Summary
The tetrameric enzyme Pyruvate Carboxylase (PC), a biotin-dependent carboxylase, produces oxaloacetate by two consecutive reactions that take place in distant active sites. Previous crystal structures revealed two different configurations for PC tetramers, the so-called symmetric and asymmetric, which were understood as characteristic molecular architectures for PC from different organisms. We have analyzed PC samples from Staphylococcus aureus while the enzyme generates oxaloacetate, expecting PC tetramers to display the conformational landscape relevant for its functioning. Using cryoEM and sorting techniques we detect previously defined symmetric and asymmetric architectures, demonstrating that PC maps both arrangements by large conformational changes. Furthermore, we observe that each configuration is coupled to one of the two consecutive enzymatic reactions. The findings describe the structural transitions relevant for the allosteric control of the multifunctional PC, and demonstrate that by cryoEM and classification we can characterize freely working macromolecules.
Introduction
Biotin-dependent carboxylases are widely distributed in all kingdoms of life since they catalyze the transfer of carboxyl groups to several substrates crucial in fatty acid, amino acid and carbohydrate metabolism (Tong, 2013; Waldrop et al., 2013). They are multifunctional and contain biotin carboxylase (BC) and carboxyltransferase (CT) domains that work in a sequential fashion. PC carboxylates pyruvate into oxaloacetate, an essential metabolite in the tricarboxylic acid cycle (Utter and Keech, 1960) which fuels several anabolic biosynthetic reactions such as gluconeogenesis, lipogenesis, insulin secretion, and synthesis of glutamate neurotransmitter (Jitrapakdee et al., 2008). In humans, PC is mitochondrial and there are several metabolic disorders associated with the deficiency of its enzymatic activity, diseases that predominantly manifest with lactic acidemia and neurological dysfunction (Marin-Valencia et al., 2010). In the first step of PC reaction (Figures 1A and 1B), the BC domain catalyzes the carboxylation of the biotin cofactor using bicarbonate as the carboxyl donor in a reaction that requires MgATP (Attwood and Wallace, 2002; Knowles, 1989). The second step is carried out by the CT component that promotes the CO2 transfer from carboxybiotin to pyruvate. The biotin prosthetic group is attached to a biotin-carboxyl carrier protein domain (BCCP) that transfers the product from the BC active site to the CT active site as substrate for the next chemical reaction (Figures 1A and 1B). The coupling between reactive centers requires large conformational changes, which may be under allosteric regulation, with structural transitions that are not well understood.
Figure 1. Crystallographic structure of PC.
A Distribution of domains in the primary structure of PC. Domains are colored: Biotin Carboxylase (BC) red; Carboxyl Transferase (CT) green; Biotin-Carboxyl Carrier Protein Domain (BCCP) blue; and Protein Tetramerization (PT) or allosteric domain, golden. The color code for domains is kept along the figures unless stated otherwise.
B One subunit of SaPC is depicted in ribbons taken from the crystallographic structure of SaPC (PDB:3BG5) (Xiang and Tong, 2008). One ATP molecule residing in the BC active site, and one molecule of Pyruvate in the CT site are shown. Biotin is represented attached to the conserved Lys residue of the BCCP domain. Below, schematic representation of the two consecutive reactions that PC catalyzes during pyruvate carboxylation.
C Crystal structure of tetrameric full-length SaPC (PDB:3BG5) (Xiang and Tong, 2008). One of the subunits is colored domain wise (monomer 1), while monomers 2–4 are seen pink, blue and yellow respectively.
D Ribbons representation for the crystal structure of SaPC tetramer (PDB:3BG5) (Xiang and Tong, 2008) showing the symmetric arrangement. The RMSD between subunits from opposing layers is 2.4 Å (excluding BBCP domains and their linkers).
E Depiction of the crystal structure of RePC tetramer (PDB:2QF7) (St Maurice et al., 2007) displaying the asymmetric architecture. The RMSD between subunits from opposing layers is 8.9 Å (excluding BBCP domains and their linkers).
For clarity, the ribbons rendering in D and E do not include the BCCP regions.
Typically, PC is found as tetramers of four identical subunits (Figure 1C), with monomers of around 120–130 kDa in size (Jitrapakdee et al., 2008). Crystallographic studies for PC from Homo sapiens (HsPC), Staphylococcus aureus (SaPC), and Rhizobium etli (RePC) describe a tetrameric rhombohedron organized in two layers, with two opposing monomers in each layer (Figure 1C) (St Maurice et al., 2007; Xiang and Tong, 2008). Each subunit contains all the three aforementioned domains, the catalytic BC and CT, and the BCCP where biotin is covalently attached (Figures 1A, 1B and 1C). The crystal structures for PC (St Maurice et al., 2007; Xiang and Tong, 2008) revealed an additional structural domain linking the other three functional ones and termed PC tetramerization (PT) domain (Figures 1A and 1B), a structural region that was not inferred from the protein sequence where its two constitutive regions are far apart from each other. In the atomic structures of SaPC and HsPC (Xiang and Tong, 2008), this region is seen to contribute to the oligomerization of the enzyme (Figure 1D).
The enzymatic activity of PC requires the BCCP domain and the tethered biotin to swing between the BC and CT active sites from opposing monomers within the same layer (St Maurice et al., 2007). X-ray crystallography showed that the biotin is carboxylated in the BC domain of its own monomer (Lietzan et al., 2011), and that the carboxyl is transferred to pyruvate in the CT domain of the opposite subunit (Xiang and Tong, 2008). Those active sites are in far distance (around 75 Å), and the needed mobility for BCCP is provided by a long disordered segment of approximately 20 amino acids that links the BCCP with the PT domain (Figures 1B and 1C). Despite considerable efforts, published crystal structures show BCCP domains located in exo binding sites far from the catalytic centers, or missing due to their flexible nature. The only exceptions to this are found in: i) HsPC and SaPC tetramers where one BCCP domain in the tetramer was found in a conformation compatible with the CT reaction (Xiang and Tong, 2008) and; ii) in RePC T882A mutant where the BCCP domain was found near the BC active site, after mutation of the CT site (Lietzan et al., 2011).
SaPC and RePC enzymes share approximately 50% sequence identity, and it is assumed that they both carry out their sequential reactions in a similar manner. However, there is a fundamental difference in their quaternary structure that is not yet understood. The PT (also known as allosteric) domain is essential to bind the allosteric activator acetyl-CoA for many PC enzymes, including RePC and SaPC (Cazzulo and Stoppani, 1968; Jitrapakdee and Wallace, 1999). Unexpectedly, this domain shows a different arrangement within SaPC and RePC tetramers. In SaPC the tetramer shows a mostly symmetrical configuration (Figure 1D) where all four PT domains have a similar arrangement and are found to mediate PT-PT contacts between subunits in different layers (Xiang and Tong, 2008). This way the subunits from the two layers are also similar with an RMSD between them of 2.4 Å. In RePC (bound to a CoA analog), however, the two layers show a considerable asymmetry, including different arrangement of the PT domains (Figure 1E) (St Maurice et al., 2007). While two PT domains are in close proximity in one layer, the other two are located at a great distance in the opposing face. The distinct arrangement of PT domains originates structural differences between PC monomers, and the RMSD between subunits from opposing layers is of 8.9 Å. In addition, the relative positions of the two monomers in each layer of RePC are very different, such that the distance between the BC and CT active sites is ~65Å in the top layer but ~80Å in the bottom layer. The structural deviation between monomers at distinct layers in PC is even larger in the structure for RePC T882A mutant where it reaches an RMSD of 9.5 Å (Lietzan et al., 2011). In comparison, the structure of SaPC in complex with CoA is even more symmetrical than the free enzyme (Yu et al., 2009), with an RMSD of 1.28 Å among the four monomers. The discrepancy between structures led to the assumption that the overall symmetry of PC tetramers was species-specific (Lietzan et al., 2011; St Maurice et al., 2007). However, a recent crystal structure of RePC lacking BC and BCCP domains showed a tetramer with a symmetrical architecture (Lietzan and St Maurice, 2013). As in SaPC, the PT domains were also found to mediate inter-layer PT-PT contacts. This leaves open the possibility that symmetrical and asymmetrical structures represent a range of conformations for general PC.
The wealth of structural data on PC has provided valuable insight into several key questions, but the overall allosteric regulation and the conformational changes that facilitate the enzymatic activity are still unclear. Here we present cryoEM structures for SaPC obtained from a sample undergoing catalysis, in the presence of all its substrates. The individual tetramers are not synchronized, but an in silico sorting of the electron microscope images detects conformational subsets of SaPC while catalyzing the sequential chemical reactions to produce oxaloacetate. The cryoEM density maps were combined with available crystal structures using molecular dynamics flexible fitting, and an ensemble of atomic models is shown for each cryoEM map. Our results confirm the presence of symmetric and asymmetric arrangements of the PT domain in SaPC tetramers during the catalytic cycle. Furthermore, the locations of BCCP domains in the different structures clearly link the asymmetric configuration with the enzymatic activity in the BC domain, while the symmetric one is compatible with the reaction at the CT center. Also, the structures show BCCP domains interacting with catalytic centers only in one layer at a time, in clear agreement with the half-site reactivity mode of action postulated for PC. Overall, the results depict the structural transitions that govern multifunctional PC.
Results
CryoEM and classification of SaPC during catalysis
In order to capture the conformational variability of PC during the carboxylation of pyruvate into oxaloacetate, we prepared samples of recombinant SaPC and added a reaction buffer containing required substrates and cofactors (KHCO3, ATP, pyruvate, and a source of Mg), and the activator acetyl-CoA. The oxaloacetate production was linked to the activity of malate dehydrogenase (Modak and Kelly, 1995), and the subsequent oxidation of NADH was monitored (Figure 2A). In the assayed conditions, tetramers of SaPC perform several catalytic cycles of pyruvate carboxylation for periods of time in the range of 3–5 min. Aliquots of the samples were taken 1 min after addition of the reaction buffer, stored transiently on ice, and cryoEM grids were prepared immediately. The presence of SaPC tetramers is apparent in the electron microscope images (Figure 2B). The tetrameric oligomers were frozen while performing their enzymatic activity, and we expect the protein to display a variety of conformational states. With this in mind, we directly performed the image processing and the calculation of 3D maps using unsupervised classification based on maximum-likelihood methods implemented in Relion software (Scheres, 2010, 2012). Initially we selected approximately 55,000 individual cryoEM images for SaPC tetramers. The particle selection was guided by template matching with one of our previous cryoEM maps for SaPC (EMD-1742) (Lasso et al., 2010), a 3D map that was also used as initial reference for the 3D classification in Relion (Figure 2C). Convergence during classification was reached when four classes were considered. In the renderings of the maps for the four classes (Figure 2D) it is clear that in class 4 the cryoEM map displays scattered densities that are not linked to the main body of the tetramer. This is understood as a symptom of heterogeneity in this subset of the images, a group that might also include defective images and/or inactive macromolecules. Thus, the data for class 4 was not further refined. On the contrary, the remaining three classes are compact, and they do not render any density outside the main envelope at the chosen density threshold. Consequently, their corresponding subsets of images were processed imposing 2-fold (C2) symmetry and refined using Spider/Spire software package (Baxter et al., 2007; Frank et al., 1996), resulting in cryoEM maps at resolutions about 11.5 Å (Figure S1). Visual inspection of the 3D maps for classes 1–3 suggests the presence of at least two different architectures, where classes 2 and 3 depict similar tetrameric arrangements that mimic the structure of the initial reference, while class 1 seems to follow a divergent design. The similarity between the four cryoEM maps can be evaluated in a dendrogram based on the correlation between them (Figure 2E), where it is clear that classes 2 and 3 have very similar 3D maps, and the map for class 4 departs the most from the rest of the cryoEM data. Since the maps for classes 2 and 3 are very similar (with very high coefficient of correlation between them), we will present and discuss the analysis only for class 2 data, although the general conclusions can also be applied to the data for class 3. Thus, we will focus the structural analysis mainly on the study and comparison of the maps calculated for classes 1 and 2.
Figure 2. CryoEM of working PC.
A Progress of SaPC catalytic activity monitored by absorbance at 340 nm (see Experimental Procedures).
B Field of an electron micrograph from the cryoEM analysis of SaPC tetramers frozen during their catalytic activity.
C Rendering of the 3D density map for SaPC tetramer (EMD-1742) (Lasso et al., 2010) used as initial reference for refinement and classification.
D CryoEM maps obtained for the four classes after classification of a total set of ~55,000 images. The number of particles that contribute to each class is included. The density maps are rendered at 4σ density threshold.
E Dendrogram based on the coefficient of correlation between the four cryoEM maps obtained in the classification and displayed in D.
See also Figure S1.
Symmetric and asymmetric tetramers for SaPC
To understand the differences between the structures for classes 1 and 2 we constructed atomic models by flexible fitting within the cryoEM maps. The first step was to explore which of the two previously reported architectures, the symmetric and the asymmetric ones, was closer to the current data. Two distinct crystallographic structures were fitted as rigid bodies inside the maps for classes 1 and 2 (Figure S2). In both maps, one of the architectures leaves large regions of PT domains outside the densities. This way, the position of the PT domains show preferred correlations: the 3D map for class 1 preferentially allocates PT domains in the asymmetric configuration shown for RePC (St Maurice et al., 2007); and class 2 structure fits better with the symmetric distribution of PT regions from the atomic structure for SaPC (Xiang and Tong, 2008). Thus, we started with different initial atomic models for the cryoEM maps for classes 1 and 2. For the fitting within the 3D map for class 1 we used two templates, both of them showing asymmetric tetramers for RePC (PDB:3TW6, 2QF7) (Lietzan et al., 2011; St Maurice et al., 2007); and for class 2, the atomic structure for the SaPC tetramer was modeled using the symmetric crystallographic structure of SaPC (PDB:3BG5) (Xiang and Tong, 2008). In the former, the combination of two atomic models was necessary to provide the four BCCP domains.
Next, we proceed with the Molecular Dynamics Flexible Fitting (MDFF) (Trabuco et al., 2008) of the initial models inside the 3D density maps. The molecular dynamic simulations were carried out for 5 ns following previously described protocol (Chan et al., 2011). In order to evaluate the accuracy in the final models, MDFF simulations were run independently five times for each cryoEM map. The flexible fittings converged very rapidly in all the runs and for the three (classes 1–3) cryoEM maps (Figure S3). The ensemble of atomic models yielded a final cross-correlation coefficient of 0.92, 0.93 and 0.96 to the corresponding cryoEM maps for classes 1, 2 and 3 respectively. Overall, the ensembles of atomic models fill completely the 3D maps, and no large density is left empty without the corresponding atomic data (Figures 3 and S4). For the map for class 1, the full length tetramer of SaPC was fitted, including all four copies of the BCCP domains (Figures 3A and 3B). In this cryoEM map small regions next to the N-terminal BC domains remain empty (with no atoms inside) in all five runs (labeled with asterisks in Figures 3A and 3B). We attribute these regions to the N-terminal tag that the SaPC construct includes, a region not present in the atomic model. This tag seems to get stabilized and apparent in the subset of tetramers included in class 1. For classes 2 and 3, there is no signal for the BCCP domains in the bottom layer (Figures 3D and S4B), and those were removed from the atomic models. We understand that these regions vanish in the 3D averages due to their flexibility.
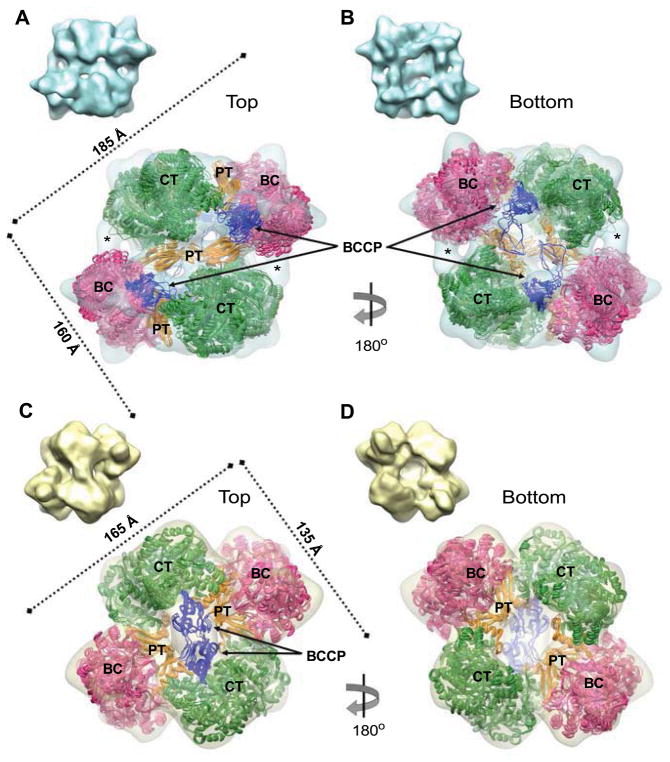
Figure 3. Ensembles of atomic models after flexible fitting.
CryoEM maps for class 1 A–B, and class 2 C–D, are depicted in semi-transparent mode to allow visualization of the atomic models generated for SaPC tetramers. There are five atomic models for each map, as the results of five independent Molecular Dynamics Flexible Fittings (MDFF) runs. The panels depict top (A and C) and bottom (B and D) views of the structures. The RMSD between the five atomic models are 3.5±0.5 Å for class 1, and 1.7±0.3 Å for class 2. The subunits from top and bottom layers show RMSD values between them (measured in the five atomic models excluding BCCP domains and their flexible linkers) of 13.3±0.7 Å (class 1) and 3.7±0.4 Å (class 2). Thumbnails of the cryoEM maps depicted in solid mode are also included in each panel. The 3D maps are rendered at 4σ density threshold.
See also Figures S2–4.
The fitted atomic coordinates fully overlap with the density maps obtained after classification. The ensemble of five atomic models calculated for each class during MDFF simulations show the same position for domains, and their low relative RMSD values (Figures 3 and S4) indicate that the cryoEM maps impose enough restrictions during the flexible fittings. In class 1 the asymmetric architecture becomes apparent since the inner PT domains fill the central density of the 3D map (Figures 3A and S2A), and the RMSD between subunits from top and bottom layers is of 13.3±0.7 Å (measured in the five atomic models generated during the flexible fitting). The pair of BCCP domains at the top layer is located near the BC active sites of their own subunit (Figure 3A), while the two BCCP domains at the bottom layer reside in a cleft between its own BC and the opposing CT regions, in a so-called exo site (Xiang and Tong, 2008) (Figure 3B). For classes 2 and 3 (Figures 3C, 3D and S4) the five atomic models keep the symmetric configuration, and the RMSD between top and bottom layer subunits is reduced to 3.7±0.4 Å (class 2) and 2.5±0.2 Å (class 3). In the top layer, two BCCP domains are seen in close contact with the catalytic CT domains in the opposite subunit (Figures 3C and S4A), but the bottom layer does not show any signal for BCCP regions (Figures 3D and S4B). Essentially, these structures of tetrameric SaPC in classes 2 and 3 are very similar to our previous cryoEM works (Lasso et al., 2010; Yu et al., 2009). The structure obtained for class 1, however, is asymmetric and shows signal for all the BCCP domains, and it has not been described before for SaPC.
The dissimilar structural design between the models for classes 1 and 2 entails changes in the relative position of domains. The distances between domains in the ensembles of atomic models (Table I) reveal that the largest differences are associated with the relative position between PT and BC domains. Remarkably, the distance between PT domains and the BC centers of the opposite subunits within the top layer increases around 20 Å from class 2 to class 1. This is the result of the outwards displacement of the two PT domains at the top layer in class 1 in the transition from a symmetric to an asymmetric arrangement. Noteworthy, there are several different levels of asymmetry when comparing the cryoEM maps and the crystal structures of SaPC and RePC. First, the RMSD between monomers in different layers increases from 8.9–9.5 Å in RePC crystal structures to 13.3 Å in our atomic models for class 1, suggesting a higher degree of asymmetry. Second, and despite the increased asymmetry in class 1, the relative position between BC and CT active centers in opposing subunits is very similar in all the models and classes (variations in the range of 5 Å), revealing that this catalytically relevant pair is conserved through the large conformational change. This is in sharp contrast to the crystal structure of RePC, where the BC-CT active site distance is 65 Å in the top layer but 80 Å in the bottom layer (St Maurice et al., 2007) but correlates with SaPC where the BC-CT active distance is similar in different layers. Thirdly, the positions of the PT domain are different in the two layers in the RePC structure. This asymmetry in the PT domains is observed in the current EM model for class 1 of SaPC, although the crystal structures of SaPC do not show it (Xiang and Tong, 2008; Yu et al., 2013; Yu et al., 2009). Regardless of the differences in the level of asymmetry, our results demonstrate that SaPC tetramers map both, the symmetric and the asymmetric configurations during its catalytic activity.
Table I. Distances between active sites and domains within the atomic models for classes 1 and 2.
The distances are presented as the averages and standard deviations for the set of five atomic models calculated for each cryoEM map during the MDFF. The position of the active sites were set by centroids defined by groups of residues surrounding the reaction centers (positions 240, 278, and 291 for BC; amino acids 577, and 878 for CT). For the PT distances, the Cα of residue 1058 was used.
| Distances between domains (Å) | Layer | Atomic models for Class 1 | Atomic models for Class 2 |
|---|---|---|---|
| BC active sites | Top | 134.1 ± 0.8 | 123.1 ± 0.4 |
| Bottom | 129.3 ± 1.3 | 123.7 ± 0.1 | |
| CT active sites | Top | 75.0 ± 1.2 | 80.6 ± 0.2 |
| Bottom | 83.9 ± 1.2 | 75.7 ± 0.7 | |
| PT <-> BC own | Top | 44.6 ± 1.0 | 47.8 ± 1.4 |
| Botom | 67.9 ± 2.7 | 43.9 ± 0.5 | |
| PT <-> BC opp | Top | 96.8 ± 1.0 | 76.9 ± 1.4 |
| Bottom | 70.1 ± 1.0 | 80.9 ± 0.5 | |
| PT <-> CT own | Top | 29.5 ± 1.6 | 33.5 ± 1.3 |
| Bottom | 40.3 ± 3.0 | 35.8 ± 0.9 | |
| PT <-> CT opp | Top | 66.3 ± 0.6 | 53.2 ± 0.5 |
| Bottom | 51.3 ± 2.8 | 49.0 ± 0.7 | |
| BC <-> CT opp | Top | 81.7 ± 1.4 | 76.8 ± 0.3 |
| Bottom | 76.3 ± 0.9 | 74.3 ± 0.6 |
Linking tetramer architecture to catalytic activity
The resolution of the 3D maps cannot define interactions in the catalytic centers at atomic resolution. The atomic models, however, allow us to explore whether the positions of BCCP domains are compatible with the enzymatic activity of PC. In the atomic models the biotin was not included, so we will judge whether the distance between the conserved Lys (Lys1112 in SaPC), where the biotin is covalently attached, and the active centers is suited for the enzymatic reactions. For clarity, we present just one out of the five atomic models calculated for each class, although the distances are averages for the five models.
In the top layer of class 1 models, the BCCP domains are in the vicinity of the BC domain of its own subunit (Figure 3A). A detailed view (Figure 4A) shows that the loop with Lys1144 enters the aperture of the BC active site. Here, the distance between the Cα of Lys1144 and the active site is ~19 Å. There is only one crystal structure with the BCCP domain resting next to the BC region, the one for RePC T882A (mutant at the CT site) (Lietzan et al., 2011), where the BCCP domain remains outside the catalytic channel (Figure 4B) and the Lys residue is almost 20 Å away. Noteworthy, in the atomic models derived from class 1 there is an opening of the B-subdomain lid within the BC domain (Figure 5) that allows for a better orientation of the BCCP domain towards the catalytic core (Figure 4A). This tilting of the lid from the BC domain is observed only in the top layer of the models for class 1 (Figure 5). The position and of the BCCP domain is still too far to be considered a catalytically competent conformation since the biotin arm just extends ~16 Å; its orientation, however, directs the biotin moiety towards the reactive center, and small local changes might allow the reaction to occur. In the bottom layer of class 1, the BCCPs are placed just between its own BC domain and the CT domain from the opposing subunit (Figure 3B). Clearly, this is an exo binding site far away from the active centers (Figure 4C). A somehow similar position was observed in the first structure reported for RePC (St Maurice et al., 2007), but there is a relative shift of around 7 Å between that crystal structure (Figure 4D) and our current model (Figure 4C). The BCCP domains at the top layer of class 2 are seen in close contact with the CT domain of the opposing subunit (Figure 3C). A closer inspection (Figure 4E) reveals that in the atomic model the β-hairpin of the BCCP domain that contains the Lys residue penetrates into the active site of the CT domain. The Cα atom of Lys1144 is ~9 Å away from the CT active site, and a very similar distance was observed in one BCCP domain from the crystallographic structure for SaPC (Figure 4F), which was understood as catalytically competent (Xiang and Tong, 2008). There is, however, a large rotation of about 40° and a shift of 13 Å when comparing these two orientations of the BCCP domain bound to the CT active site (Figures 4E and 4F).
Figure 4. Position of BCCP domains.
Along the panels, ribbon representations for atomic data are seen within semi-transparent cryoEM maps for classes 1 and 2 rendered at 5σ density threshold. Thumbnails are depicted solid and also semi-transparent as orientation guides.
A Depiction of one atomic model calculated for class 1 that shows the BCCP domain bound to the BC region of its own monomer. The average distance in the five models between the Lys where the biotin is attached and the BC active site is 19.0±1.8 Å.
B The crystallographic structure for RePC T882A mutant (PDB:3TW6) (Lietzan et al., 2011) is shown after rigid body fitting within the cryoEM map for class 1. The distance between the Lys residue and the BC active center is of 19.7 Å.
C Atomic model for class 1 showing in the bottom layer of the tetramer the BCCP domain in the exo binding site, between BC and CT centers.
D Depiction of the crystal structure of RePC (PDB:2QF7) (St Maurice et al., 2007) after rigid body fitting in the cryoEM map for class 1. It shows also a BCCP domain in an exo binding site between BC and CT domains.
E One of the atomic models from the MDFF for class 2 is seen. The zoom-in view shows the BCCP near the CT active site of the opposite subunit. Among the five models calculated for class 2, the distance between the Cα of conserved Lys and the CT active site is 9.0±1.3 Å.
F The ribbons from the crystal structure of SaPC (PDB:3BG5) (Xiang and Tong, 2008) are seen after rigid body fitting in the map for class 2. Here, the distance between Lys and the active site is 9.4 Å.
Figure 5. Structural differences in the BC domains.
A Renderings of the cryoEM maps for classes 1 and 2 focused on the side view of the BC regions. The map for class 1 (blue) is depicted in semi-transparent mode. The cryoEM map for class 2 (yellow) is seen solid.
B Comparison between one of the atomic models fitted in the map for class 1 (colored domain wise) and one atomic model fitted within the 3D map for class 2 (ribbons in grey). There is a tilt of the B-subdomain lid of BC domain between the atomic models.
C The map for class 1 is rendered semi-transparently to visualize one of the fitted atomic models.
D Semi-transparent representation of the 3D map for class 2 together with the ribbon display of one of the fitted atomic coordinates.
The panels conserve the same orientation.
Essentially, our atomic models display the BCCP domains engaged with the BC active site of its own monomer, bound to the CT center of the opposite subunit, and between those two domains. These three locations are similar to those previously reported for SaPC (Xiang and Tong, 2008) and RePC (Lietzan et al., 2011; St Maurice et al., 2007), and go along with the expected movements of BCCP domains during PC functioning. Additionally, the BCCP is bound to its own BC domain in the context of the asymmetric tetramer, while in the symmetric architecture, the BCCP is engaged with the opposite CT active site. This clearly suggests that the transitions between asymmetric and symmetric configurations are coupled with the movements of the BCCP domains during PC function. Also, BCCP domains are seen bound to catalytic domains only in one of the faces, what supports a half-site reactivity mode for PC tetramers, where only one of the layers at a time is engaged in enzymatic reactions.
A structural transition model for PC subunits
There are four different conformations for SaPC subunits among the atomic models for classes 1 and 2 (Figure 6), essentially one conformation per layer and per class. Two of them might be catalytically competent and display the BCCP regions on its own BC (Figure 6A) and on the opposite CT (Figure 6B) centers. In the transition between them (compare Figures 6A and 6B), the Lys1144 in the BCCP domain moves ~65 Å to reach the CT domain of the opposing subunit. This movement is facilitated by a relative rotation of ~20° between the BC and CT domains within monomers (Figure 7A) and is coupled with the inwards movement of the PT domain by ~19 Å (Figure 7B). A third monomer structure has the BCCP in the exo binding site (Figure 6C). In this subunit, the rotations between the BC and CT regions, and the displacement of the PT domain follow the same trend, with additional ~19° rotation and ~11 Å movement respectively (Figures 7A and 7C). Thus, the same conformational change can produce the three structures starting in the context of an asymmetric tetramer (with its PT domain shifted outwards and the BCCP in its own BC domain; Figure 6A), passing through a symmetric (with the BCCP in the opposite CT domain; Figure 6B), and returning again to an asymmetric one (with its PT shifted inwards and the BCCP in exo position; Figure 6C). These three conformations are to be achieved by a gradual increase of the rotation angle between the BC and CT domains within each monomer. The fourth structure for the SaPC subunit does not include the BCCP and its linker (Figure 6D), since the cryoEM map for class 2 did not show density for these regions in the bottom layer. The conformation of this subunit is very similar to the monomer with the BCCP engaged on the CT domain (Figure 6B), with an RMSD between them of 3.7±0.4 Å (within the five models generated for class 2), underpinning the similarity between subunits in the symmetric tetramer.
Figure 6. The four structures for SaPC subunits derived from the atomic models.
The panels A–D show in the left side ribbons representations for four distinct SaPC subunits after the MDFF in the top and bottom layers of cryoEM maps for classes 1 and 2. The monomers correspond to: A, top layer of class 1; B, top layer of class 2; C, bottom layer of class 1; and D, bottom layer of class 2. All the BCCP domains establish their interactions within their layer, and their location is indicated. Structural transitions related to the relative rotation between CT and BC domains within the subunits are indicated by arrows. In the right side of the panels, the arrangement of SaPC tetramers is depicted in cartoons. In these drawings, the tetramers are colored as in figure 1C. The architecture of the tetramers has been observed in: class 1 (A); class 2 (B); class 1 upside-down (C) and class 2 upside-down (D).
Figure 7. Structural changes between atomic models for SaPC.
A Relative rotation between BC and CT domains of the same subunit observed in the transition between the subunits depicted in figures 6A, 6B, and 6C. The monomers where aligned by the CT regions, and the rotation measured at the BC domains. The BC are colored: red for the subunit with the BCCP on the BC of the same subunit (as in figure 6A); magenta in the monomer with the BCCP engaged on the CT of the opposing subunit (as in figure 6B); and purple in the subunit with the BCCP bound to the exo binding site (as in figure 6C). The BCCP and its flexible linker are not depicted. The fourth subunit, with the BCCP flexible (as in figure 6D) is very similar to the one with the BCCP on CT.
B Movements of BCCP and PT domains in SaPC subunits during the transition of the BCCP domain from the BC of the same subunit, to the CT of the opposing one.
C Movements of BCCP and PT domains in SaPC subunits during the transition of the BCCP domain from the CT of the opposing subunit to the exo site.
In B and C the BC domains are not depicted.
The relationships and possible communications between subunits within PC tetramers also change. When the PT is retracted in the asymmetric state (as in Figure 6A), the subunit does not show any PT-PT contact. Transition to a symmetric design (Figures 6B and 6D) allows for PT-PT interactions between subunits from different layers. When the PT domain goes central in the asymmetric configuration (Figure 6C), the communication between PT domains is established within the same layer. Thus, there is an interplay of connections through PT regions that varies between inter- or intra-layer, and the position of this domain defines the landscape of regions accessible for the BCCP domains. The rotations between BC and CT regions that drive the movement of PT domains are necessarily coupled to relative rotations of the BC and CT domains in the subunits of the opposite layer. Since the PC tetramer is arranged with the four polypeptides running antiparallel on each layer and perpendicularly between layers, the rotations that move the PT domains inwards in one face, also displace the same region outwards in the other layer. Also, the orientations of the catalytic domains, and hence, the accessibility of the BBCP region to the active sites, are altered between layers in a coordinated manner.
Discussion
Our previous attempts to characterize by cryoEM the conformational landscape in PC functioning did not reveal any large structural change of the oligomer (Lasso et al., 2010; Yu et al., 2009). In those works we tried to halt SaPC tetramers in distinct steps of its enzymatic reaction in a way that all the molecules were synchronized. By using several combinations of substrates and cofactors we calculated some 3D density maps looking for individual snapshots. In those results none of the 3D maps displayed the BCCP domain close to the BC active site, the first reaction of the sequence for pyruvate carboxylation. In the current approach, however, the study of freely working SaPC is intended to fish several movie frames within a single sample. This way we might have access to conformations that were elusive in the single snapshot approach. We are aware that the sorting of cryoEM images might provide just some of the intermediates, and with no information about the sequential link between them. In the context of known PC functioning, however, we can infer an overall model in agreement with our structural data.
The sorted subsets of electron microscope images might still be very heterogeneous, and the isolated class 4 fits well with a mixture of conformations (Figure 2D). The rest of the classes display compact tetramers and include more homogeneous subsets of images. The maps for classes 2 and 3 are very similar and depict the same symmetric architecture (Figures 3C, 3D and S4). We could have merged both classes, but class 3 shows smaller densities for BCCP domains that could be caused by lower occupancy of the domain. We also prefer to preserve the raw outcome from the classification. The presence of redundant structures after the sorting indicates that the chosen number of classes is large enough to describe the variability at the achieved resolution (Scheres, 2010). It is remarkable that BCCP domains have been caught in the vicinity of active sites in classes 1–3, which include almost 80 % of the images. The sample for cryoEM was transiently stored on ice, what keeps the thermally driven conformational fluctuations at a minimum, and thus, the swinging of the BCCP domains might be reduced. The limited movement of BCCP domains favors the capture of this region stably bound to catalytic domains.
In the description of the cryoEM maps we refer to “top” and “bottom” layers to guide the analysis, we cannot, however, directly correlate layers between different maps. We have used the term “top” for the layers that show BCCP domains close to BC or CT reaction centers. Nevertheless, we can assume, following the catalytic activity of the enzyme, that a transition occurs between two states: from the BCCP engaged on the BC center of its own subunit (Figure 6A); to the BCCP bound to the CT active site of the opposing one (Figure 6B). When this transition happens in one of the faces of the tetramer (as in the cartoons at the right side of Figure 6), the other layer moves: from the state with the BCCP domains in exo sites (Figure 6C); to again a symmetric tetramer with the BCCP flexible and not seen (Figure 6D). This is clear since those two pairs of states appear coupled in the layers of the two cryoEM maps analyzed (for classes 1 and 2). Thus, in a full sequence of events, PC tetramers start in the asymmetric state with BCCP domains bound to the BC site (class 1). Next, a transition to a symmetric configuration allows for the binding of BCCP domains to the CT active sites (class 2). This latter step fulfils the enzymatic reaction in the layer. An additional structural change to an asymmetric architecture leave the BCCP resting in the exo binding site and permits the BC reaction to occur in the opposing layer (class 1 upside-down). And, finally, a new transformation to an again symmetric tetramer concludes the reaction at the bottom layer (class 2 upside-down).
Our structural data clearly shows a coupling between the architecture and the position of the BCCP domains, and suggest that the relative rotations between BC and CT domains in PC subunits govern the large movements required to communicate the distant active sites. At the top layer of the asymmetric tetramer the distance between the PT and the BC centers differs considerably (Table I). The active site in the subunit is about 45 Å away from the PT, while the BC site from the opposing subunit in the layer is almost at 100 Å distance. In this configuration a BC reaction within the same subunit is favored. Also, in this same state, the BC domains from top and bottom layers are distinct. At the top layer, the B-subdomain lid of the BC domain tilts and allows the access of the BCCP domain (Figure 5), while in the bottom face this region is closed. Similar differences have been observed for BC domain dimers of acetyl-CoA carboxylase (Mochalkin et al., 2008), a demonstration of its half-site reactivity. Although this mode of action has not been demonstrated for PC, the current structures clearly support this type of negative cooperativity between layers. Noteworthy, truncation of BC and BCCP domains in RePC resulted in tetramers arranged in symmetric configuration (Lietzan and St Maurice, 2013) with CT-CT dimers identical to the observed in the full-length protein arranged in asymmetric mode. This pointed out the role of the BC domain in regulating the conformational landscape of PC. Our results suggest that BC domains define the active layer by BC-BC communication, and that the configuration of the asymmetric architecture also determines which BC active site the BCCP domain can reach. The progress to the CT reaction is linked to the transition to a symmetric architecture. In this case, the distances between PT and CT sites cannot rule the selection of the active site from the same subunit, but the rotations of domains that promote the changes between asymmetric and symmetric states expose the CT catalytic cavity towards the opposite subunit.
Our cryoEM maps show SaPC tetramers in different architectures. The crystallographic structures for full length PC, however, clearly split between the symmetric architecture for SaPC (Xiang and Tong, 2008; Yu et al., 2009), and the asymmetric one for RePC (Lietzan et al., 2011; St Maurice et al., 2007). The propensity of those PC samples to adopt specific structural states might be caused by crystallization conditions. It is also possible that contacts between tetramers in the crystal packing restrict their conformation. We observe that the large BC and CT domains rotate in the conformational changes, therefore the side surfaces exposed for crystal contacts vary between symmetric and asymmetric states. The residues in the surface are less conserved between PC samples from distinct species, and this difference might guide preferred crystal contacts between adjacent tetramers in the crystals.
Altogether, the current structural study reveals that: PC tetramers map asymmetric and symmetric architectures; those are coupled to the consecutive BC and CT enzymatic reactions respectively; and the catalytic activity occurs one layer at a time. Through the entire set of structural transformations of PC tetramers, the PT domains play a central role since they: integrate the relative changes between BC and CT regions; change the inter- and intra-layer contacts between subunits; and move the anchor points for the linker of the BCCP domains. Also, the differences between the BC domains from opposing layers block or allow the binding of the BCCP domain. Interestingly, the allosteric activator acetyl-CoA binds to the PT domain near the BC-BC dimer interface (St Maurice et al., 2007), a binding site that is only preserved in two subunits of the asymmetric tetramer (Lietzan et al., 2011; St Maurice et al., 2007), but appears occupied by the activator in the four PC subunits in the context of a symmetric architecture (Yu et al., 2009). This correlation between the number of binding sites for acetyl-CoA and the architecture of PC tetramers might be essential for the allosteric control of the enzyme and the movements of PT domains, but unraveling the precise role of acetyl-CoA activator requires further future insights.
Experimental Procedures
Protein expression and purification
Full length wild-type S. aureus PC was expressed and purified as described previously (Lasso et al., 2010). Essentially, SaPC was expressed in E. coli BL21 Star (DE3) with an N-terminal hexa-histidine tag by cloning the protein in pET28a vector. After purification of the construct by Ni-NTA agarose beads, the eluted SaPC was further purified through a Sephacryl S300 gel filtration column. SaPC samples were stored at −80°C in a buffer containing 20 mM Tris (pH 7.5), 200 mM NaCl, 2 mM dithiothreitol (DTT), and 5% (v/v) glycerol.
Enzyme activity assay
The catalytic activity of SaPC was determined at 10°C by monitoring the production of oxaloacetate, which was coupled to NADH oxidation catalyzed by malate dehydrogenase (Modak and Kelly, 1995). The reaction mixture contained 20 mM Tris-HCl (pH=7.5), 200 mM NaCl, 50 mM KHCO3, 20 mM Pyruvate, 5 mM MgCl, 2 mM DTT, 2 mM acetyl-CoA, 2 mM ATP, 0.2 mM NADH, 15U of malate dehydrogenase, and various SaPC concentrations ranging from 0.01 μM to 0.1 μM. Oxaloacetate reduction to malate by malate dehydrogenase was coupled to NADH oxidation to NAD+. The decreasing concentration of NADH was monitored by measuring the absorbance at 340 nm.
Sample preparation for cryo-EM
SaPC at a concentration of 1 mg/ml was first incubated in a solution containing 20 mM Tris-HCl (pH=7.5), 200 mM NaCl, 5 mM MgCl2, 2 mM DTT, and 2 mM acetyl-CoA for 20 minutes. Following this, the same buffer containing 20 mM pyruvate was added. Finally, a reaction buffer containing 50 mM KHCO3 and 10 mM ATP was added to the SaPC sample. After 1 min of incubation the sample was stored on ice and cryoEM grids were immediately prepared. The final SaPC concentration was 0.05 mg/ml in a buffer solution containing 16.6 mM Tris-HCl (pH=7.5), 1.7 mM acetyl-CoA, 15.7 mM pyruvate, 4.1 mM MgCl2, 1.7 mM DTT, 166.6 mM NaCl, 8.3 mM KHCO3 and 1.66 mM ATP.
Cryo-EM and image processing
Cryo-EM grids were prepared following standard procedures and vitrified samples were examined on a JEM-2200FS/CR transmission electron microscope (JEOL Europe, Croissy-sur-Seine, France) at an acceleration voltage of 200 kV. Micrographs were taken on Kodak films under low-dose conditions at a magnification of 40,000X. Digitalization of micrographs was carried out on a Z/I Photoscan (ZEIS) scanner obtaining a final pixel size of 1.75 Å. Particles were selected using a semi-automated procedure from the digitized micrographs in Spider/Spire package (Baxter et al., 2007; Frank et al., 1996), which was also used to determine the defocus of the micrographs. Classification of the dataset into four subsets and initial 3D reconstruction of each subset was carried out with Relion (Scheres, 2012), using as the initial reference map a cryo-EM density map for SaPC tetramer (EMD-1742) filtered at 60 Å (Lasso et al., 2010). Whenever necessary cryo-EM maps obtained during classification were refined further using Spider/Spire (Baxter et al., 2007; Frank et al., 1996) by imposing 2-fold (C2) symmetry. Resolutions of the cryo-EM density maps were estimated using a cutoff of 0.15 in the FSC (Rosenthal and Henderson, 2003). The cryoEM maps calculated for classes 1 and 2 have been deposited in the 3D-EM database under accession codes EMD-5945 and EMD-5944 respectively.
SaPC homology modeling
The initial atomic model of class 1 was modeled based on the asymmetric crystal structure of RePC (PDB:3TW6) (Lietzan et al., 2011). Both the target and the template sequences were aligned by ClustalW (Goujon et al., 2010; Larkin et al., 2007). Homology modeling was carried out using SwissModel (Arnold et al., 2006). The BCCP domain was modeled near the BC active site in monomers A and B (Lietzan et al., 2011). In monomers C and D, the BCCP was modeled in the RePC exo binding site (PDB: 2QF7) (St Maurice et al., 2007). The initial atomic model of the SaPC tetramer in classes 2 and 3 were modeled using the symmetric crystal structure of SaPC (Xiang and Tong, 2008) as the template structure. Monomers A and B were modeled with their BCCP domain pointing towards the CT active site as in the crystal structure of SaPC. Monomers C and D were modeled without the BCCP domains. Connecting loops between the PT and BCCP domain were modeled with Loopy (Soto et al., 2008; Xiang et al., 2002). The protonation state of histidine residues in the assembled initial models were predicted using the Propka software from pdb2pqr package (Bas et al., 2008; Li et al., 2005).
Rigid and flexible fittings of atomic models
The assembled initial models were rigidly fitted into the corresponding cryo-EM maps using UCSF Chimera (Pettersen et al., 2004). Molecular dynamic simulations were carried out with NAMD 2.9 (Phillips et al., 2005) through the MDFF plug-in (Trabuco et al., 2008). Simulations were run with the CHARMM27 force field with CMAP corrections (Mackerell et al., 2004) in generalized Born implicit solvent (Tanner et al., 2011). For each class, five separate MDFF simulations were carried out, all of them were run for 5 ns. Non-hydrogen atoms were coupled to the UEM potential derived from the corresponding cryo-EM density maps with a grid scaling of 0.3 kcal/mol. Simulations used restraints for secondary structure, chirality and cispeptide derived from the initial assembled atomic models. During the initial 0.5 ns of each simulation no symmetrical restraint was applied between monomers. A symmetry restraint force constant k was applied during the next 0.5 ns, linearly increasing from 0 to 10 (kcal/mol)/Å2 (Chan et al., 2011). Following this, the symmetry restraint force constant k was kept at 10 (kcal/mol)/Å2 during the next four ns. Finally, 10,000 steps of energy minimization were performed with a grid scaling of 0 in order to increase the stability of the resulting structure. All simulation parameters were kept as specified by the MDFF plug-in except to those required in intrinsic solvent simulations: the dielectric constant was set to 1; cutoff 16 Å; switchdist 15 Å; pairlistdist 18 Å; GIBS on; ionConcentration 0.3 M; alphaCutoff 14 Å; sasa on; and surfaceTension 0.006 kcal/mol/Å2.
Supplementary Material
Highlights.
Tetramers of PC are observed by cryoEM during catalysis
Classification reveals symmetric an asymmetric architectures for tetramers
The different architectures are linked to sequential enzymatic reactions
The tetramers are active in one layer at a time
Acknowledgments
This work was supported by grants: BFU2012-34873 from the Spanish Ministry of Economy and Competitiveness (to M.V.); and by a grant from the NIH (DK067238) to LT. LPCY was also supported by an NIH training program in Cellular and Molecular Foundations of Biomedical Science (GM008798).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Attwood PV, Wallace JC. Chemical and catalytic mechanisms of carboxyl transfer reactions in biotin-dependent enzymes. Accounts of chemical research. 2002;35:113–120. doi: 10.1021/ar000049+. [DOI] [PubMed] [Google Scholar]
- Bas DC, Rogers DM, Jensen JH. Very fast prediction and rationalization of pKa values for protein-ligand complexes. Proteins. 2008;73:765–783. doi: 10.1002/prot.22102. [DOI] [PubMed] [Google Scholar]
- Baxter WT, Leith A, Frank J. SPIRE: the SPIDER reconstruction engine. Journal of structural biology. 2007;157:56–63. doi: 10.1016/j.jsb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Cazzulo JJ, Stoppani AO. The regulation of yeast pyruvate carboxylase by acetyl-coenzyme A and L-aspartate. Arch Biochem Biophys. 1968;127:563–567. doi: 10.1016/0003-9861(68)90263-4. [DOI] [PubMed] [Google Scholar]
- Chan KY, Gumbart J, McGreevy R, Watermeyer JM, Sewell BT, Schulten K. Symmetry-restrained flexible fitting for symmetric EM maps. Structure. 2011;19:1211–1218. doi: 10.1016/j.str.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. Journal of structural biology. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic acids research. 2010;38:W695–699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S, St Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, mechanism and regulation of pyruvate carboxylase. The Biochemical journal. 2008;413:369–387. doi: 10.1042/BJ20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S, Wallace JC. Structure, function and regulation of pyruvate carboxylase. The Biochemical journal. 1999;340(Pt 1):1–16. doi: 10.1042/bj3400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JR. The mechanism of biotin-dependent enzymes. Annual review of biochemistry. 1989;58:195–221. doi: 10.1146/annurev.bi.58.070189.001211. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lasso G, Yu LP, Gil D, Xiang S, Tong L, Valle M. Cryo-EM analysis reveals new insights into the mechanism of action of pyruvate carboxylase. Structure. 2010;18:1300–1310. doi: 10.1016/j.str.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Robertson AD, Jensen JH. Very fast empirical prediction and rationalization of protein pKa values. Proteins. 2005;61:704–721. doi: 10.1002/prot.20660. [DOI] [PubMed] [Google Scholar]
- Lietzan AD, Menefee AL, Zeczycki TN, Kumar S, Attwood PV, Wallace JC, Cleland WW, St Maurice M. Interaction between the biotin carboxyl carrier domain and the biotin carboxylase domain in pyruvate carboxylase from Rhizobium etli. Biochemistry. 2011;50:9708–9723. doi: 10.1021/bi201277j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietzan AD, St Maurice M. A substrate-induced biotin binding pocket in the carboxyl transferase domain of pyruvate carboxylase. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M113.477828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerell AD, Jr, Feig M, Brooks CL., 3rd Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. Journal of computational chemistry. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- Marin-Valencia I, Roe CR, Pascual JM. Pyruvate carboxylase deficiency: mechanisms, mimics and anaplerosis. Molecular genetics and metabolism. 2010;101:9–17. doi: 10.1016/j.ymgme.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Mochalkin I, Miller JR, Evdokimov A, Lightle S, Yan C, Stover CK, Waldrop GL. Structural evidence for substrate-induced synergism and half-sites reactivity in biotin carboxylase. Protein Sci. 2008;17:1706–1718. doi: 10.1110/ps.035584.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak HV, Kelly DJ. Acetyl-CoA-dependent pyruvate carboxylase from the photosynthetic bacterium Rhodobacter capsulatus: rapid and efficient purification using dye-ligand affinity chromatography. Microbiology (Reading, England) 1995;141(Pt 10):2619–2628. doi: 10.1099/13500872-141-10-2619. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. Journal of computational chemistry. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Scheres SH. Classification of structural heterogeneity by maximum-likelihood methods. Methods in enzymology. 2010;482:295–320. doi: 10.1016/S0076-6879(10)82012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH. RELION: implementation of a Bayesian approach to cryo-EM structure determination. Journal of structural biology. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto CS, Fasnacht M, Zhu J, Forrest L, Honig B. Loop modeling: Sampling, filtering, and scoring. Proteins. 2008;70:834–843. doi: 10.1002/prot.21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Maurice M, Reinhardt L, Surinya KH, Attwood PV, Wallace JC, Cleland WW, Rayment I. Domain architecture of pyruvate carboxylase, a biotin-dependent multifunctional enzyme. Science (New York, NY. 2007;317:1076–1079. doi: 10.1126/science.1144504. [DOI] [PubMed] [Google Scholar]
- Tanner DE, Chan KY, Phillips JC, Schulten K. Parallel Generalized Born Implicit Solvent Calculations with NAMD. Journal of chemical theory and computation. 2011;7:3635–3642. doi: 10.1021/ct200563j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci. 2013;70:863–891. doi: 10.1007/s00018-012-1096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabuco LG, Villa E, Mitra K, Frank J, Schulten K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure. 2008;16:673–683. doi: 10.1016/j.str.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter MF, Keech DB. Formation of oxaloacetate from pyruvate and carbon dioxide. The Journal of biological chemistry. 1960;235:PC17–18. [PubMed] [Google Scholar]
- Waldrop GL, Holden HM, St Maurice M. The enzymes of biotin dependent CO(2) metabolism: what structures reveal about their reaction mechanisms. Protein Sci. 2013;21:1597–1619. doi: 10.1002/pro.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S, Tong L. Crystal structures of human and Staphylococcus aureus pyruvate carboxylase and molecular insights into the carboxyltransfer reaction. Nat Struct Mol Biol. 2008;15:295–302. doi: 10.1038/nsmb.1393. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Soto CS, Honig B. Evaluating conformational free energies: the colony energy and its application to the problem of loop prediction. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7432–7437. doi: 10.1073/pnas.102179699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LP, Chou CY, Choi PH, Tong L. Characterizing the importance of the biotin carboxylase domain dimer for Staphylococcus aureus pyruvate carboxylase catalysis. Biochemistry. 2013;52:488–496. doi: 10.1021/bi301294d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LP, Xiang S, Lasso G, Gil D, Valle M, Tong L. A symmetrical tetramer for S. aureus pyruvate carboxylase in complex with coenzyme A. Structure. 2009;17:823–832. doi: 10.1016/j.str.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.