the pneumoperitoneum, an effect that was no longer observed with higher oxygen concentrations (e.g., 12%) (Elkelani et al., 2004; Molinas et al., 2001; Molinas and Koninckx, 2000; Yesildaglar et al., 1999; Yesildaglar and Koninckx, 2000).
To understand the role of peritoneal hypoxia it is important to bear in mind that in normal conditions peripheral cells receive oxygen from the vascular network and have a partial pressure of oxygen (pO2) of 23 mm Hg (5-40 mm Hg depending on the cells type and on the distance to the capillaries). This intracellular pO2 is the result of a progressive decrease of the pO2 from 160 mm Hg in the air to 95 mm Hg in the arterial end of the capillaries, and to 40 mm Hg in the interstitial fluid (Guyton and Hall, 2000). The pneumoperitoneum, depending on the pressure and on the time of exposure, and independently on the insufflation gas, will compress the capillary flow in the superficial peritoneal layers, decreasing tissue perfusion, inducing ischemia and reducing the pO2 in the mesothelial cells up to hypoxic levels (Caldwell and Ricotta, 1987; Eleftheriadis et al., 1996a, 1996b; Kotzampassi et al., 2000). In addition, the insufflation gas present in the abdominal cavity (e.g., CO2) will diffuse through the apical surface of the mesothelial cells to the bloodstream, decreasing the mesothelial pO2 and inducing hypercarbia and acidosis if not adequately corrected by the assisted ventilation. During standard laparoscopy (100% CO2 pneumoperitoneum), mesothelial cells do not receive sufficient oxygen supply from the capillaries and the pure CO2 present in the abdominal cavity diffuses into the mesothelial cells. During laparoscopy with CO2 pneumoperitoneum with 3% of oxygen, mesothelial cells do not receive sufficient oxygen from the capillaries either, but since the insufflation gas has a pO2 of 23 mm Hg, which is remarkably similar to the normal intracellular pO2, they could absorb the oxygen present in the abdominal cavity, raising the intracellular pO2 up to more physiologic levels. During laparoscopy with CO2 pneumoperitoneum with 12% of oxygen, mesothelial cells do not receive adequate oxygen supply from the capillaries either, but since the insufflation gas has a pO2 of 92 mm Hg the oxygen would diffuse into the mesothelial cells increasing the intracellular pO2 to levels higher than normal.
The hypothesis of peritoneal hypoxia is supported by the absence of CO2 pneumoperitoneum-enhanced adhesions in mice deficient for some factors regulated by cellular hypoxia, such as hypoxia inducible factors (HIFs) (i.e., HIF-1 and HIF-2) (Molinas et al., 2003b), PAI-1 (Molinas et al., 2003c) and members of the vascular endothelial growth factor (VEGF) family (i.e. VEGF-A, VEGF-B and placental growth factor (PlGF)) (Molinas et al., 2003a), and by blocking pneumoperitoneum-enhanced adhesions in mice treated with antibodies against placental growth factor (Molinas et al., 2003a) and against the VEGF receptor 1 (Flt-1) (Molinas et al., 2004a). Other studies in rats demonstrate that the intra-peritoneal administration of small interfering RNA (siRNAs), which downregulate HIF-1 and PAI-1 expression, prevents postoperative adhesions, confirming the role of hypoxia, HIF-1 and PAI-1 (Segura et al., 2007).
The key role of peritoneal hypoxia is also supported by the report of tissue pO2 measured with a flexible micro-catheter implanted in the abdominal wall in rats in which it was demonstrated that both CO2 and helium pneumoperitoneum decreases the pO2 to about 5 mm Hg whereas insufflation with a non-hypoxic gas mixture (80% CO2 and 20% O2) induces no significant changes (Wildbrett et al., 2003).
Although the suggested CO2 pneumoperitoneum-induced peritoneal hypoxia was not confirmed by other authors using a non-injured peritoneum laparoscopic mouse model (Bourdel et al., 2007), subsequent studies of the same group, using the same model with both injured and non-injured peritoneum, clearly demonstrate peritoneal tissue and cellular hypoxia during CO2 pneumoperitoneum at high insufflation pressures at both the injured and the distant peritoneal sites (Matsuzaki et al., 2010).
Pneumoperitoneum-induced acidosis/hypercarbia as a driving mechanism
The relation between CO2 pneumoperitoneum-induced acidosis/ hypercarbia and adhesion formation has been addressed in a laparoscopic mouse model in which animals with endotracheal intubation were mechanically ventilated with different patterns (Molinas et al., 2004b). In a first series of experiments, mice were exposed to pure CO2 pneumoperitoneum during laparoscopic surgery for induction of adhesions, which were evaluated on postoperative day 7. In a second series of experiments, mice were exposed to anaesthesia only or to anaesthesia plus CO2 pneumoperitoneum and arterial blood gases were measured at the end of the procedures. Adhesions formation was higher in animals poorly ventilated and decreased with higher ventilation rates (ml/min). In comparison with animals who underwent anaesthesia only, the CO2 pneumoperitoneum increases the pCO2 and decreases the pH, as has been reported in pigs (Liem et al., 1996), dogs (Kotzampassi et al., 1993), rabbits (Mynbaev et al., 2002), rats (Hazebroek et al., 2002a) and humans (Neuberger et al., 1996). These effects were more pronounced in mice poorly ventilated and counteracted by appropriate ventilation (i.e., higher ventilation rates).
These data demonstrate an association between CO2 pneumoperitoneum-induced acidosis/hypercarbia and adhesion formation. The mechanism whereby this acidosis/hypercarbia becomes a cofactor in adhesion formation remains unclear. CO2 pneumoperitoneum induces respiratory acidosis that, if not corrected, leads to metabolic acidosis and metabolic hypoxia (Mynbaev et al., 2002). This could enhance the ischemic hypoxia in the peritoneum, which was suggested to be a driving mechanism in CO2 pneumoperitoneum-enhanced adhesion formation. Obviously a direct effect of acidosis/ hypercarbia upon cells and molecules involved in adhesion formation cannot be excluded. Indeed, acidosis affects lymphocyte and macrophage functions altering cellular and humoral immune function (Lardner, 2001) and up-regulates VEGF expression independently from hypoxia (Fukumura et al., 2001), which has been reported to be involved in adhesion formation (Molinas et al., 2003a, 2004a; Rout et al., 2000; Saltzman et al., 1996; Wiczyk et al., 1998).
Although these kind of studies are difficult to reproduce in humans and that the clinical significance of the data is unclear, the importance of CO2 pneumoperitoneum-induced acidosis/hypercarbia should be taken into account for patients in steep Trendelenburg position or with limited cardiovascular adaptation, such as obese and heavy smoker patients, and for laparoscopic surgery of the retroperitoneum or of long duration.
Pneumoperitoneum-induced desiccation and temperature’s changes as driving mechanisms
The abdominal insufflation with the standard dry and cold CO2 for creating the pneumoperitoneum determines that the gas entering the cavity will be warmed to reach an equilibrium in temperature (between the cold gas and the warm peritoneum) and will be humidified to reach and equilibrium in humidity (between the dry gas and the wet peritoneum). Both processes occur at expenses of the patient and more specifically of the peritoneum. Indeed, the peritoneum will lose temperature and water to reach the equilibrium, which consumes energy and consequently induces hypothermia in the patient (Bessell et al., 1995; Bessell et al., 1999). The energy required to warm the cold gas (0.00003 cal to heat 1 mL of CO2 by 1°C) is much less than the energy needed to humidify the dry gas (577 cal to vaporize 1 g of water) (Binda et al., 2006). Therefore, the pneumoperitoneum-induced hypothermia is to a large extent caused by the pneumoperitoneum-induced desiccation, both effects being intimately associated.
Desiccation of the peritoneum will tends to continue till the pneumoperitoneum reaches 100% of relative humidity (amount of water held by a gas in relation with the maximum amount that can be hold at certain temperature). Hence, it will depend on the time of exposure to the insufflation gas (i.e., more desiccation with longer times), the flow rate through the abdominal cavity (i.e., more desiccation with more gas leakage) and the temperature of the insufflation gas. The effect of this later factor is crucial because the absolute humidity of a gas (mg of water in a litre of gas) is higher at higher temperatures and therefore 100% relative humidity will only be reached with more water (i.e. the higher the insufflation gas temperature the more the water will be needed to reach the equilibrium, and thus the more desiccation will occur).
It was postulated that the desiccation caused by the dry and cold CO2 pneumoperitoneum will favour the development of peritoneal adhesions. In vitro studies confirm that the degree of desiccation depends on the flow rate of the gas through the humidified surface. Indeed, when dry and cold CO2 circulates through water-filled flaks water is lost depending on the flow rate; the higher the flow the more desiccation is observed (Yesildaglar et al., 1999). Knowing that desiccation is flow-dependent, the effect of dry CO2 with different flow rates through the abdominal cavity upon adhesion formation was evaluated in rabbits (Yesildaglar and Koninckx, 2000) and mice (Yesildaglar et al., 1999). Since adhesion formation increases with higher flow rates, the key role of desiccation in the pathogenesis of the process was evident. However, because desiccation and hypothermia are intimately linked, the specific contribution of each factor to adhesion formation was difficult to determine.
During a series of experiments performed in a laparoscopic mouse model it become accidentally apparent that mice with lower body temperatures develop fewer adhesions than normothermic mice. Since these totally unexpected observations were very provocative, the effect of body temperature upon adhesion formation was investigated more deeply in subsequent studies.
To address the specific effect of hypothermia without the confounding effect of desiccation, we performed a study using humidified insufflation gas and strictly controlling mouse body temperature throughout the entire surgery. The reduction of adhesion formation with hypothermia was confirmed (Binda et al., 2004). Consistent with these observations, other animal data demonstrated that peritoneal infusion with cold saline decreased postoperative adhesions (Fang et al., 2010), whereas irrigation with warm saline increased postoperative adhesions (Kappas et al., 1988). In humans, local hypothermia after laparotomy was reported to decrease the inflammatory reaction and to increase intestinal peristalsis, thus decreasing adhesion formation (Gataullin et al., 1971).
To address the pure effect of desiccation without the normally associated hypothermia we have designed an experiment in mice in which animals exposed to dry and cold CO2 pneumoperitoneum with different flow rates through the abdominal cavity (for having different level of desiccation) were protected against hypothermia by covering them with warmed body blankets. As expected, adhesion formation increases with desiccation. Furthermore, this desiccation-induced adhesion formation was prevented by using humidified gas (Binda et al., 2006), which was confirmed by other groups in rats (Peng et al., 2009). Interestingly, this desiccation-induced adhesion formation was also reduced by leaving the animals to develop the normally associated hypothermia (Binda et al., 2006), indicating that both desiccation and hyperthermia contributes independently to adhesion formation. In addition, this experiment also confirmed excellent humidifying capacity of the peritoneal cavity since the intraperitoneal relative humidity of mice in all groups with dry insufflation gas was 100%.
Several mechanisms might be involved in this beneficial effect of hypothermia and detrimental effect of desiccation. Hypothermia might reduce adhesion formation by protecting tissues and cells from the pneumoperitoneum-induced hypoxia, since cell oxygen consumption decreases with temperature. Indeed, hypothermia decreases the global cerebral metabolic rate during ischaemia, slowing the breakdown of glucose, phosphocreatine and ATP and the formation of lactate and inorganic phosphate (Erecinska et al., 2003). In addition, hypothermia reduces the production of ROS during reperfusion in several tissues and organs (Horiguchi et al., 2003; Prasad et al., 1992; Zhao et al., 1996), improves the recovery of energetic parameters during reperfusion (Erecinska et al., 2003), and suppresses the inflammatory response decreasing the infiltration of PMN cells and the production of tumour necrosis factor-a, interleukin-1b and macrophage inflammatory protein-2 (Kato et al., 2002; Patel et al., 2000).
This hypothesis of desiccation as a driving mechanism in adhesion formation is supported by the data demonstrating that the dry and cold CO2 pneumoperitoneum alters the morphology of the mesothelium (i.e., destroys the hexagonal pattern, reduces the microvilli and bulges up the cells) (Hazebroek et al., 2002b; Mouton et al., 1999; Suematsu et al., 2001; Volz et al., 1999), which can favour the development of postoperative adhesions.
Discussion and conclusions
If these pneumoperitoneum-induced changes contributes to adhesion formation because of purely local effects or because of more general and systemic effects are still difficult to determine. There is emerging evidence suggesting that the pathogenesis of peritoneal adhesion formation is not restricted to the operative site (classical model) and that the entire peritoneal cavity, and maybe the entire organism, could be involved. Consistent with this hypothesis it was recently demonstrated in mice that adhesion formation increases at the lesion site (i.e., uterine horns and pelvic side walls) when the distant peritoneum (i.e., omentum and bowels) is manipulated and that this effect depends on the severity of the manipulation (Schonman et al., 2009).
Although most of these data derives from small animal studies, the relevance of the peritoneal environment in the pathogenesis of adhesion formation is clear and the importance of its proper modulation for adhesion prevention becomes evident.
Pneumoperitoneum-induced peritoneal hypoxia can be reduced by adding 3% of oxygen to the CO2 pneumoperitoneum, whereas its direct consequences, such as the up-regulation of HIFs, PAI-1, VEGF, can be prevented by specific antibodies against these factors. Some preliminary data in humans indicate that the addition of oxygen could also have some beneficial effects in terms of postoperative pain reduction (unpublished observations).
Pneumoperitoneum-induced ischemia-reperfusion generate ROS, which can be minimize by reducing the insufflation pressure (Kaya et al., 2002; Sare et al., 2002) or by “ischemic preconditioning”, a concept that consists of short periods of inflation and deflation upon establishment of pneumoperitoneum (Cevrioglu et al., 2004; Sahin et al., 2007).
Pneumoperitoneum-induced peritoneal desiccation can be reduced by humidifying the insufflation gas, which in addition to reduce adhesion formation will have other local and systemic beneficial effects, such as less destruction of the mesothelium, less postoperative pain and less hypothermia.
The alteration of the peritoneal surface reported in mice, rats and pigs depends on the time of exposure, the insufflation pressure, and the type of insufflation gas (Hazebroek et al., 2002b; Mouton et al., 1999; Suematsu et al., 2001; Volz et al., 1999) and are highly influenced by the temperature and humidity of the insufflation gas. Indeed, some animal studies shows less peritoneal damage with warm and humidified CO2 (Erikoglu et al., 2005; Mouton et al., 1999; Peng et al., 2009). This protective effect is, however, not conclusive because others studies failed to reach the same conclusions (Glew et al., 2004; Hazebroek et al., 2002b).
The dry and cold gas used for creating the pneumoperitoneum was claimed to contribute to postoperative pain because the dominant source of pain and discomfort after laparoscopy comes from the peritoneum rather than from the skin or the abdominal wall (Helvacioglu and Weis, 1992). Several human studies have demonstrated that the use warm and humidified insufflation gas is associated with less postoperative pain and analgesic requirements (Sajid et al., 2008; Sammour et al., 2008). In addition, other studies reported that warm and humidified gas reduces the recovering room stay (Ott et al., 1998) and the hospitalisation length (Savel et al., 2005), and that it is associated with a faster return to normal activities (Mouton et al., 1999).
Human data also confirms that the CO2 pneumoperitoneum-induced hypothermia is prevented with warm and humidified insufflation gas (Sajid et al., 2008). However, there are some evidences that cold and humidified CO2 is as efficaciously as warm and humidified gas for preventing hypothermia (Schlotterbeck et al., 2008).
Since the side effects of the hypothermia are well known (Insler and Sessler, 2006), surgeons, anaesthesiologists and nurses working in the operating room have always pretended to keep the patient normothermic. Recent data, however, demonstrated that keeping the abdominal cavity slightly cold (i.e., 32ºC) and humidified rather than warm and humidified can be useful, or even better, for preventing adhesion formation (Binda et al., 2004; Binda et al., 2006; Fang et al., 2010; Kappas et al., 1988) because this local hypothermia at the trauma site will minimize the local inflammation, and the toxic effects of the hypoxia and of the ischemia-reperfusion processes. The optimal way to achieve this challenging and provocative approach (i.e., normothermic patient with slightly cold abdominal cavity) still has to be determined.
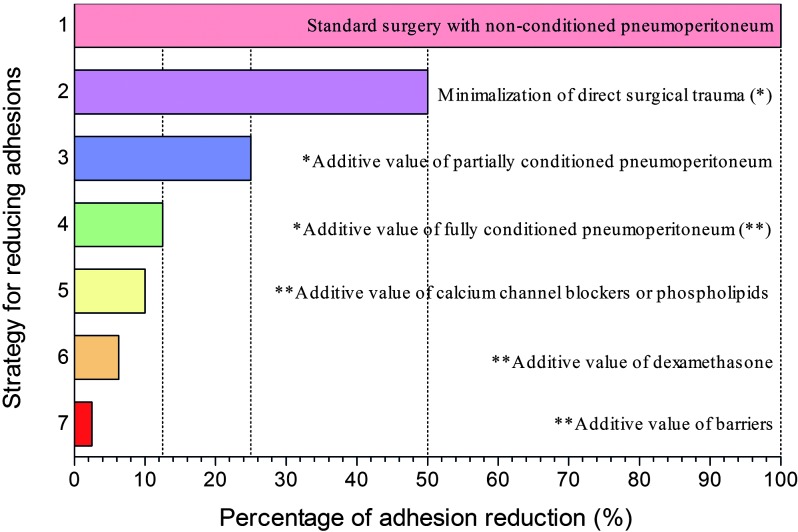
Our studies in the laparoscopic mouse model demonstrate that good surgical technique, avoiding as much as possible collateral damage and unnecessary manipulation, and which can only be achieved by appropriate training, reduces adhesion by some 50%, whereas CO2 pneumoperitoneum conditioning (i.e., humidification, addition of 3-4% of oxygen, and/or slight cooling) has an additional preventive effect (50% for partial conditioning and another 50% for full conditioning) (Fig. 2) (Binda et al., 2004; Binda et al., 2006; Binda and Koninckx, 2009). The efficacy of adhesion prevention agents were also investigated in mice in two different models, the standard CO2 pneumoperitoneum and the conditioned CO2 pneumoperitoneum. The available data already demonstrate that the effects of most products varies with the model (Binda et al., 2007a, 2007b, 2009), indicating that the role of the peritoneal environment is crucial not only for adhesion formation but also for adhesion prevention. Interestingly, it was demonstrated that the combination of good surgical technique, conditioned pneumoperitoneum and some adhesion prevention agents (e.g., calcium channel blocker, phospholipids, dexametasone, Hyalobarrier gel) can reduce adhesion formation up to some 90% (Fig. 2) (Binda and Koninckx, 2009).
2. Strategies for reducing adhesions (data from a laparoscopic mouse model).
From the maximum adhesions that can be observed in the model (1) good surgical technique (reduction of collateral damage and unnecessary manipulation) reduces adhesions by some 50% (2). Partial pneumoperitoneum conditioning (addition of 3% of oxygen, humidification or slight cooling) further reduces adhesions by another 50% (3), whereas with full pneumoperitoneum conditioning an additional 50% of reduction is observed (4). From this already reduced amount of adhesions calcium channel blockers or phospholipids (5), dexametasone (6) or hyalobarrier gel (7) provide some additive effect.
In conclusion, there is growing evidence indicating that in addition to the direct surgical trauma the entire peritoneal environment plays a key role in postoperative adhesion formation (Fig. 1), although it alone would not be able to induce de novo adhesions. The importance of the peritoneal environment is crucial for both adhesion formation and adhesion prevention after both open and laparoscopic surgery. The important role of the peritoneal cavity environment only recently became apparent and is not yet incorporated in adhesion reducing strategies. The practical implications of this peritoneal environment are more relevant for laparoscopy because the pneumoperitoneum, being a closed environment, can be evaluated, modulated and conditioned more easily than the open environment at laparotomy. Although human studies are lacking, animal data indicate that the standard dry and cold CO2 pneumoperitoneum is a cofactor in adhesion formation because it induces hypoxia, acidosis and desiccation. The pure effect of desiccation, however, is underestimated because it is associated with hypothermia, which surprisingly has a protective effect against adhesion formation. Our data in the laparoscopic mouse model indicate that peritoneal adhesions can be reduced to a large extent with a proper surgical technique, with adequate pneumoperitoneum conditioning (i.e., addition of 3% of oxygen, humidification and slightly cooling of the insufflation gas) and with the use of adhesion prevention agents (Fig. 2). They also indicate that the efficacy of these agents varies according to the local environment. The ideal “low” temperature for the pneumoperitoneum and the optimal approach to keep the patient normothermic while having a moderate local hypothermia remains to be elucidated. The relevance of all these strategies for peritoneal environment conditioning to reduce adhesion formation and the efficacy of adhesion prevention agents under specific peritoneal conditions still have to be confirmed in larger animals and in humans. If the key role of the peritoneal environment is confirmed in humans, research and strategies for improving patient compliance (e.g., postoperative pain) in general and for adhesion prevention in particular will be directed more to conditioning the peritoneal cavity than to the use of agents.
References
- Bessell JR, Karatassas A, Patterson JR, et al. Hypothermia induced by laparoscopic insufflation. A randomized study in a pig model. Surg Endosc. 1995;9:791–796. doi: 10.1007/BF00190083. [DOI] [PubMed] [Google Scholar]
- Bessell JR, Ludbrook G, Millard SH, et al. Humidified gas prevents hypothermia induced by laparoscopic insufflation: a randomized controlled study in a pig model. Surg Endosc. 1999;13:101–105. doi: 10.1007/s004649900914. [DOI] [PubMed] [Google Scholar]
- Binda MM, Hellebrekers BW, Declerck PJ, et al. Effect of Reteplase and PAI-1 antibodies on postoperative adhesion formation in a laparoscopic mouse model. Surg Endosc. 2009;23:1018–1025. doi: 10.1007/s00464-008-0111-x. [DOI] [PubMed] [Google Scholar]
- Binda MM, Koninckx PR. Prevention of adhesion formation in a laparoscopic mouse model should combine local treatment with peritoneal cavity conditioning. Hum Reprod. 2009;24:1473–1479. doi: 10.1093/humrep/dep053. [DOI] [PubMed] [Google Scholar]
- Binda MM, Molinas CR, Bastidas A, et al. Efficacy of barriers and hypoxia-inducible factor inhibitors to prevent CO(2) pneumoperitoneum-enhanced adhesions in a laparoscopic mouse model. J Minim Invasive Gynecol. 2007a;14:591–599. doi: 10.1016/j.jmig.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Binda MM, Molinas CR, Bastidas A, et al. Effect of reactive oxygen species scavengers, antiinflammatory drugs, and calcium-channel blockers on carbon dioxide pneumoperi-toneum-enhanced adhesions in a laparoscopic mouse model. Surg Endosc. 2007b;21:1826–1834. doi: 10.1007/s00464-007-9296-7. [DOI] [PubMed] [Google Scholar]
- Binda MM, Molinas CR, Hansen P, et al. Effect of desiccation and temperature during laparoscopy on adhesion formation in mice. Fertil Steril. 2006;86:166–175. doi: 10.1016/j.fertnstert.2005.11.079. [DOI] [PubMed] [Google Scholar]
- Binda MM, Molinas CR, Mailova K, et al. Effect of temperature upon adhesion formation in a laparoscopic mouse model. Hum Reprod. 2004;19:2626–2632. doi: 10.1093/humrep/deh495. [DOI] [PubMed] [Google Scholar]
- Bourdel N, Matsuzaki S, Bazin JE, et al. Peritoneal tissue-oxygen tension during a carbon dioxide pneumoperitoneum in a mouse laparoscopic model with controlled respiratory support. Hum Reprod. 2007;22:1149–1155. doi: 10.1093/humrep/del482. [DOI] [PubMed] [Google Scholar]
- Brokelman WJ, Lensvelt M, Rinkes IH, et al. Peritoneal changes due to laparoscopic surgery. Surg Endosc. doi: 10.1007/s00464-010-1139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulletti C, Polli V, Negrini V, et al. Adhesion formation after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1996;3:533–536. doi: 10.1016/s1074-3804(05)80163-x. [DOI] [PubMed] [Google Scholar]
- Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res. 1987;43:14–20. doi: 10.1016/0022-4804(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Cevrioglu AS, Yilmaz S, Koken T, et al. Comparison of the effects of low intra-abdominal pressure and ischaemic preconditioning on the generation of oxidative stress markers and inflammatory cytokines during laparoscopy in rats. Hum Reprod. 2004;19:2144–2151. doi: 10.1093/humrep/deh380. [DOI] [PubMed] [Google Scholar]
- Chegini N. TGF-beta system: the principal profibrotic mediator of peritoneal adhesion formation. Semin Reprod Med. 2008;26:298–312. doi: 10.1055/s-0028-1082388. [DOI] [PubMed] [Google Scholar]
- Diamond MP. Incidence of postsurgical adhesions. In diZerega GS (eds) Peritoneal surgery. New York, USA: Springer-Verlag; 2000. pp. 217–220. [Google Scholar]
- Diamond MP, Daniell JF, Feste J, et al. Adhesion reformation and de novo adhesion formation after reproductive pelvic surgery. Fertil Steril. 1987;47:864–866. doi: 10.1016/s0015-0282(16)59181-x. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Nezhat F. Adhesions after resection of ovarian endometriomas. Fertil Steril. 1993;59:934–935. [PubMed] [Google Scholar]
- diZerega GS. Biochemical events in peritoneal tissue repair. Eur J Surg Suppl. 1997:10–16. [PubMed] [Google Scholar]
- diZerega GS. New York, USA: Springer-Verlag; 2000. Peritoneum, peritoneal healing and adhesion formation. In diZerega GS (eds) Peritoneal surgery; pp. 3–38. [Google Scholar]
- Eleftheriadis E, Kotzampassi K, Botsios D, et al. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996a;10:324–326. doi: 10.1007/BF00187381. [DOI] [PubMed] [Google Scholar]
- Eleftheriadis E, Kotzampassi K, Papanotas K, et al. Gut ischemia, oxidative stress, and bacterial translocation in elevated abdominal pressure in rats. World J Surg. . 1996b;20:11–16. doi: 10.1007/s002689900002. [DOI] [PubMed] [Google Scholar]
- Elkelani OA, Binda MM, Molinas CR, et al. Effect of adding more than 3% oxygen to carbon dioxide pneumoperitoneum on adhesion formation in a laparoscopic mouse model. Fertil Steril. 2004;82:1616–22. doi: 10.1016/j.fertnstert.2004.07.933. [DOI] [PubMed] [Google Scholar]
- Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997:5–9. [PubMed] [Google Scholar]
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- Erikoglu M, Yol S, Avunduk MC, et al. Electron-microscopic alterations of the peritoneum after both cold and heated carbon dioxide pneumoperitoneum. J Surg Res. 2005;125:73–77. doi: 10.1016/j.jss.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Fang CC, Chou TH, Lin GS, et al. Peritoneal infusion with cold saline decreased postoperative intra-abdominal adhesion formation. World J Surg. 2010;34:721–727. doi: 10.1007/s00268-009-0378-7. [DOI] [PubMed] [Google Scholar]
- Filmar S, Gomel V, McComb PF. Operative laparoscopy versus open abdominal surgery: a comparative study on postoperative adhesion formation in the rat model. Fertil Steril. 1987;48:486–489. doi: 10.1016/s0015-0282(16)59423-0. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Xu L, Chen Y, et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- Garrard CL, Clements RH, Nanney L, et al. Adhesion formation is reduced after laparoscopic surgery. Surg Endosc. 1999;13:10–13. doi: 10.1007/s004649900887. [DOI] [PubMed] [Google Scholar]
- Gataullin NG, Khunafin SN, Mukhamedrakhimov RF. [Prevention of postoperative peritoneal adhesions] Vestn Khir Im I I Grek. 1971;106:58–62. [PubMed] [Google Scholar]
- Glew PA, Campher MJ, Pearson K, et al. The effect of warm humidified CO2 on the dissipation of residual gas following laparoscopy in piglets. Am Assoc Gynecol Laparosc. 2004;11:204–210. doi: 10.1016/s1074-3804(05)60200-9. [DOI] [PubMed] [Google Scholar]
- Gray RI, Ott DE, Henderson AC, et al. Severe local hypothermia from laparoscopic gas evaporative jet cooling: a mechanism to explain clinical observations. JSLS. 1999;3:171–177. [PMC free article] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Transport of oxygen and carbon dioxide in the blood and body fluids. In Guyton AC and Hall JE (eds) Textbook of medical physiology. Philadelphia, USA: WB Saunders; 2000. pp. 463–473. [Google Scholar]
- Hazebroek EJ, Haitsma JJ, Lachmann B, et al. Impact of carbon dioxide and helium insufflation on cardiorespiratory function during prolonged pneumoperitoneum in an experimental rat model. Surg Endosc. 2002;16:1073–1078. doi: 10.1007/s00464-001-8248-x. [DOI] [PubMed] [Google Scholar]
- Hazebroek EJ, Schreve MA, Visser P, et al. Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A. 2002b;12:355–364. doi: 10.1089/109264202320884108. [DOI] [PubMed] [Google Scholar]
- Helvacioglu A, Weis R. Operative laparoscopy and postoperative pain relief. Fertil Steril. 1992;57:548–552. [PubMed] [Google Scholar]
- Holmdahl L. The role of fibrinolysis in adhesion formation. Eur J Surg Suppl. 1997:24–31. [PubMed] [Google Scholar]
- Holmdahl L, Falkenberg M, Ivarsson ML, et al. Plasminogen activators and inhibitors in peritoneal tissue. APMIS. 1997;105:25–30. doi: 10.1111/j.1699-0463.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Shimizu K, Ogino M, et al. Postischemic hypothermia inhibits the generation of hydroxyl radical following transient forebrain ischemia in rats. J Neurotrauma. 2003;20:511–520. doi: 10.1089/089771503765355577. [DOI] [PubMed] [Google Scholar]
- Horvath KD, Whelan RL, Lier B, et al. The effects of elevated intraabdominal pressure, hypercarbia, and positioning on the hemodynamic responses to laparoscopic colectomy in pigs. Surg Endosc. 1998;12:107–114. doi: 10.1007/s004649900608. [DOI] [PubMed] [Google Scholar]
- Ichinose A, Takio K, Fujikawa K. Localization of the binding site of tissue-type plasminogen activator to fibrin. J Clin Invest. 1986;78:163–169. doi: 10.1172/JCI112546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insler S R, Sessler D I. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin. 2006;24:823–837. doi: 10.1016/j.atc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Lalak NJ, Hunt DR. s laparoscopy associated with a lower rate of postoperative adhesions than laparotomy? A comparative study in the rabbit. Aust N Z J Surg. 1995;65:342–344. doi: 10.1111/j.1445-2197.1995.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Junghans T, Bohm B, Grundel K, et al. Effects of pneumoperitoneum with carbon dioxide, argon, or helium on hemodynamic and respiratory function. Arch Surg. 1997;132:272–278. doi: 10.1001/archsurg.1997.01430270058012. [DOI] [PubMed] [Google Scholar]
- Kappas AM, Fatouros M, Papadimitriou K, et al. Effect of intraperitoneal saline irrigation at different temperatures on adhesion formation. Br J Surg. 1988;75:854–856. doi: 10.1002/bjs.1800750908. [DOI] [PubMed] [Google Scholar]
- Kato A, Singh S, McLeish KR, et al. Mechanisms of hypothermic protection against ischemic liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G608–616. doi: 10.1152/ajpgi.00454.2001. [DOI] [PubMed] [Google Scholar]
- Kaya Y, Coskun T, Demir MA, et al. Abdominal insufflation-deflation injury in small intestine in rabbits. Eur J Surg. 2002;168:410–417. doi: 10.1080/110241502320789104. [DOI] [PubMed] [Google Scholar]
- Kotzampassi K, Kapanidis N, Kazamias P, et al. Hemodynamic events in the peritoneal environment during pneumoperitoneum in dogs. Surg Endosc. 1993;7:494–499. doi: 10.1007/BF00316688. [DOI] [PubMed] [Google Scholar]
- Kotzampassi K, Paramythiotis D, Eleftheriadis E. Deterioration of visceral perfusion caused by intra-abdominal hypertension in pigs ventilated with positive end-expiratory pressure. Surg Today. 2000;30:987–992. doi: 10.1007/s005950070018. [DOI] [PubMed] [Google Scholar]
- Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69:522–530. [PubMed] [Google Scholar]
- Levrant SG, Bieber EJ, Barnes RB. Anterior abdominal wall adhesions after laparotomy or laparoscopy. J Am Assoc Gynecol Laparosc. 1997;4:353–356. doi: 10.1016/s1074-3804(05)80227-0. [DOI] [PubMed] [Google Scholar]
- Liem TK, Krishnamoorthy M, Applebaum H, et al. A comparison of the hemodynamic and ventilatory effects of abdominal insufflation with helium and carbon dioxide in young swine. J Pediatr Surg. 1996;31:297–300. doi: 10.1016/s0022-3468(96)90020-2. [DOI] [PubMed] [Google Scholar]
- Lu HR, Wu Z, Pauwels P, et al. Comparative thrombolytic properties of tissue-type plasminogen activator (t-PA), single-chain urokinase-type plasminogen activator (u-PA) and K1K2Pu (a t-PA/u-PA chimera) in a combined arterial and venous thrombosis model in the dog. J Am Coll Cardiol. 1992;19:1350–1359. doi: 10.1016/0735-1097(92)90344-m. [DOI] [PubMed] [Google Scholar]
- Lucas PA. Stem cells for mesothelial repair: an understudied modality. Int J Artif Organs. 2007;30:550–556. doi: 10.1177/039139880703000613. [DOI] [PubMed] [Google Scholar]
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220–224. [PubMed] [Google Scholar]
- Lundorff P. Treatment of ectopics and subsequent adhesion formation. Prog Clin Biol Res. 381:139–147. [PubMed] [Google Scholar]
- Marana R, Luciano AA, Muzii L, et al. Laparoscopy versus laparotomy for ovarian conservative surgery: a randomized trial in the rabbit model. Am J Obstet Gynecol. 1994;171:861–864. doi: 10.1016/0002-9378(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Marana R, Muzii L. New York, USA: Springer-Verlag; 2000. Infertility and adhesions. In diZerega GS (eds) Peritoneal surgery; pp. 329–333. [Google Scholar]
- Matsuzaki S, Jardon K, Maleysson E, et al. Carbon dioxide pneumoperitoneum, intraperitoneal pressure, and peritoneal tissue hypoxia: a mouse study with controlled respiratory support. Surg Endosc. 2010;24:2871–2880. doi: 10.1007/s00464-010-1069-z. [DOI] [PubMed] [Google Scholar]
- Molinas CR, Binda MM, Carmeliet P, et al. Role of vascular endothelial growth factor receptor 1 in basal adhesion formation and in carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in mice. Fertil Steril. 2004;82(Suppl 3):1149–1153. doi: 10.1016/j.fertnstert.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Molinas CR, Campo R, Dewerchin M, et al. Role of vascular endothelial growth factor and placental growth factor in basal adhesion formation and in carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80 Suppl 2:803–811. doi: 10.1016/s0015-0282(03)00768-4. [DOI] [PubMed] [Google Scholar]
- Molinas CR, Campo R, Elkelani OA, et al. Role of hypoxia inducible factors 1alpha and 2alpha in basal adhesion formation and in carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80(Suppl 2):795–802. doi: 10.1016/s0015-0282(03)00779-9. [DOI] [PubMed] [Google Scholar]
- Molinas CR, Elkelani O, Campo R, et al. Role of the plasminogen system in basal adhesion formation and carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril . 2003;80:184–192. doi: 10.1016/s0015-0282(03)00496-5. [DOI] [PubMed] [Google Scholar]
- Molinas CR, Koninckx PR. Hypoxaemia induced by CO(2) or helium pneumoperitoneum is a co-factor in adhesion formation in rabbits. Hum Reprod. 2000;15:1758–1763. doi: 10.1093/humrep/15.8.1758. [DOI] [PubMed] [Google Scholar]
- Molinas CR, Mynbaev O, Pauwels A, et al. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76:560–567. doi: 10.1016/s0015-0282(01)01964-1. [DOI] [PubMed] [Google Scholar]
- Molinas CR, Tjwa M, Vanacker B, et al. Role of CO(2) pneumoperitoneum-induced acidosis in CO(2) pneumoperitoneum-enhanced adhesion formation in mice. Fertil Steril. 2004b;81:708–711. doi: 10.1016/j.fertnstert.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Mouton WG, Bessell JR, Pfitzner J, et al. A randomized controlled trial to determine the effects of humidified carbon dioxide insufflation during thoracoscopy. Surg Endosc. 1999;13:382–385. doi: 10.1007/s004649900994. [DOI] [PubMed] [Google Scholar]
- Murphy G, Atkinson S, Ward R, et al. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann N Y Acad Sci. 1992;667:1–12. doi: 10.1111/j.1749-6632.1992.tb51590.x. [DOI] [PubMed] [Google Scholar]
- Mynbaev OA, Molinas CR, Adamyan LV, et al. Pathogenesis of CO(2) pneumoperitoneum-induced metabolic hypoxemia in a rabbit model. J Am Assoc Gynecol Laparosc. 2002;9:306–314. doi: 10.1016/s1074-3804(05)60409-4. [DOI] [PubMed] [Google Scholar]
- Neuberger TJ, Andrus CH, Wittgen CM, et al. Prospective comparison of helium versus carbon dioxide pneumoperitoneum. Gastrointest Endosc. 1996;43:38–41. doi: 10.1016/s0016-5107(96)70258-4. [DOI] [PubMed] [Google Scholar]
- Neuhaus SJ, Watson DI. Pneumoperitoneum and peritoneal surface changes: a review. Surg Endosc. 2004;18:1316–1322. doi: 10.1007/s00464-003-8238-2. [DOI] [PubMed] [Google Scholar]
- Norrman B, Wallen P, Ranby M. Fibrinolysis mediated by tissue plasminogen activator. Disclosure of a kinetic transition. Eur J Biochem. 1985;149:193–200. doi: 10.1111/j.1432-1033.1985.tb08911.x. [DOI] [PubMed] [Google Scholar]
- Ordonez JL, Dominguez J, Evrard V, et al. The effect of training and duration of surgery on adhesion formation in the rabbit model. Hum Reprod. 1997;12:2654–2657. doi: 10.1093/humrep/12.12.2654. [DOI] [PubMed] [Google Scholar]
- Ott DE. Correction of laparoscopic insufflation hypothermia. J Laparoendosc Surg. 1991;1:183–186. doi: 10.1089/lps.1991.1.183. [DOI] [PubMed] [Google Scholar]
- Ott DE. Laparoscopic hypothermia. J Laparoendosc Surg. 1991b;1:127–131. doi: 10.1089/lps.1991.1.127. [DOI] [PubMed] [Google Scholar]
- Ott DE. Laparoscopy and tribology: the effect of laparoscopic gas on peritoneal fluid. J Am Assoc Gynecol Laparosc. 2001;8:117–123. doi: 10.1016/s1074-3804(05)60560-9. [DOI] [PubMed] [Google Scholar]
- Ott DE, Reich H, Love B, et al. Reduction of laparoscopic-induced hypothermia, postoperative pain and recovery room length of stay by pre-conditioning gas with the Insuflow device: a prospective randomized controlled multi-center study. JSLS. 1998;2:321–329. [PMC free article] [PubMed] [Google Scholar]
- Patel S, Pachter HL, Yee H, et al. Topical hepatic hypo-thermia attenuates pulmonary injury after hepatic ischemia and reperfusion. J Am Coll Surg. 2000;191:650–656. doi: 10.1016/s1072-7515(00)00736-5. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zheng M, Ye Q, et al. Heated and humidified CO2 prevents hypothermia, peritoneal injury, and intra-abdominal adhesions during prolonged laparoscopic insufflations. J Surg Res. 2009;151:40–47. doi: 10.1016/j.jss.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Prasad MR, Liu X, Rousou JA, et al. Reduced free radical generation during reperfusion of hypothermically arrested hearts. Mol Cell Biochem. 1992;111:97–102. doi: 10.1007/BF00229579. [DOI] [PubMed] [Google Scholar]
- Ray NF, Denton WG, Thamer M, et al. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1–9. doi: 10.1016/s1072-7515(97)00127-0. [DOI] [PubMed] [Google Scholar]
- Rout UK, Oommen K, Diamond MP. Altered expressions of VEGF mRNA splice variants during progression of uterine-peritoneal adhesions in the rat. Am J Reprod Immunol. 2000;43:299–304. doi: 10.1111/j.8755-8920.2000.430509.x. [DOI] [PubMed] [Google Scholar]
- Sahin DA, Haliloglu B, Sahin FK, et al. Stepwise rising CO2 insufflation as an ischemic preconditioning method. J Laparoendosc Adv Surg Tech A. 2007;17:723–729. doi: 10.1089/lap.2007.0008. [DOI] [PubMed] [Google Scholar]
- Sajid MS, Mallick AS, Rimpel J, et al. Effect of heated and humidified carbon dioxide on patients after laparoscopic procedures: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2008;18:539–546. doi: 10.1097/SLE.0b013e3181886ff4. [DOI] [PubMed] [Google Scholar]
- aksela O, Rifkin DB. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990;110:767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman AK, Olson TA, Mohanraj D, et al. Prevention of postoperative adhesions by an antibody to vascular permeability factor/vascular endothelial growth factor in a murine model. Am J Obstet Gynecol. 1996;174:1502–1506. doi: 10.1016/s0002-9378(96)70596-3. [DOI] [PubMed] [Google Scholar]
- Sammour T, Kahokehr A, Hill AG. Meta-analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95:950–956. doi: 10.1002/bjs.6304. [DOI] [PubMed] [Google Scholar]
- Sammour T, Mittal A, Loveday BP, et al. Systematic review of oxidative stress associated with pneumoperitoneum. Br J Surg. 2009;96:836–850. doi: 10.1002/bjs.6651. [DOI] [PubMed] [Google Scholar]
- Sare M, Hamamci D, Yilmaz I, et al. Effects of carbon dioxide pneumoperitoneum on free radical formation in lung and liver tissues. Surg Endosc. 2002;16:188–192. doi: 10.1007/s004640090103. [DOI] [PubMed] [Google Scholar]
- Savel RH, Balasubramanya S, Lasheen S, et al. Beneficial effects of humidified, warmed carbon dioxide insufflation during laparoscopic bariatric surgery: a randomized clinical trial. Obes Surg. 2005;15:64–69. doi: 10.1381/0960892052993530. [DOI] [PubMed] [Google Scholar]
- chafer M, Krahenb HL, Buchler MW. Comparison of adhesion formation in open and laparoscopic surgery. Dig Surg. 1998;15:148–152. doi: 10.1159/000018609. [DOI] [PubMed] [Google Scholar]
- Schippers E, Tittel A, Ottinger A, et al. Laparoscopy versus laparotomy: comparison of adhesion-formation after bowel resection in a canine model. Dig Surg. 1998;15:145–147. doi: 10.1159/000018608. [DOI] [PubMed] [Google Scholar]
- Schlotterbeck H, Schaeffer R, Dow WA, et al. Cold nebulization used to prevent heat loss during laparoscopic surgery: an experimental study in pigs. Surg Endosc. 2008;22:2616–2620. doi: 10.1007/s00464-008-9841-z. [DOI] [PubMed] [Google Scholar]
- Schonman R, Corona R, Bastidas A, et al. Effect of upper abdomen tissue manipulation on adhesion formation between injured areas in a laparoscopic mouse model. J Minim Invasive Gynecol. 2009;16:307–312. doi: 10.1016/j.jmig.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Segura T, Schmokel H, Hubbell JA. RNA interference targeting hypoxia inducible factor 1alpha reduces post-operative adhesions in rats. J Surg Res. 2007;141:162–170. doi: 10.1016/j.jss.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Steege J. Clinical significance of adhesions in patients with chronic pelvic pain. In diZerega GS (eds) Peritoneal surgery . New York, USA: Springer-Verlag; 2000. pp. 343–348. [Google Scholar]
- Suematsu T, Hirabayashi Y, Shiraishi N, et al. Morphology of the murine peritoneum after pneumoperitoneum vs laparotomy. Surg Endosc. 2001;15:954–958. doi: 10.1007/s004640090100. [DOI] [PubMed] [Google Scholar]
- Taskin O, Buhur A, Birincioglu M, et al. The effects of duration of CO2 insufflation and irrigation on peritoneal microcirculation assessed by free radical scavengers and total glutathion levels during operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:129–133. doi: 10.1016/s1074-3804(98)80078-9. [DOI] [PubMed] [Google Scholar]
- Volz J, Koster S, Spacek Z, et al. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc. 1999;13:611–614. doi: 10.1007/s004649901052. [DOI] [PubMed] [Google Scholar]
- Volz J, Koster S, Weiss M, et al. Pathophysiologic features of a pneumoperitoneum at laparoscopy: a swine model. Am J Obstet Gynecol. 1996;174:132–140. doi: 10.1016/s0002-9378(96)70385-x. [DOI] [PubMed] [Google Scholar]
- Weibel MA, Majno G. Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg. 1973;126:345–353. doi: 10.1016/s0002-9610(73)80123-0. [DOI] [PubMed] [Google Scholar]
- Wiczyk HP, Grow DR, Adams LA, et al. Pelvic adhesions contain sex steroid receptors and produce angiogenesis growth factors. Fertil Steril. 1998;69:511–516. doi: 10.1016/s0015-0282(97)00529-3. [DOI] [PubMed] [Google Scholar]
- Wildbrett P, Oh A, Naundorf D, et al. Impact of laparoscopic gases on peritoneal microenvironment and essential parameters of cell function. Surg Endosc. 2003;17:78–82. doi: 10.1007/s00464-002-9015-3. [DOI] [PubMed] [Google Scholar]
- Wong AP, Cortez SL, Baricos WH. Role of plasmin and gelatinase in extracellular matrix degradation by cultured rat mesangial cells. Am J Physiol. 1992;263:F1112–1118. doi: 10.1152/ajprenal.1992.263.6.F1112. [DOI] [PubMed] [Google Scholar]
- Yesildaglar N, Koninckx PR. Adhesion formation in intubated rabbits increases with high insufflation pressure during endoscopic surgery. Hum Reprod. 2000;15:687–691. doi: 10.1093/humrep/15.3.687. [DOI] [PubMed] [Google Scholar]
- Yesildaglar N, Ordonez JL, Laermans I, et al. The mouse as a model to study adhesion formation following endoscopic surgery: a preliminary report. Hum Reprod. 1999;14:55–59. doi: 10.1093/humrep/14.1.55. [DOI] [PubMed] [Google Scholar]
- Yung S, Chan TM. Mesothelial cells. Perit Dial Int. 2007;27(Suppl 2):S110–115. [PubMed] [Google Scholar]
- Zhao W, Richardson JS, Mombourquette MJ, et al. Neuroprotective effects of hypothermia and U-78517F in cerebral ischemia are due to reducing oxygen-based free radicals: an electron paramagnetic resonance study with gerbils. J Neurosci Res. 1996;45:282–288. doi: 10.1002/(SICI)1097-4547(19960801)45:3<282::AID-JNR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]