Abstract
Leukocyte adhesion deficiency Type I (LAD-I), a disease syndrome associated with frequent microbial infections, is caused by mutations on the CD18 subunit of β2 integrins. LAD-I is invariably associated with severe periodontal bone loss, historically attributed to lack of neutrophil surveillance of the periodontal infection. Here, we challenge this dogma by showing that the cytokine IL-17 plays a major role in the oral pathology of LAD-I. Defective neutrophil recruitment in LAD-I patients, or in LFA-1 (CD11a/CD18)-deficient mice that exhibit the LAD-I periodontal phenotype, was associated with excessive production of predominantly T cell-derived IL-17 in the periodontal tissue. The pathological elevation of IL-17 in the LFA-1–deficient periodontal tissue derived also from innate lymphoid cells. Strikingly, local treatment with anti-IL-17 (or anti-IL-23) in LFA-1-deficient mice not only blocked inflammatory periodontal bone loss but also caused a reduction in the total bacterial burden, suggesting that the IL-17-driven pathogenesis of LAD-I periodontitis leads to dysbiosis. Our findings therefore support an IL-17-targeted therapy for this condition.
INTRODUCTION
Neutrophils, the most abundant white blood cells, form the first line of defense against pathogenic insults (1). Upon their mobilization from the bone marrow, neutrophils circulate in the blood and can rapidly migrate to peripheral tissues (e.g., skin, gut, lungs, or gingiva) in response to infection or inflammation. The extravasation process is a cascade of low- and high-affinity adhesive interactions between the neutrophils and the vascular endothelium of the infected or inflamed tissue and involves distinct steps, including tethering and rolling, activation, firm adhesion, intraluminal crawling, and transmigration (1, 2). Firm adhesion and crawling are largely dependent on interactions between leukocyte β2-integrins (heterodimers comprising a distinct CD11 subunit and a common CD18 subunit) and endothelial counter-receptors, such as the intercellular adhesion molecules-1 and -2. The LFA-1 integrin (CD11a/CD18) plays a crucial role in firm adhesion, which is essential to the subsequent extravasation of the neutrophils to peripheral tissues (1, 2).
Although important in innate immune defense, neutrophils can also cause tissue damage if their activity is not properly regulated. In this regard, neutrophil homeostasis entails more than regulation of extravasation, also including tight control of granulopoiesis, release of mature neutrophils from the bone marrow, and clearance of apoptotic neutrophils (3-5). Ley and colleagues proposed a feedback loop mechanism that coordinates the peripheral clearance of transmigrated apoptotic neutrophils with neutrophil production and release from the bone marrow (6). According to this neutrophil rheostat (‘neutrostat’) model, the phagocytosis of transmigrated apoptotic neutrophils by tissue phagocytes suppresses their expression of IL-23 leading in turn to downregulation of IL-17 and G-CSF production and ultimately of granulopoiesis (6). This regulatory circuit is disrupted in leukocyte adhesion molecule-deficient mice resulting in unrestrained tissue expression of IL-17 (6). However, this homeostatic breakdown has yet to be linked to an IL-17-driven disease in animal models or in humans. Moreover, it has remained uncertain whether IL-17 is excessively elevated in humans with leukocyte adhesion deficiency (LAD) or contributes to any of the clinical manifestations of this disorder. Here we show that dysregulated local overproduction of IL-17 in LAD type I (LAD-I) drives periodontal bone loss, which has been historically attributed to defective innate immune surveillance of the periodontal infection (7-10).
LAD-I is an autosomal recessive immunodeficiency caused by mutations in the CD18-encoding ITGB2 gene that result in defective neutrophil adhesion and transmigration (11, 12). Affected individuals display neutrophilia, suffer from recurrent infections, and invariably develop early-onset generalized aggressive periodontitis featuring severe bone loss and premature loss of primary and permanent teeth (8, 11-13). However, successful bone marrow transplantation in LAD-I patients reverses the periodontal disease phenotype (14). Mice deficient in CD18 or CD11a exhibit neutrophilia and defective neutrophil adhesion and extravasation (6, 15) and are thus useful models to study mechanisms of LAD-I-associated pathology. Our clinical findings in LAD-I patients and mechanistic studies in mouse models, reported here, collectively show that defective neutrophil recruitment is associated with IL-17-driven inflammatory periodontal bone loss, and suggest promising treatment options for the hitherto intractable LAD-I periodontitis.
RESULTS
Human LAD-I periodontitis exhibits an IL-17-dominated gene expression signature
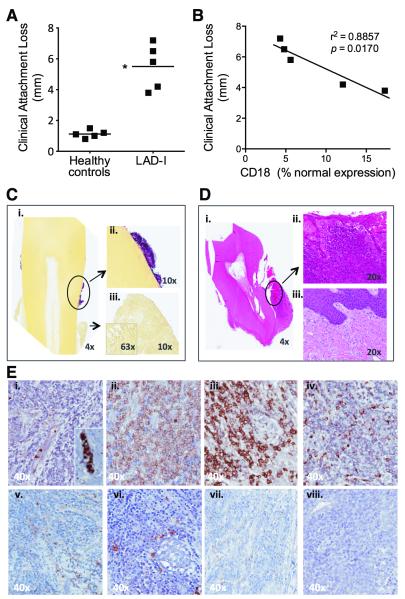
To investigate the mechanistic basis of LAD-I periodontitis, we clinically evaluated and followed a cohort of patients diagnosed with LAD-I based on the identification of CD18 mutations, and diminished CD18 expression on peripheral neutrophils (table S1), as well as history of recurrent infections since childhood. Periodontitis with severe loss of tooth-supporting connective tissue and bone was clinically diagnosed in all patients in our LAD-I cohort (Fig. 1A and fig. S1). Importantly, the severity of periodontal tissue destruction (“clinical attachment loss”; measured in mm) in LAD-I patients (ages 11-13) inversely correlated with the remaining expression of CD18 on their peripheral neutrophils (Fig. 1B). This observation suggests a direct link between the patients’ defective neutrophil phenotype and severity of LAD-I periodontitis.
Fig. 1. Clinical and histological profile of LAD-I periodontitis.
(A) Clinical attachment loss (marker of bone loss) was measured on all teeth of five 11-13 year-old LAD-I patients and healthy controls. Shown are mean measurements per individual (*P < 0.01; unpaired t test). (B) Correlation between clinical attachment loss and CD18 expression on peripheral neutrophils of LAD-I patients (Pearson correlation coefficient r2=0.8857; P=0.0170). CD18 expression on neutrophils evaluated by flow cytometry and expressed as percent of normal. (C) Gram staining (Brown and Hoops) on an extracted tooth and surrounding soft tissues: (i) Blue/pink positive staining on the tooth encircled and shown in higher magnification (ii); Soft tissues with no visible gram staining (iii). (D) H&E of extracted tooth and surrounding soft tissues: (i) Soft tissue surrounding the entire tooth indicating total loss of bone support. Encircled soft tissue in higher magnification (ii) revealing dense inflammatory infiltrate in the lesion compared to healthy gingival tissue from a control subject (iii). (E) Immunohistochemistry for major cell populations in the lesion: (i) neutrophils, inset showing positive cells within a vessel; (ii) T cells; (iii) plasma cells; (iv) macrophages; (v) γδ T cells; (vi) mast cells; (vii) DC; (viii) NK cells. C, D, and E representative of four patients and multiple extracted teeth. All original magnifications indicated.
LAD-I periodontitis has been historically attributed to lack of neutrophil innate immune surveillance (ostensibly rendering the patients susceptible to periodontal infections), although it has proven unresponsive to antibiotics and/or mechanical removal of the tooth-associated biofilm (8). We observed progressive periodontal disease in all patients evaluated, despite prophylactic antibiotic use since infancy and access to dental care. Gram staining of extracted teeth and surrounding tissues from LAD-I patients suggested profound microbial colonization of tooth surfaces but not of underlying diseased gingival tissue (Fig. 1C), consistent with absence of a raging infection within the gingiva. Moreover, quantification of bacterial counts in gingival tissue sections from LAD-I patients and healthy controls, using real-time PCR of the 16S rRNA gene, revealed that the bacterial load within the tissue of LAD-I patients was comparable to (if not less than) that of healthy controls (fig. S2). The diseased gingival tissue, however, harbored immunopathological lesions featuring a dense inflammatory infiltrate (Fig. 1D). The infiltrate comprised primarily lymphocytes, whereas neutrophils were confined within vessels (Fig. 1E). These data suggested that LAD-I periodontitis is not likely to result from an unusual tissue-invasive periodontal infection but rather could represent an immunopathological entity, potentially involving mechanism(s) distinct from the slowly progressive chronic or adult-type periodontitis (16).
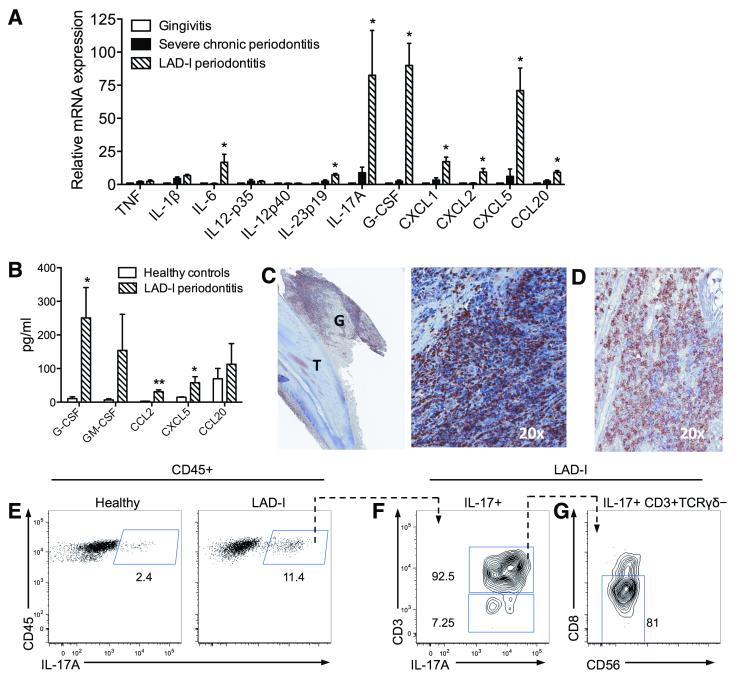
To investigate the underlying immunopathological process, we first compared the periodontal cytokine/chemokine gene expression profile of LAD-I patients with that of systemically healthy patients with severe chronic periodontitis or with gingivitis, i.e., periodontal inflammation without bone loss. We detected a striking upregulation of IL-17A expression in LAD-I periodontitis, as compared to both gingivitis and periodontitis (Fig. 2A). Cytokines linked to the induction and amplification of IL-17A expression, such as IL-1β, IL-6, and IL-23 (17) were readily expressed in LAD-I periodontitis, although only IL-6 and IL-23p19 were significantly elevated relative to both gingivitis and periodontitis (Fig. 2A). While IL-12p40 was not upregulated in LAD-I periodontitis, it was detected (at comparable levels to those expressed in gingivitis and periodontitis). Consistent with the high levels of IL-17A expression, IL-17A-dependent cytokines or chemokines linked to neutrophil granulopoiesis or recruitment, such as G-CSF, CXCL2, and CXCL5, also displayed significant upregulation in LAD-I periodontitis (Fig. 2A). High production of granulopoiesis factors and chemokines was also observed in the local inflammatory exudate bathing the space between the teeth and the free gingiva (gingival crevicular fluid) of LAD-I patients (Fig. 2B).
Fig. 2. IL-17 signature in LAD-I periodontitis.
(A) Quantitative real-time PCR analysis of indicated cytokine mRNA expression levels in the lesions of LAD-I periodontitis compared to those from severe chronic periodontitis (severe inflammatory bone loss) or gingivitis (gingival inflammation without associated bone loss); results were normalized to HPRT mRNA and presented as fold induction relative to gingivitis, assigned an average value of 1. Data are means ± SEM (n=4 per group). *P < 0.05 vs. both chronic periodontitis and gingivitis (one-way ANOVA). (B) The indicated cytokines/chemokines were measured in gingival crevicular fluid from healthy control subjects and LAD-I patients, using multiplex luminex assays. Data are means ± SEM (n=5 per group) *P < 0.05 and **P < 0.01; unpaired t test. (C) Immunohistochemistry for IL-17A in LAD-I gingiva [G] surrounding an extracted tooth [T] (left). Numerous IL-17A+ cells are seen throughout the lesion, shown in larger magnification (right). (D) Immunohistochemistry for CD3 in IL-17A+ cell regions. C and D are serial sections, representative of 4 patients and multiple tooth sites. (E-G) Characterization of IL-17-producing cells in LAD-I periodontitis. (E) Flow cytometry after intracellular staining for IL-17A in isolated CD45+ gingival cells, from healthy (left) or LAD-I (right) subjects, stimulated with PMA and ionomycin. Plots F and G show further characterization of IL-17+ populations in LAD-I: (F) IL-17A versus CD3 staining gated on CD45+IL-17+ cells; (G) CD56 versus CD8 staining gated on CD45+IL-17+CD3+TCRγδ− cells. Representative of two separate experiments with paired LAD-I versus healthy control comparisons.
Human LAD-I periodontitis is characterized by an abundance of IL-17-expressing cells
Consistent with the high levels of IL-17A mRNA expression in LAD-I periodontitis (Fig. 2A), immunohistochemical analysis revealed the presence of abundant IL-17-positive cells throughout the inflammatory lesion (Fig. 2C). IL-17-positive cells were seen predominantly among CD3+ cell-rich regions (Fig. 2D). Flow cytometry of mononuclear cells isolated from oral biopsies of LAD-I patients demonstrated considerably higher numbers of IL-17-producing cells as compared to healthy control subjects (Fig. 2E). Characterization of the IL-17-producing cells revealed predominantly CD3+ IL-17 producers (Fig. 2F), specifically within the CD3+CD8−CD56−TCRγδ− compartment (Fig. 2G). This T cell population is presumed to be CD4+, although we were unable to directly stain for CD4 likely due to its downregulation upon activation (18). CD3− IL-17 producers were also present albeit at a lower frequency (Fig. 2F).
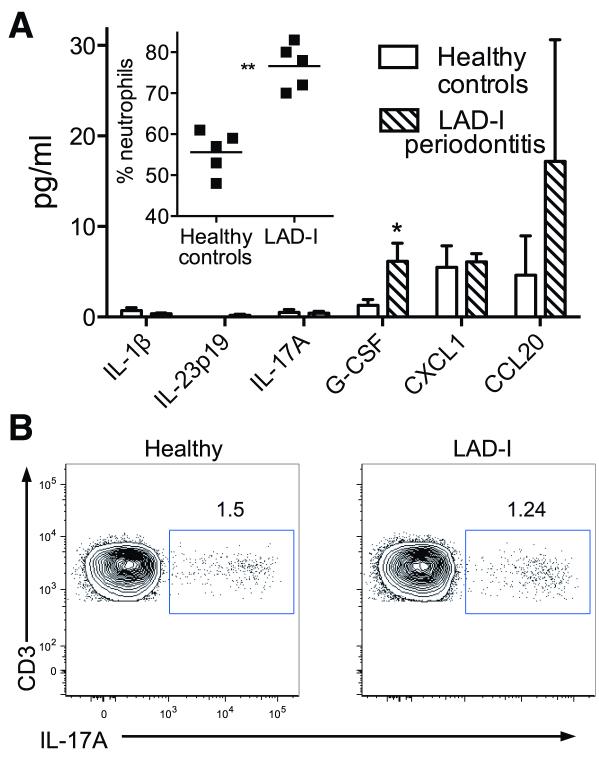
In contrast to oral mucosal areas, blood plasma from LAD-I patients contained little or no IL-23 or IL-17 (Fig. 3A) and the percentages of IL-17-secreting blood mononuclear cells were similar between LAD-I patients and healthy subjects (Fig. 3B). These data reveal that the elevated expression of IL-17 in LAD-I is compartmentalized rather than systemic. In contrast, IL-17-induced granulopoiesis factors and chemokines were readily detected in blood plasma of LAD-I patients (Fig. 3A), correlating with significant neutrophilia relative to healthy subjects (Fig. 3A inset).
Fig. 3. Systemic responses in LAD-I patients.
(A) The indicated cytokines/chemokines were measured in blood plasma from healthy control subjects and LAD-I patients using multiplex luminex assays. The inset shows the percentage of neutrophils in the peripheral blood of the two groups (each point represents an individual). Data are means ± SEM (n = 5 per group). *P < 0.05 and **P < 0.01; unpaired t test. (B) Flow cytometry after intracellular staining for IL-17A in PBMC, from healthy (left) or LAD-I (right) subjects, stimulated with PMA and ionomycin. The PBMC were gated on CD45+CD3+ cells and the plots are representative of four separate experiments with paired LAD-I versus healthy control comparisons.
In summary, our clinical findings demonstrate that LAD-I periodontitis prominently includes aberrant expression of IL-17, consistent with dysregulation of the IL-23-IL-17 axis in mouse models of LAD-I (6). To address whether IL-17 drives LAD-I periodontitis would require an intervention study, which could not be performed at present in human LAD-I patients. We therefore sought evidence for a causal link between IL-17 and LAD-I periodontitis using preclinical models.
Mice with defective neutrophil adhesion or recruitment mirror the IL-17-dominated signature of LAD-I periodontitis
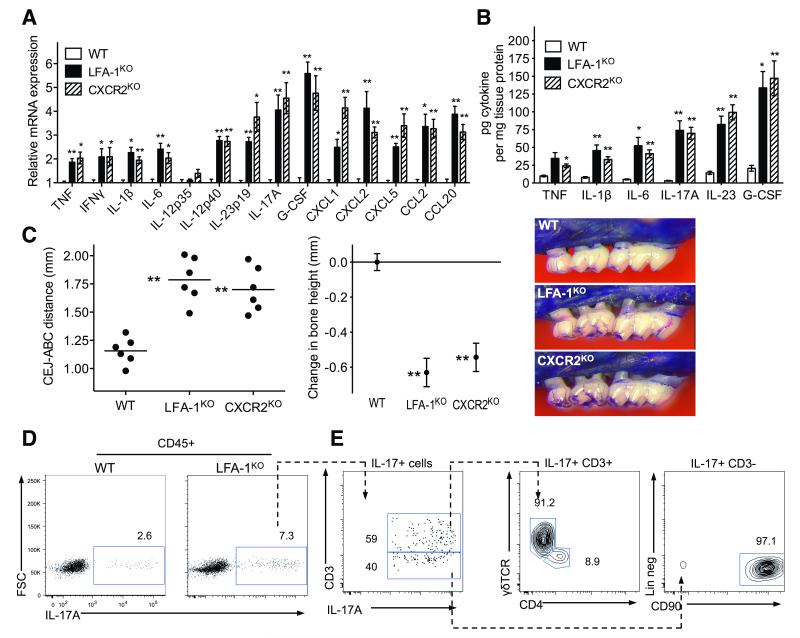
Similar to LAD-I patients, mice deficient in CD11a (LFA-1 knockout; LFA-1KO) experience spontaneous severe periodontal bone loss early in life, although we have not previously analyzed their periodontal cytokine profile or the underlying pathomechanism (19). We have now shown that 18-week-old LFA-1KO mice express significantly higher levels of IL-23 and IL-17A mRNA (Fig. 4A) and protein (Fig. 4B) than wild-type (WT) C57BL/6 controls, correlating with increased periodontal bone loss in the former group (Fig. 4C). Flow cytometry of mononuclear cells isolated from the gingiva showed increased numbers of IL-17-producing cells in LFA-1KO as compared to WT mice (Fig. 4D). Consistent with our findings in LAD-I patients, the dominant source of IL-17 in LFA-1KO mice was CD45+CD3+ T cells, yet in mice the predominant T cell source were γδ T cells (Fig. 4E). We also identified a significant population of CD45+CD3− cells producing IL-17 (Fig. 4E). Further characterization of this population indicated that they were predominantly innate lymphoid cells (CD45+CD3−CD19−CD5−NK1.1−CD11b−Ly6G−CD117− CD90+) (20). Consistent with the elevated IL-17, LFA-1KO mice also showed higher expression of IL-17-dependent genes encoding IL-6, G-CSF and chemokines (Fig. 4A). TNF, IL-1β, and IFNγ were also detected in the periodontal tissue of LFA-1KO mice but their expression was not as pronounced as IL-17 and IL-23 (Fig. 4, A and B). Consistent with a preferential dysregulation of IL-23 production, the mRNA expression of the IL-12p35 subunit (which is unique to IL-12) was not upregulated in the periodontal tissue of LFA-1KO mice, whereas the IL-12/IL-23-shared p40 subunit as well as IL-23p19 were elevated relative to WT mice (Fig. 4A).
Fig. 4. Cytokine profiles in periodontitis associated with defective neutrophil adhesion and/or recruitment.
18-week-old WT control mice were compared with age-matched LFA-1KO and CXCR2KO mice for cytokine levels and bone loss. (A, B) Gingiva were dissected to assess the indicated cytokine responses at the mRNA (A) or protein (B) level. Cytokine mRNA expression levels were normalized against GAPDH mRNA and expressed as fold induction relative to the transcript levels of 18-week-old WT mice, which were assigned an average value of 1. (C) Measurement of periodontal bone heights (CEJ-ABC distance) in the indicated mouse groups (left), calculation of relative bone loss (middle), and representative images of maxillae (right). In the left panel, each symbol represents an individual mouse and small horizontal lines indicate the mean. In the middle panel, bone loss was calculated as bone height in WT control mice (0 baseline) minus bone height in experimental mice. Data are means ± SEM (n = 6 mice/group) from one of two independent experiments with similar results. *P< 0.05 and **P< 0.01 compared with WT controls (one-way ANOVA). (D, E) Flow cytometry of cell preparations isolated from mouse gingiva stimulated with PMA and ionomycin. (D) IL-17 staining in CD45+ cells from WT and LFA-1KO mice. (E) Further characterization of IL-17+ populations in LFA-1KO mice. Plots shown from left to right; staining for CD3 versus IL-17 gated on CD45+IL-17+ cells; TCRγδ versus CD4 gated on CD45+IL-17+CD3+ cells; and lineage staining (CD3−CD19−CD5−NK1.1−CD11c−CD11b−Ly6G−CD117−) versus CD90 gated on CD45+IL-17+CD3− cells. Data are representative of three independent experiments.
Mice deficient in the chemokine receptor CXCR2 (CXCR2KO), which fail to recruit neutrophils to the periodontal tissue (21), displayed a cytokine expression profile and bone loss similar to LFA-1KO mice (Fig. 4, A-C), suggesting that defective neutrophil recruitment rather than defective neutrophil adhesion per se causes these abnormalities. Taken together, these data show that LFA-1KO and CXCR2KO mice share the periodontal phenotype of LAD-I patients in terms of early-onset severe bone loss associated with defective neutrophil recruitment and elevated IL-17.
IL-23 or IL-17 is required for bone loss and increased bacterial burden in mouse LAD-I periodontitis
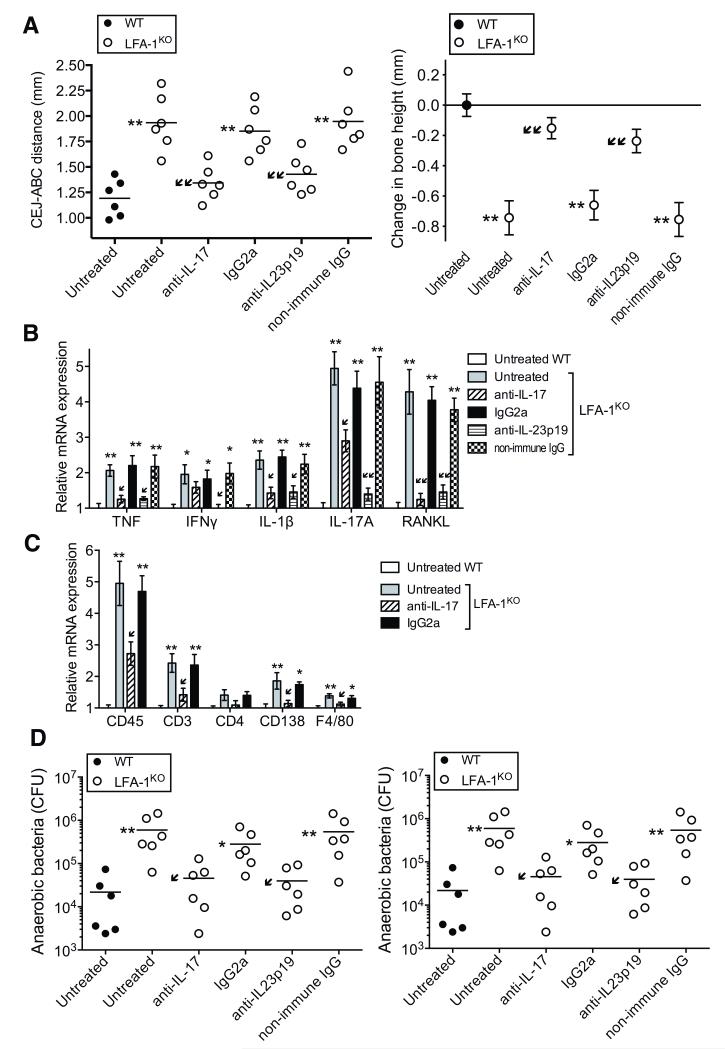
To determine whether IL-23 and/or IL-17 are causally linked to LAD-I periodontitis, we performed intervention experiments in LFA-1KO mice, which were validated as a model of LAD-I periodontitis (Fig. 4). LFA-1KO mice treated locally in the gingiva with anti-IL-17A or anti-IL-23p19 antibody (three times weekly from the age of 4 to 18 weeks) were protected from bone loss, now resembling the WT mouse phenotype, while untreated or control-treated LFA-1KO mice had progressive disease (Fig. 5A). The antibody-mediated inhibition of bone loss correlated with significantly decreased expression of receptor activator of nuclear factor-κB ligand (RANKL), a key osteoclastogenic factor, and other proinflammatory cytokines in the periodontal tissue (Fig. 5B). Moreover, the expression of IL-17 was diminished by anti-IL-23p19 treatment (Fig. 5B), indicating that IL-17 overproduction in untreated LFA-1KO mice was dependent on IL-23. Quantitative real-time PCR analysis of cell-specific marker mRNA expression in the periodontal tissue of anti-IL-17-treated LFA-1KO mice revealed significantly reduced expression of CD45, CD3, CD138, and F4/80, compared with untreated LFA-1KO mice (Fig. 5C), suggesting reduced infiltration of cells of haematopoietic origin (identified by CD45), including inflammatory cells, such as CD3+ T cells, plasma cells (CD138), and macrophages (F4/80). Since CD3+ T cells are major IL-17 producers (Fig. 4E), the reduction of CD3+ T cells by anti-IL-17 could explain, at least in part, why anti-IL-17 treatment also suppressed IL-17 mRNA expression (Fig. 5B). LFA-1KO mice exhibited a higher periodontal bacterial load than WT controls (Fig. 5D), suggestive of dysbiosis (similarly, the tooth-associated periodontal microbiota of LAD-I patients exhibited higher total counts in comparison with age-matched healthy controls; fig. S3). Intriguingly, the antibody treatments caused a reduction in the numbers of cultivable anaerobic bacteria (Fig. 5D, left) and in total periodontal bacterial counts as determined by 16S rRNA real-time PCR (Fig. 5D, right). The combined anti-inflammatory and anti-microbial effect of anti-IL-23 or anti-IL-17 is consistent with the notion that IL-17-driven periodontal inflammation fosters bacterial growth, presumably through the generation of tissue breakdown products used as nutrients by the bacteria (22, 23). These data also suggest that the elevated bacterial burden in untreated LFA-1KO mice (relative to WT controls) may not necessarily or exclusively result from impaired neutrophil surveillance of the periodontal tissue; rather, higher bacterial burden could represent the consequence of increased inflammation. Taken together, the data in figures 4 and 5 suggest that, in the absence of normal neutrophil recruitment, IL-23 and IL-17 are upregulated in the periodontal tissue and cause bone loss associated with increased bacterial burden.
Fig. 5. Treatment of LFA-1KO mice with anti-IL-17 or anti-IL-23 inhibits bone loss and reduces the bacterial burden.
LFA-1KO mice were either left untreated or were treated locally in the gingiva with anti-IL-17A mAb (or IgG2a isotype control) or anti-IL-23p19 polyclonal antibody (or non-immune IgG), three times weekly from the age of 4 to 18 weeks. (A) Periodontal bone heights (CEJ-ABC distance) in the indicated mouse groups (left) and bone loss (right), calculated as bone height in 18-week-old WT control mice (0 baseline) minus bone height in experimental mice. (B, C) Quantitative real-time PCR analysis of the indicated cytokine (B) and cell-specific marker (C) mRNA expression in the periodontal tissue of mice treated as shown; results were normalized to those of GAPDH mRNA and are presented as fold induction relative to the transcript levels of untreated WT controls, which were assigned an average value of 1. (D) Quantification of cultivatable oral anaerobic bacteria (left) and determination of total bacterial burden in the periodontal tissue of the indicated mouse groups by real-time PCR of the 16S rRNA gene (right). Data were pooled from two independent experiments with three mice per group in each experiment (i.e., total of six mice per group). In A (left) and D, each symbol represents an individual mouse and small horizontal lines indicate the mean. In A (right), B, and C data are means ± SEM (n = 6 mice/group). *P< 0.05 and **P< 0.01 compared with untreated WT control (one-way ANOVA). ↙P< 0.05 and ↙↙P< 0.01 compared to untreated LFA-1KO (one-way ANOVA).
DISCUSSION
Our analyses of the periodontal tissue from LAD-I patients and relevant animal models collectively demonstrate a primary role for IL-17 in the oral pathology of LAD-I. Our intervention studies in mice exhibiting the LAD-I periodontal phenotype unequivocally demonstrate that the IL-23-IL-17 axis is driving dysbsiosis and inflammatory bone loss, a finding with obvious translational implications for the treatment of the hitherto intractable human LAD-I periodontitis. Our findings not only confer biological relevance to the neutrostat concept established in mice (6) but also provide a human (and animal) disease correlate of this mechanism. Periodontitis was a highly relevant condition to test the biological importance of the neutrostat mechanism due to its particular mucosal environment (16), where the presence of IL-23–inducing bacteria (24) can potentially unleash unregulated production of IL-23 and hence local overexpression of IL-17, a notion that was substantiated by our findings.
Importantly, our findings challenge the traditional concept that LAD-associated periodontitis is mainly attributed to impaired neutrophil surveillance of infection. Since continuous prophylactic antibiotic treatment of LAD-I patients and mechanical removal of tooth-associated microbial biofilm have not been successful in the treatment and prevention of LAD-I periodontitis (8, 25, 26), there is urgent need for alternative therapies in this condition. In this regard, IL-17-targeting regimens are promising candidates according to our present data. Additionally, our demonstration that defective neutrophil recruitment can lead to IL-17–dependent immunopathology may shed light to the understanding of other diseases associated with LAD-I, such as colitis (27).
The dysbiosis developed in LFA-1KO mice was reversible by anti-IL-17 treatment, suggesting that it was driven by IL-17-dependent inflammation (22, 23) rather than by defective immune surveillance due to the lack of neutrophils. In this regard, patients with chronic granulomatous disease (CGD) who have defective neutrophil oxygen-dependent bactericidal activity and suffer from recurrent or persistent infections (e.g., pneumonia and abscesses of the skin) are not susceptible to periodontitis, in contrast to LAD patients (9). This observation suggests that defective immune surveillance by neutrophils may not be a dominant factor in susceptibility to periodontitis, possibly because neutrophils could be compensated for, in that regard, by other phagocytic cells (e.g., macrophages). In stark contrast, the extravasation competence of neutrophils appears to be essential for periodontal tissue homeostasis. Our recent demonstration that neutrophils migrate normally to the periodontal tissue in the absence of bacterial colonization (i.e., in germ-free mice) (21) further suggests that neutrophil recruitment may serve homeostatic function(s) that are not necessarily related to infection control.
Our microbiological findings from human patients further support that LAD-I periodontitis is unlikely to result from an unusual tissue-invasive periodontal infection. This, however, does not exclude the involvement of the tooth-associated microbiota in the pathogenic process. The local microbiota can act as the initial stimulus to unleash the disinhibited IL-23-IL-17 axis. It should be noted that the bacteria do not have to invade the periodontal tissue to stimulate inflammatory cells. This is because released bacterial products capable of activating inflammatory cells (e.g., lipopolysaccharide) can readily penetrate through the gingival junctional epithelium, which is highly porous as the cells are interconnected by only a few desmosomes and occasional gap junctions (28).
To strengthen the notion that defective neutrophil recruitment underlies IL-17-mediated pathology in LAD-I, we performed animal model studies in two distinct models with neutrophil defects, LFA-1KO and CXCR2KO. The chemokine receptor CXCR2 is essential for tissue recruitment of neutrophils (29, 30), consistent with the observation that the extravascular sites in the periodontal tissue of CXCR2KO mice are nearly completely void of neutrophils (21). The LFA-1 integrin is crucial for the recruitment of neutrophils to peripheral tissues (15, 31, 32), but less important for other leukocytes which use different or additional adhesion molecules (e.g., VLA-4; very late antigen-4) for this function (33-37). This explains why the inflammatory infiltrates in the periodontium of LAD-I patients are specifically devoid of neutrophils, which are confined in vessels, whereas lymphocytes (including plasma cells) and other cells of hematopoietic origin can be found in abundance within the periodontium.
We found increased numbers of plasma cells in the lesions of LAD-I periodontitis; whether their abundance can be attributed to the overexpression of IL-17 is uncertain. In this respect, IL-17 was shown to enhance the survival and proliferation of human B cells and their differentiation into plasma cells (38). Given that B cells/plasma cells constitute a major source of RANKL in the bone resorptive lesions of chronic periodontitis (39), the abundance of plasma cells in LAD-I periodontitis might contribute to inflammatory bone loss. This notion is consistent with the correlation between decreased periodontal expression of CD138 (a plasma cell marker) and decreased bone loss in anti-IL-17-treated LFA-1KO mice.
Our findings that LAD-I patients as well as both LFA-1KO and CXCR2KO mice share an IL-17-dominated periodontal disease phenotype suggest that an IL-17-driven mechanism for periodontal tissue destruction might be relevant to additional conditions (i.e., other than LAD-I) associated with poor or no accumulation of neutrophils in extravascular sites, owing to defective chemotaxis (e.g., Chediak-Higashi syndrome and Papillon-Lefèvre syndrome) or neutropenic states (e.g., congenital agranulocytosis, autoimmune neutropenia, HIV-associated neutropenia, and neutropenia in cancer patients under chemotherapy or radiation therapy). Similar to LAD-I individuals, patients with defective chemotaxis develop rapidly advancing inflammatory periodontal bone loss at young age, and the same is observed in neutropenic patients unless their neutrophil count is appropriately corrected (7-10, 13, 25, 26, 40).
Our studies also aimed at characterizing the nature of the IL-17 response in LAD-I periodontitis and revealed that T cells were the primary source of IL-17, consistent with the recognition of T cell-derived IL-17 in the pathogenesis of inflammatory bone loss in humans and animal models of arthritis (41, 42). In mice, these cells were identified as CD3+ γδ T-cells which, in the presence of IL-23, constitute an important source of IL-17 in murine mucosal tissues (43) including the periodontal tissue (44). In LAD-I patients, the identified CD3+CD8−CD56−TCRγδ− IL-17 producers were presumed to be CD4+ (Th17); however, we cannot rule out that these might in fact be a population of CD3+CD4−CD8− cells similar to the ones identified in patients with systemic lupus erythematosus or Sjogren’s syndrome as dominant sources of pathogenic IL-17 (45, 46). Importantly, our data support that the pathogenic role in LAD-I periodontitis of CD3+ IL-17-producing cells (whether Th17 or γδ T cells) is primarily related to their capacity to produce IL-17, a potent osteoclastogenic cytokine (42). Interestingly, a secondary source of IL-17 in the mucosal setting of LAD-I periodontitis were CD3− IL-17-producing cells. In LFA-1KO mice, CD3− IL-17-producing cells were further defined to be lineage-negative innate lymphoid cells, consistent with the capacity of IL-23 to upregulate IL-17 in both adaptive and innate immune cells (47). Innate lymphoid cells have recently emerged as innate cell populations with key barrier functions at mucosa sites (20). Although the exact function of this cell population in the gingival tissue is not clear, our findings imply the possible association of innate lymphoid cells with an inflammatory disease state.
In conclusion, we have demonstrated a link between defective neutrophil recruitment in LAD-I and dysregulation of local IL-17 production, which is therapeutically important. Indeed, our preclinical studies support the notion that the neutralization of IL-17 (or IL-23) could effectively serve as an adjunctive therapy for generalized aggressive periodontitis associated with LAD-I, and perhaps other leukocyte adhesion deficiencies (i.e., LAD-II involving defective glycosylation of selectin ligands (10, 48)), or other conditions associated with poor or no recruitment of neutrophils (e.g., Chediak-Higashi syndrome featuring defective chemotaxis) (7-9, 13).
MATERIALS AND METHODS
Study design
The objective of this study was to understand the mechanism(s) underlying LAD-I periodontitis and identify candidate therapeutic targets. Research was performed under Institutional Review Board-approved protocols. Five LAD-I patients with defined CD18 mutations (table S1) and an equal number of control subjects were included in the studies and their periodontal status was clinically evaluated. Oral tissues, fluids, dental plaque, and peripheral blood were obtained for gene expression analysis, (immuno)histological and microbiological studies, and flow cytometry of cell populations. The oral biopsies were performed either during therapeutic procedures or as elective research procedures. Preclinical studies including intervention experiments with anti-IL-23 or anti-IL-17 were performed in LFA-1KO mice, which exhibit the LAD-I periodontal phenotype. Animal experiments involved six mice per group (determined by GraphPad StatMate power analysis for a P value of 0.05 and a power of 0.80). All procedures described in this study were approved by the Institutional Animal Care and Use Committee, in compliance with established federal and state policies.
LAD diagnostics and clinical data
Patients were diagnosed with LAD-I based on a defined CD18 mutation and flow cytometric analysis of CD18 expression on peripheral neutrophils, as previously described (49). Neutrophil counts were extracted from routine patients’ CBCs.
Clinical examination and determination of bone loss in humans
Loss of tooth-supporting connective tissue and bone (“Clinical Attachment Loss”; CAL) was clinically measured, using a straight probe calibrated in mm, at six sites per tooth in all teeth per patient and expressed as mean measurement per patient. Periodontal disease diagnosis was performed according to the criteria of the American Academy of Periodontology (50), with sites exhibiting CAL >5mm considered severe periodontitis.
Human tissue specimens
Periodontitis-involved tissue samples from patients diagnosed with severe chronic periodontitis or LAD-I periodontitis were obtained during therapeutic surgery. Inclusion criteria for diseased tissues were evidence of inflammation (bleeding on probing) and advanced loss of tooth-supporting structures (CAL >5 mm). Inflamed tissues without associated bone loss (i.e., gingivitis) and healthy tissue (non-inflamed) samples were obtained as oral biopsies. Research involving human subjects was reviewed and approved by the Institutional Review Boards of NIH. All patients provided written informed consent for participation. Tissues were either immediately placed in zinc-formalin (Anatech) for histology, snap-frozen for RNA studies, or placed in RPMI media (InVitrogen) for flow cytometry.
Immunohistochemistry
Formalin-fixed tissues were embedded in paraffin and sectioned into 5-μm sections, deparaffinized and rehydrated, followed by heat-induced epitope retrieval. Methanol containing 3% H2O2 was used to block the endogenous peroxidase for 15-30 minutes. Sections were then blocked with BSA and incubated for 1h at room temperature or overnight at 4°C with primary antibody to CD3, CD138, CD68, DC-SIGN (Abcam), IL-17A (R&D Systems), mast cell tryptase (Dako), CD15 (Novus), CD56 (Thermo Scientific), TCRγδ (eBioscience) or isotype controls. After washing with PBS three times, immunolabeling was performed using the ImPress detection system (Vector) followed by visualization with ImmPACT DAB peroxidase substrate. Finally, the specimens were counterstained with Mayer’s hematoxylin, mounted with Permount (Fisher), and sections were scanned using a ScanScope (Aperio).
Cell isolation from gingiva and flow cytometry
All human or mouse tissue samples were digested in a collagenase (Invitrogen) and DNase (Sigma) mix for 1 h at 37°C with agitation. A single-cell suspension was then generated by mashing digested samples through a 70-μm filter (BD Biosciences). Single-cell suspensions from gingival tissues, or human PBMC, were stimulated for 3.5h with or without PMA (50 ng/ml; Sigma) and ionomycin (2.5 μg/ml; Sigma) in the presence of brefeldin A. At the end of the incubation period, human cells were stained with Live/Dead Cell Viability assay (Invitrogen) and anti-human CD3, CD14 (Biolegend), CD45, CD4, CD8, TCRγδ, CD161, CD56, CD19, CD117, CD127, IL-17A (eBioscience). Mouse cells were stained with Viability stain (Zombie yellow, Biolegend), anti- CD45, TCRαβ, TCRγδ, CD4, NK1.1, CD3, CD5, CD11b, CD11c, CD19, CD90, CD117 (eBioscience), Ly6G (BD Bioscience), IL-17 (Biolegend). All samples were analyzed using a FACS Fortessa cytometer (BD Biosciences). Data analysis was performed using FlowJo software (Treestar).
Cytokine protein measurements
(a) Humans: Cytokines were measured in human blood plasma and gingival crevicular fluid using a custom-designed Panomics Procarta Cytokine Profiling Assay, based on Luminex technology (Affymetrix). (b) Mice: Gingival tissue was excised from around the maxillary molars and homogenized as previously described (51). Cytokine levels were determined in soluble extracts by ELISA using commercially available kits (eBioscience). Cytokine protein concentrations were normalized to the total protein concentrations in the tissue homogenates, as measured using the Coomassie Plus (Bradford) protein assay kit (Pierce).
Cytokine mRNA expression in periodontal tissues
Total RNA was extracted from human or mouse gingival tissue using TRIzol Reagent (Invitrogen) or the PerfectPure RNA cell kit (5 Prime, Fisher) and quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems) and real-time PCR with cDNA was performed using the ABI 7500 Fast System, according to the manufacturer’s protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for detection and quantification of cytokine genes by quantitative real-time PCR were purchased from Applied Biosystems. Data were analyzed using the 2−ΔCT method (2^((−1)*(mean cycle number of target gene - mean cycle number of housekeeping gene)).
Bacteria quantification
(a) Human samples: Bacterial DNA was extracted from human tooth-associated subgingival biofilm (dental plaque) samples and from 5-μm-thick gingival tissue sections using the MasterAmp DNA Extraction Kit (Epicenter Biotechnologies, Madison, WI). Total bacterial load was determined via real-time PCR using 16S universal primers (52). (b) Mouse samples: Genomic DNA isolated from maxillary palatal and buccal gingiva and hard tissue (teeth and immediately surrounding bone) was used to determine total bacterial counts by quantitative real-time PCR of the 16S rRNA gene (19, 53). Bacterial samples from mouse oral cavities were also obtained using sterile fine-tipped cotton swabs held against the gum line for 30 s. Serial dilutions of the swab extracts were plated onto blood agar plates for anaerobic growth and determination of colony-forming units.
Mice and determination of periodontal bone loss
LFA-1KO mice on C57BL/6 background were generously provided by Dr. C.M. Ballantyne (Baylor College of Medicine) (15). CXCR2KO mice on C57BL/6 background and WT C57BL/6 controls were obtained from the Jackson Laboratory. Periodontal bone heights were assessed in defleshed maxillae under a dissecting microscope (objective, x40) fitted with a video image marker measurement system (VIA-170K; Boeckeler Instruments). The distance between the cementoenamel junction and alveolar bone crest (CEJ-ABC distance) was measured at 14 predetermined maxillary sites (54). For calculation of relative bone loss (e.g., LFA-1KO mice versus WT controls), the 14-site total CEJ-ABC distance for each mouse was subtracted from the mean CEJ-ABC distance of control mice. The results are presented in mm; negative values indicate bone loss relative to controls.
Intervention experiments in mice
A neutralizing mAb to IL-17A (rat IgG2a; M210) was generously provided by Amgen, and azide-free rat IgG2a (RTK2758; Biolegend) served as isotype control. Purified polyclonal goat IgG antibody to IL-23p19 subunit and non-immune IgG control were purchased from R&D Systems. The antibodies or their controls were microinjected locally into the murine palatal gingiva (5 μg per site), three times weekly, using a 28.5-gauge MicroFine needle (BD Biosciences). Microinjections were performed on the mesial of the first molar and in the papillae between first and second and third molars on both sides of the maxilla (19).
Statistical analysis
Data were evaluated by one-way analysis of variance (ANOVA) and the Dunnett multiple-comparison test with the InStat program (GraphPad Software). Where appropriate (comparison of two groups only), two-tailed t-tests were performed. P values < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank T. Maekawa for assistance and comments.
Funding: Supported in part by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research (NIDCR) and National Institute of Allergy and Infectious Diseases (NIAID), the Deutsche Forschungsgemeinschaft (CH279/5-1) and the European Research Council (to TC), and U.S. Public Health Service Grants DE015254, DE017138, and DE021685 from the NIH/NIDCR (to GH).
Footnotes
Author contributions: N.M. and G.H. conceived of, designed and supervised the research and wrote the paper; W.C., T.C., and S.M.H. analyzed and interpreted data; G.U. performed clinical studies, J.K. established relevant assays and performed experiments with human and animal specimens, M.S., T.W., N.D., L.A., performed experiments with human specimens and microbial samples, A.E., C.Z., K.B.H. and T.A. performed animal experiments, tissue processing, and host response and microbiota analyses.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: Materials will be made available upon request, subject to a material transfer agreement.
LIST OF SUPPLEMENTARY MATERIALS
Fig. S1. Clinical picture and radiographs of an 11-year-old boy with LAD-I. Fig. S2. Bacterial load in tissue sections from LAD-I periodontitis.
Fig. S3. Tooth-associated bacterial load in LAD-I periodontitis.
Table S1. Genotype and neutrophil characterization in LAD-I patients.
REFERENCES
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G, Chavakis T. Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 2013;34:1–6. doi: 10.1016/j.it.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J. Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugl S, Wirths S, Muller MR, Radsak MP, Kopp HG. Current insights into neutrophil homeostasis. Ann. N. Y. Acad. Sci. 2012;1266:171–178. doi: 10.1111/j.1749-6632.2012.06607.x. [DOI] [PubMed] [Google Scholar]
- 5.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Hart TC, Atkinson JC. Mendelian forms of periodontitis. Periodontol. 2000;2007;45:95–112. doi: 10.1111/j.1600-0757.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 8.Deas DE, Mackey SA, McDonnell HT. Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol. 2003;32:82–104. doi: 10.1046/j.0906-6713.2003.03207.x. 2000. [DOI] [PubMed] [Google Scholar]
- 9.Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J. Clin. Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann. N. Y. Acad. Sci. 2012;1250:50–55. doi: 10.1111/j.1749-6632.2011.06389.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol. Immunol. 2012;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 13.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J. Dent. Res. 2013 doi: 10.1177/0022034513507956. Epub ahead of print, doi: 10.1177/0022034513507956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas C, Le Deist F, Cavazzana-Calvo M, Benkerrou M, Haddad E, Blanche S, Hartmann W, Friedrich W, Fischer A. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood. 1995;86:1629–1635. [PubMed] [Google Scholar]
- 15.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 16.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 17.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 18.Pelchen-Matthews A, Parsons IJ, Marsh M. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J. Exp. Med. 1993;178:1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 21.Zenobia C, Luo XL, Hashim A, Abe T, Jin L, Chang Y, Jin ZC, Sun JX, Hajishengallis G, Curtis MA, Darveau RP. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell. Microbiol. 2013;15:1419–1426. doi: 10.1111/cmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 24.Allam JP, Duan Y, Heinemann F, Winter J, Gotz W, Deschner J, Wenghoefer M, Bieber T, Jepsen S, Novak N. IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J. Clin. Periodontol. 2011;38:879–886. doi: 10.1111/j.1600-051X.2011.01752.x. [DOI] [PubMed] [Google Scholar]
- 25.Dababneh R, Al-Wahadneh AM, Hamadneh S, Khouri A, Bissada NF. Periodontal manifestation of leukocyte adhesion deficiency type I. J. Periodontol. 2008;79:764–768. doi: 10.1902/jop.2008.070323. [DOI] [PubMed] [Google Scholar]
- 26.Sollecito TP, Sullivan KE, Pinto A, Stewart J, Korostoff J. Systemic conditions associated with periodontitis in childhood and adolescence. A review of diagnostic possibilities. Me.d Oral Patol. Oral Cir. Bucal. 2005;10:142–150. [PubMed] [Google Scholar]
- 27.Uzel G, Kleiner DE, Kuhns DB, Holland SM. Dysfunctional LAD-1 neutrophils and colitis. Gastroenterology. 2001;121:958–964. doi: 10.1053/gast.2001.28022. [DOI] [PubMed] [Google Scholar]
- 28.Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J. Dent. Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 29.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect. Immun. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 2000;68:4289–4296. doi: 10.1128/iai.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basit A, Reutershan J, Morris MA, Solga M, Rose CE, Jr., Ley K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L200–207. doi: 10.1152/ajplung.00346.2005. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J. Clin. Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J. Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- 34.Kuchroo VK, Martin CA, Greer JM, Ju ST, Sobel RA, Dorf ME. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J. Immunol. 1993;151:4371–4382. [PubMed] [Google Scholar]
- 35.Hyun YM, Chung HL, McGrath JL, Waugh RE, Kim M. Activated integrin VLA-4 localizes to the lamellipodia and mediates T cell migration on VCAM-1. J. Immunol. 2009;183:359–369. doi: 10.4049/jimmunol.0803388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luscinskas FW, Ding H, Tan P, Cumming D, Tedder TF, Gerritsen ME. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-α-activated vascular endothelium under flow in vitro. J. Immunol. 1996;157:326–335. [PubMed] [Google Scholar]
- 37.Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- 38.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldrop TC, Anderson DC, Hallmon WW, Schmalstieg FC, Jacobs RL. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J. Periodontol. 1987;58:400–416. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- 41.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009;5:549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 43.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alunno A, Bistoni O, Bartoloni E, Caterbi S, Bigerna B, Tabarrini A, Mannucci R, Falini B, Gerli R. IL-17-producing CD4−CD8− T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren’s syndrome. Ann. Rheum. Dis. 2013;72:286–292. doi: 10.1136/annrheumdis-2012-201511. [DOI] [PubMed] [Google Scholar]
- 46.Crispin JC, Tsokos GC. Interleukin-17-producing T cells in lupus. Curr. Opin. Rheumatol. 2010;22:499–503. doi: 10.1097/BOR.0b013e32833c62b0. [DOI] [PubMed] [Google Scholar]
- 47.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 48.Price TH, Ochs HD, Gershoni-Baruch R, Harlan JM, Etzioni A. In vivo neutrophil and lymphocyte function studies in a patient with leukocyte adhesion deficiency type II. Blood. 1994;84:1635–1639. [PubMed] [Google Scholar]
- 49.Uzel G, Tng E, Rosenzweig SD, Hsu AP, Shaw JM, Horwitz ME, Linton GF, Anderson SM, Kirby MR, Oliveira JB, Brown MR, Fleisher TA, Law SK, Holland SM. Reversion mutations in patients with leukocyte adhesion deficiency type-1 (LAD-1) Blood. 2008;111:209–218. doi: 10.1182/blood-2007-04-082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. 2000. [DOI] [PubMed] [Google Scholar]
- 51.Lin X, Han X, Kawai T, Taubman MA. Antibody to receptor activator of NF-κB ligand ameliorates T cell-mediated periodontal bone resorption. Infect. Immun. 2011;79:911–917. doi: 10.1128/IAI.00944-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McIntosh ML, Hajishengallis G. Inhibition of Porphyromonas gingivalis-induced periodontal bone loss by CXCR4 antagonist treatment. Mol Oral Microbiol. 2012;27:449–457. doi: 10.1111/j.2041-1014.2012.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 2000;68:5864–5868. doi: 10.1128/iai.68.10.5864-5868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.