Introduction
An open wound injury triggers a healing process that requires the well-orchestrated integration of complex biological and molecular events, and impairment of this process results in pathological conditions (Falanga, 2005; Martin, 1997; Singer and Clark, 1999). Despite advances in wound care, around 6.5 million patients suffer from pathological conditions and over 72,000 amputations occur each year in the United States alone (Brem et al., 2006). Current medical interventions, such as systemic (e.g. hyperbaric oxygen therapy) or topical (e.g. growth factor; PDGF) therapy, and mechanical devices for wound protection, attain only a 50% recovery, and fail in many cases to prevent chronic wound-related lower limb amputations. The cost of treatment is staggering, at $25 billion annually in the U.S., and the burden is rapidly growing (Sen et al., 2009).
Inflammation is crucial to the normal wound healing process, however, persistent inflammation leads to impaired healing (Barone et al., 1998; Stadelmann et al., 1998; Trengove et al., 2000; Zhou et al., 2000). Several pro-inflammatory factors, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), were found in significantly higher concentrations in human (Tarnuzzer and Schultz, 1996; Trengove et al., 2000) and in murine (Zhou et al., 2000) wound fluid from non-healing leg ulcers compared to healing ulcers. Fibroblasts act as sentinel cells (Cooney et al., 1997) and it is evident that most of the pro-inflammatory factors are transcriptionally regulated by a nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB)-mediated pathway (Kleinert et al., 1996; Xie et al., 1994). Interleukin (IL)-10 is one of the most important anti-inflammatory molecules that acts to inhibit the production of pro-inflammatory cytokines (Wang et al., 1995) through the suppression of NF-κB activation and also promote regenerative healing in a cutaneous wound model (Peranteau et al., 2008). The activation and transloca-tion of NF-κB to the nucleus is followed by transcription of iNOS (Kleinert et al., 1996) and pro-inflammatory cytokines (Baldwin, 1996; Ghosh and Karin, 2002). Previous studies have identified NF-κB transcription factors as key regulators of TNF-α -induced inflammatory gene expression in fibroblasts and other cellular systems (Kleinert et al., 1996; Xie et al., 1994). Thus inhibition of NF-κB activity can be a potential mechanism for regulating inflammatory responses. Studies indicate that IL-10 inhibits NF-κB activation upon TNF-α stimulation in various cell types (Dhingra et al., 2009; Wang et al., 1995).
As stem cells are increasingly recognized for their regener-ative properties in clinical applications, the use of NEHUCB-CD34+ cells would be considered a promising and novel therapeutic approach to overcome the economic and social burden of wound-related treatment. CD133 is a cell surface glycoprotein which is co-expressed with the CD34 antigen on the hematopoietic stem cell population and is believed to be a phenotypically primitive stem cell marker (Miraglia et al., 1997; Potgens et al., 2001; Yin et al., 1997). We previously reported about a stem cell expansion technology, developed in our laboratory, which allowed us to isolate a pure population of CD133+ cells from human umbilical cord blood, and to expand them ex vivo up to 250-fold in serum-free medium on aminated poly-ether sulfone (PES) nanofiber coated plates over a period of 10 days (Das et al., 2009a). Flowcytometric analysis showed that more than 90% of these expanded cells express CD34 where as 23% express CD133 (Das et al., 2009a), leading us to refer to these cells as nanofiber expanded cord blood-derived (NEHUCB-) CD34+ cells. Previously, our labora-tory has shown that NEHUCB-CD34+ cell therapy restores functionality and enhances neo-vascularization more efficient-ly than freshly isolated counterparts in NOD/SCID mice in various ischemic models (Das et al., 2009a,b). Expression of CXCR4, a chemokine receptor on the surface of HSCs and their lineages, helps their preferential migration to the inflammatory or ischemic areas, which express higher levels of the SDF-1 molecule, a ligand for CXCR4 (Aiuti et al., 1997; Jo et al., 2000). NEHUCB-CD34+ cells constitutively express high levels of pro-migratory (CXCR4) and pro-adhesive (LFA-1) surface molecules, which equip them for efficient homing to the challenged area, and higher mobilization in response to the SDF-1 molecule (Das et al., 2009a). Conversely, anti-CXCR4 administration also facilitates mobilization and recruitment of endogenous bone marrow progenitor cells to the wound bed (Fiorina et al., 2010). Although, these stem/progenitor cells play important roles in the improved functionality observed in various preclinical models, their role in limiting inflammatory responses is not well understood. Previous reports indicate that cord blood mesenchymal stem cells possess a variety of immunomodulatory and anti-inflammatory activities (Fiorina et al., 2011; Francese and Fiorina, 2010). To assess the efficacy of NEHUCB-CD34+ cells for treating excisional wounds in NOD/SCID mice and thereby address mechanism, we show herein that NEHUCB-CD34+ cells home to the wound site and significantly accelerate the wound-healing process. Acceler-ated wound closure was associated with re-epithelialization and increased angiogenesis. Additionally, NEHUCB-CD34+ cell-therapy decreased the expression of TNF-α, IL-1β, IL-6 and NOS2A with a concomitant increase in the expression of IL-10 in the wound bed. Moreover, NEHUCB-CD34+ cells attenuated NF-κB activation and nuclear translocation in dermal fibroblasts through enhanced secretion of IL-10, which is known to regulate NF-κB by suppressing its transcriptional activity. Collectively, these data provide novel mechanistic insights into how NEHUCB-CD34+ cells mediate accelerated wound healing.
Materials and methods
CD133+ cell isolation
Fresh human umbilical cord blood was obtained from Wexner Medical Center at The Ohio State University after IRB approval and written consent from donors and processed following an earlier described protocol (Das et al., 2009a). Briefly, after Ficoll separation, CD133+ cells were isolated by using an AutoMACS device (Miltenyi Biotec, Auburn, CA), and the purity of the isolated cells as well as their phenotypes after expansion were determined by flowcytometry.
Ex-vivo CD133+ cell expansion
Freshly isolated CD133+ cells were expanded according to the protocol described previously (Das et al., 2009a). Briefly, eight hundred CD133+ cells were seeded onto each well containing nanofiber mesh (kind gift from Hai-Quan Mao, PhD, Johns Hopkins University, Baltimore, MD) in 600 μl of StemSpan serum-free expansion medium (SFEM, Stem Cell Technologies, Vancouver, BC, Canada), containing essential supplements. Cells were cultured without change of culture medium for 10 days at 37 °C in an atmosphere containing 5% CO2, and harvested after 10 days. Flowcytometric analysis of expanded cells was performed before experiments.
In vitro culture of NEHUCB-CD34+ cells
NEHUCB-CD34+ cells were collected along with media (expansion media) as described earlier (Das et al., 2009a) on day 10. Around 1 × 106 NEHUCB-CD34+ cells were then cultured for 24 h in serum-free DMEM media. After 24 h, NEHUCB-CD34+ cells were collected by centrifugation, and m-RNA was isolated. Cell culture supernatant was stored at −80 °C for enzyme-linked immuno sorbent assay (ELISA) to estimate the levels of IL-10.
Isolation of NEHUCB-CD34 (−) cells
Nanofiber expanded human umbilical cord blood-derived CD34 (−) cells were isolated from the 10-day expanded cells using AutoMACS device and negative selection methods (Miltenyi Biotec, Auburn, CA). Briefly, 10 day expanded cells were collected and incubated with CD34+ magnetic bead conjugated Ab for 30 min, passed through the columns, and the negative fraction was collected from the AutoMACS device. The unbound magnetic beads were removed from the CD34 (−) cells by adhering to the magnet. Cells were washed with 1x PBS and used for subsequent experiments.
Labeling of NEHUCB-CD34+ cells
NEHUCB-CD34+ cells were transfected with GFP containing vector (pmaxGFP) using a human CD34 cell Nucleofector kit (Amaxa Inc.) following the manufacturer’s protocol (Das et al., 2009a). After transfection, cells were cultured overnight with serum-free complete media.
Fibroblast cell culture
A primary human dermal fibroblast cell line was established from skin punch biopsies of a healthy donor. Primary human dermal fibroblast cells (Powell and Boyce, 2007) (a generous gift from Dr. Heather M. Powell, Department of Materials Science and Engineering, Department of Biomedical Engi-neering, The Ohio State University, Columbus, Ohio) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen Corporation, Carlsbad, CA, USA), supplemented with 4% fetal calf serum (FCS) (Sigma-Aldrich, St. Louis, MO, USA), 2 mM glutamine (Invitrogen Corporation), 5 μg/ml insulin (Sigma-Aldrich, St. Louis, MO, USA), 0.5 μg/ml Hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA), 0.1 mM ascorbic acid-2-phosphate (Sigma-Aldrich, St. Louis, MO, USA), 50 U/ml penicillin, and 50 μg/ml streptomycin (Invitrogen Corpora-tion) and grown in 5% CO2 at 37 °C and used from passage 3 to 7.
Co-culture of NEHUCB-CD34+ cells and dermal fibroblasts
Primary human dermal fibroblasts were co-cultured with NEHUCB-CD34+ or NEHUCB-CD34 negative (−) cells in separate six-well plates using fibroblast culture media. For all studies, dermal fibroblasts were seeded at 3 × 105 cells/well in a 6-well plate and maintained in DMEM for 6 h. Fibroblast cells were serum starved for 24 h prior to any experiment. After serum starvation, fibroblast cells were stimulated with 10 ng/ml recombinant human TNF-α (PeproTech, Rocky Hill, NJ, USA) in the presence or absence of 3 × 105 NEHUCB-CD34+ or NEHUCB-CD34 (−) cells. The following 4 different treatment groups were devised: untreated controls, fibroblasts treated with 10 ng/ml recombinant human TNF-α, and fibroblasts treated with 3 × 105 NEHUCB-CD34+ cells with or without 10 ng/ml TNF-α. Similar groups were considered in a separate experiment using NEHUCB-CD34 (−) cells. Then, fibroblasts were cultured for 30, 60 and 90 min under the previously specified culture conditions. Fibroblasts were harvested at each specified time point, and total, cytoplasmic, and nuclear proteins, as well as m-RNA were isolated and analyzed as mentioned. Fibroblasts were harvested from the co-culture wells after removal of NEHUCB-CD34+ cells by thoroughly washing with 1x PBS. In some experiments, media were collected from the co-cultured wells and stored at −80 °C for IL-10 estimation after removal of NEHUCB-CD34+ cells by centrifugation.
Addition of rIL-10 to human dermal fibroblast cell culture
Human dermal fibroblasts were seeded at 5 × 105 cells in 6-cm dishes and cultured in DMEM for 6 h. Twenty-four hours prior to initiating experiments, medium in plates was replaced with serum-free DMEM. After 24 h of serum starvation, fibroblast cells were treated with or without 10 ng/ml human recombi-nant IL-10 (R&D Systems, USA) 5 min before treating with 10 ng/ml recombinant human TNFα (PeproTech, Rocky Hill, NJ, USA). Four different groups were assigned: 1) untreated controls, 2) cells treated with recombinant human IL-10, 3) cells treated with TNFα, and 4) cells treated with IL-10 and TNFα. Fibroblast cells were harvested after 30, 60, or 90 min of TNFα treatment to perform further assays.
Full thickness excision cutaneous wound model
All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of The Ohio State University. NOD/SCID mice were purchased from Jackson laboratory (Bar Harbor, ME) and 8–10 week old male mice were used for this study. Under anesthesia, the mouse dorsum was clipped, hair was removed, and the area was wiped with Betadine solution. A full-thickness 8-mm skin punch biopsy (Acuderm Inc. Fort Lauderdale, FL) was created on the dorsal skin of each mouse. Mice were sacrificed on the 3rd, 5th, 7th and 9th days post-excision. At each time point, samples that included the wound and 2 mm of the surrounding skin were harvested.
Transplantation of labeled NEHUCB-CD34+ cells
After 2 h of cutaneous excision stem cells were injected to the mice through the lateral tail vein. Ten-day nanofiber-expanded cells (0.5 × 106 cells/mouse), or GFP transfected (24 h prior to injection) nanofiber-expanded cells (0.5 × 106 cells/mouse) in 200 μl volume of media were injected into each group (15) of mice, with media alone serving as a control.
Evaluation of wound area
Imaging of wounds was performed on days 0, 3, 5, 7, and 9 days post-wounding, with a digital camera (Sony cyber-shot DSC-H10) from a fixed distance. Wound closure rate was measured by tracing the wound area onto acetate paper on each day as described elsewhere (Marrotte et al., 2010). The tracings were scanned and digitalized, and the areas were calculated using the UTHSCSA (University of Texas Health Science Center at San Antonio) image tool (Version 3.00) and converted to percent wound closure. The investiga-tors measuring samples were blinded. The percentage of wound closure was calculated as follows: (Area of wound on day 0–Area of wound on day of examination) / Area of wound on day 0 × 100).
Immunohistochemistry
At the end of the experiments, mice were sacrificed and skin tissues were harvested and part of the tissue was fixed in formalin-PBS buffer, paraffin-embedded, and wound-edge specimens were sectioned with 4 μm diameter. After de-paraffinization, sections were stained with hematoxylin & eosin (H & E) by standard procedures and examined under light microscopy. For immunofluorescence staining, antigen retrieval was performed using citrate buffer, pH 6.0, and baking for 5 min in a microwave, followed by cooling for 3 min. After non-specific blocking, specific staining was performed by using vWF (Dako, Carpinteria, CA) Ab, followed by incubation with secondary antibody alexa fluor 594-conjugated IgG (Invitrogen, Molecular Probe, Carlsbad, CA). Counterstaining was performed with DAPI (Invitrogen) and slides were imaged by fluorescent microscope (Nikon E800 with MetaMorph version 4.5 software, Universal Imaging Corp.). GFP staining procedures were performed following methods of VECTASTAIN Elite ABC kits (Vector laboratories Inc, Burlingame, CA) after using anti-GFP primary antibody (Zymed, Invitrogen). Immunohistochemical and Immunofluo-rescence data were analyzed using an image analysis software program (ImageJ, NIH).
Immunofluorescence
Nuclear translocation of NF-κB p65 was assessed by immuno-fluorescence using mouse anti- NF-κB p65 IgG (Santa Cruz Biotechnology) as primary antibody followed by incubation with secondary antibody alexa fluor 594-conjugated IgG (Invitrogen, Molecular Probes, Carlsbad, CA). The stained cells were visualized under a Zeiss Axioimage epifluorescence microscope.
Quantitative RT-PCR analysis
Total RNA was extracted from wound-edge tissue harvested on days 3, 5, 7 and 9, and from dermal fibroblasts and NEHUCB-CD34+ cells using TRIzol reagent (Invitrogen) fol-lowing the manufacturer’s protocol. The reverse-transcription of 1 μg of mRNA to cDNA was performed using the ‘High Capacity cDNA Reverse Transcription Kit’ (Applied Biosystems, Foster City, CA). One 20th of the cDNA was used for the real time PCR analysis using appropriate primers. Reactions were performed using SYBR Green PCR master mix (Applied Biosystems) in a Light Cycler 480 (Roche Applied Science) detection system. mRNA expression levels were normalized to β-actin for mouse samples and GAPDH for human samples.
Western blot analysis
Western blot (WB) analysis of various proteins isolated from wound tissue from animals with or without stem cell therapy was performed following the standard procedures. Primary antibodies used were against p65, Lamin B (both from Santa Cruz, CA), Phospho p65 (Ser536), β-actin, and GAPDH (all from Cell Signaling, Beverly, MA). HRP-conjugated anti-mouse, and -rabbit IgG (Cell Signaling, Beverly, MA) as well as anti-goat IgG-HRP conjugated (Santa Cruz, CA) secondary Abs were used and specific bands were detected by enzyme-linked chemiluminescence (Pierce, IL). Densitometric analysis of developed bands was performed using a standard scanner and analyzed with UN-SCAN-IT (gel 6.1 version) software. Relative density was calculated by comparison to their respective GAPDH/β-actin/Lamin B levels.
Enzyme-linked immunosorbent assay (ELISA)
Human IL-10 ELISA assay was performed to analyze the media collected from co-culture (fibroblast and NEHUCB-CD34+ cell) experiments, NEHUCB-CD34+ expansion media from day 10 and from NEHUCB-CD34+ stimulation experiments using an ELISA kit (Quantikine, R&D Systems, USA) and following manufacturer’s protocol. The values were expressed as (pg/ml) for each sample measured by comparison to an IL-10 standard curve.
Statistical analysis
All values were expressed as mean ± SEM. Student’s ‘t’ test was performed for comparison of data of unpaired samples. A probability (p) value b0.05 was considered significant.
Results
Isolation, expansion and labeling of human umbilical cord blood-derived progenitor cells
CD133+ cells were isolated from freshly collected human umbilical cord blood using Auto MACS system, and showed greater than 95% purity. Isolated CD133+ cells, which also co-express CD34 were expanded on nanofiber matrices following earlier mentioned protocols shown to preserve stem cell phenotypes and characteristics (Das et al., 2009a).
After nanofiber expansion, the purity of the CD34 + cell populations was more than 90% as determined by flow cytometry (Das et al., 2009a). Nanofiber-expanded CD34+ cells were transfected using the Amaxa electroporation protocol described earlier (Das et al., 2009a). Transfection efficiency with pmaxGFP vector was more than 90% and cell viability was ~70% (Das et al., 2009b).
NEHUCB-CD34+ cell therapy accelerates wound healing
Evidence supports the potential of adult or fetal stem/ progenitor cells as therapeutic agents for healing cutaneous wounds (Barcelos et al., 2009; Sivan-Loukianova et al., 2003; Suh et al., 2005). However, potential therapeutic efficacies of nanofiber-expanded stem cells have yet to be assessed. Herein, we have evaluated the potential of NEHUCB-CD34+ cells to promote the healing of an excisional cutaneous wound using a NOD/SCID murine model. NOD/SCID mice have been well known for lower graft rejection and are a well-established animal model in which to study the effect of human cell transplantation for various disease states including wound healing. Morphological image analysis revealed that NEHUCB-CD34+ cell therapy significantly improved the rate of wound closure as early as day 3 post-wounding and became more evident on day 7 post-wounding (Fig. 1A). Cumulative image analysis revealed a statistically significant improvement in wound healing on days 3, 5, 7 and 9 after NEHUCB-CD34+ cell therapy compared to the vehicle-treated control animals (Fig. 1B), indicating a potential application of these cells in cutaneous wound treatment.
Figure 1.
NEHUCB-CD34+ cell therapy enhances wound healing in NOD/SCID mice. A. Morphological images of cutaneous wounds (8 mm punch biopsies) in NOD/SCID mice at various time points (days 0, 3, 5, 7 and 9) with or without NEHUCB-CD34+ cell therapy. B. Graphical presentation of cumulative measurement of wounds area (% closure) on days 0, 3, 5, 7 and 9 with or without nanofiber-expanded stem cell therapy. Statistical analysis revealed that significant (p < 0.005 at any time point) enhancement of closure in the animals received NEHUCB-CD34+ cell therapy compared to the control animals without CD34+ cell therapy (on day 3, n = 15; day 5, n = 12; day 7, n = 9; and day 9, n = 6).
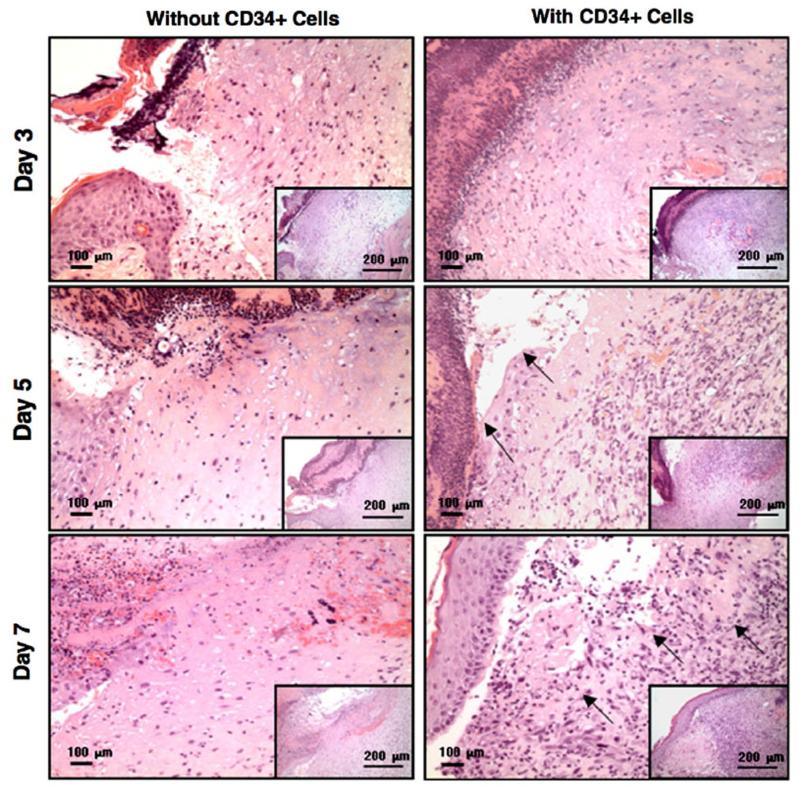
NEHUCB-CD34+ cell therapy enhances re-epithelialization, granulation and vascularization of wounds To investigate the effectiveness of the transplanted NEHUCB-CD34+ cells at the cellular level, we performed histopathological analysis using H & E staining, and immu-nohistochemical analysis of the wound sections using the vascularization specific marker von Willebrand factor (vWF). Histological evaluation revealed that enhanced re-epithelialization had occurred in the wounds of animals treated with NEHUCB-CD34+ cells compared to vehicle treated animals at various time points tested such as days 3, 5 and 7. Higher granularity was also observed in the wounds of animals treated with NEHUCB-CD34+ cells compared to vehicle treated animals on days 5 and 7, suggesting an enhanced cellular regeneration in these animals (Fig. 2). Quantification of vWF positive staining revealed that a significant (p b 0.05) increase in vascularization was visible on days 3, 5 and 7 in the wounds of animals treated with NEHUCB-CD34+ cells compared to the control animals treated with vehicle only (Figs. 3A & B), indicating that neo-vascularization contributes to accelerated wound healing.
Figure 2.
NEHUCB-CD34+ cell therapy enhances re-epithelialization and granulation of wounds. Hematoxylin and eosin staining of wound sections with or without NEHUCB-CD34+ cell therapy at various time points. Arrowheads on day 5, indicate re-epithelialization, on day 7, indicate granulation in the wound. Inset images are of lower magnification views of the same sections.
Figure 3.
Increased vasculogenesis in wound bed after NEHUCB-CD34+ cell therapy. A. Immunofluorescence-based detection of neovascularization in the wound bed using anti-von Willebrand factor (vWF, green) Ab at various time points (days 3, 5 and 7) in animals with or without NEHUCB-CD34+ cell therapy. B. Bar graph shows quantitative values of vascular density of wound tissue at days 3, 5, 7 post-wounding. Values were measured by stained percent area in 10 randomly chosen high power microscopic fields within the sections (n = 3). Data are presented as mean ± SEM.
Homing of NEHUCB-CD34+ cells into the wound bed
We next assessed whether transplanted NEHUCB-CD34+ cells were migrating to the wound bed. To detect homing, human CD34+ cells were transiently transfected with pmaxGFP + vector using the Amaxa electroporation system. We found that more than 90% of cells were GFP positive after 24 h and that cell survivability was more than 70%. Within 24 h of pmaxGFP transfected NEHUCB-CD34 + cells were used for transplanta-tion. Immunohistochemical staining in the wound tissue sections of the animals transplanted with NEHUCB-CD34+ cells using anti-GFP Ab revealed that a substantial number of GFP+ cells were homed to the wound bed as early as 3 h of post-transplantation, and remained as late as 7 days of post-treatment (Fig. 4A). At the initial time point (3 h), GFP+ cells were primarily observed around the blood vessels, whereas GFP+ cells were distributed throughout the dermis area at days 3, 5 and 7 post-wounding (Fig. 4A). Quantification showed that 2238 ± 200 GFP+ cells/mm2 were present at 3 h, while the number of GFP+ cells slightly decreased at 24 h (2129 ± 167 GFP+ cells/mm2 at 6 h; 1815 ± 67 GFP+ cells/mm2 at 24 h) (Fig. 4B). Additionally, the number of GFP+ cells was gradually decreased at later time points, at days 3, 5 and 7 (1074 ± 160 GFP+ cells/mm2 at day3; 630 ± 100 GFP+ cells/mm2 at day5; and 50 ± 10 GFP+ cells/mm2 at day7) (Fig. 4B). These results indicate that a substantial number of NEHUCB-CD34+ cells home to the wound bed and remain there during the wound healing process without further increasing in number.
Figure 4.
NEHUCB-CD34+ cells home to the wound area. A. GFP over-expressing NEHUCB-CD34+ cells were assessed for homing to the wound bed at various time points using α-GFP Ab and immunohistochemical methods. B. Bar graph shows quantification of GFP+ cells in the wound bed at various time points. GFP+ cells were counted in 16 randomly chosen high power microscopic fields within the sections (n = 3). Data are presented as mean ± SEM.
NEHUCB-CD34+ cell therapy reduces expression of pro-inflammatory factors in the wound
A well-regulated and controlled inflammatory response is required for the normal wound healing process. However, sustained inflammation at the wound site results in delayed or inefficient wound closure and develops into pathogenesis (Menke et al., 2007). We therefore first sought to test the gene expression pattern of pro-inflammatory factors such as IL-1β, IL-6, TNF-α, and NOS2A in the wound tissue of mice treated with or without NEHUCB-CD34+ cells during the course of the healing process. Quantitative RT-PCR analysis revealed that there was a significant decrease in the expression of pro-inflammatory factors such as IL-1β, IL-6, TNF-α, and NOS2A in wound tissues of the animals treated with NEHUCB-CD34+ cells on various days (such as days 3, 5 and 7) during the wound healing process compared to wounds of animal treated with vehicle only (Fig. 5). This result indicated that NEHUCB-CD34+ cell therapy mediates a reduction in pro-inflammatory activity in the wound bed.
Figure 5.
Modulation of pro- and anti-inflammatory factors in the wounds after NEHUCB-CD34+ cell therapy. Graphical presentation of real-time quantitative PCR analysis of the expression of pro-inflammatory genes such as IL-1β, IL-6, TNF-α and NOS2A, as well as anti-inflammatory gene IL-10, in wounds of animals with or without NEHUCB-CD34+ cell therapy at various time points (days 3, 5, and 7). The housekeeping gene encoding β-actin was used as an internal reference for normalization. Data expressed as ± SEM (n = 3 in triplicate). The expression level of each target gene at day 3, without stem cell therapy, was considered the base line. This experiment was repeated at least 3 times.
NEHUCB-CD34+ cell therapy increases expression of anti-inflammatory cytokine in wound bed
To explore the possibility that NEHUCB-CD34+ cells mediate anti-inflammatory gene expression in vivo, we investigated levels of IL-10, a well established anti-inflammatory factor, in mouse wound tissues collected in the presence or absence of NEHUCB-CD34+ cell therapy. Quantitative RT-PCR analysis revealed a significant increase in the expression of IL-10 in the wound tissues of the mice treated with NEHUCB-CD34+ cells on various days (such as days 3, 5 and 7) during the wound healing process compared to wound tissues from animals treated with vehicle only (Fig. 5). These results suggest NEHUCB-CD34+ cell treatment may trigger the expression of anti-inflammatory genes in vivo to suppress inflammation in the wound.
NEHUCB-CD34+ cells express IL-10 mRNA and protein in vitro
We next designed a series of in vitro experiments using NEHUCB-CD34+ cells and human primary dermal fibroblastsin the presence or absence of pro-inflammatory stimulus to test the hypothesis that NEHUCB-CD34+ cells are contrib-uting to elevated levels of the anti-inflammatory molecule IL-10 within the wound. Quantitative RT-PCR analysis revealed that mRNA expression of IL-10 was significantly (p b 0.05) higher in CD34+ cells at 1 h or 24 h after TNF-α stimulation compared to the unstimulated NEHUCB-CD34+ cells (Fig. 6A). Importantly, without pro-inflammatory stimulus at 24 h time point, we observed a 3.5-fold increase in expression of IL-10 compared to the 1 h time point (Fig. 6A). This result indicates that NEHUCB-CD34+ cells inherently express the IL-10 gene, and that upon inflamma-tory stimulus, the expression level of IL-10 is significantly increased.
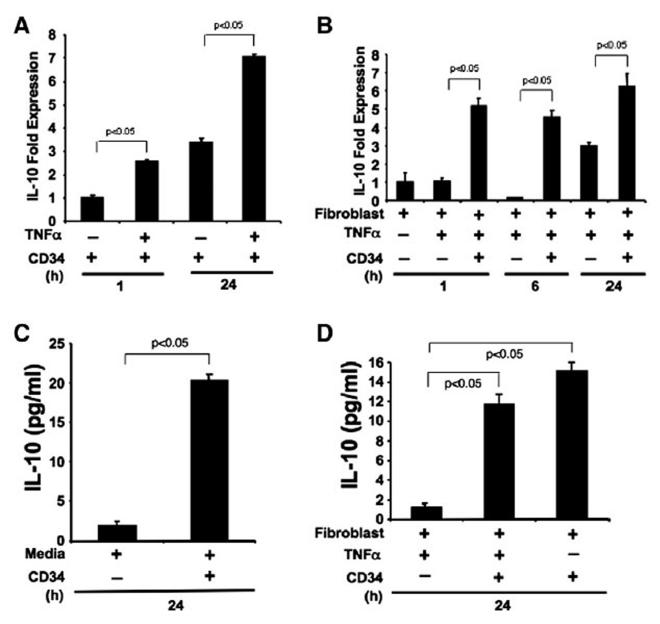
Figure 6.
NEHUCB-CD34+ cells express and secrete IL-10. A. IL-10 expression was analyzed by real-time PCR in NEHUCB-CD34+ cells, which were cultured in a serum free-medium without growth factors for 1 h and 24 h, with or without TNF-α (10 ng/ml) stimulation B. IL-10 expression was analyzed in human primary dermal fibroblast cells with or without TNF-α (10 ng/ml) and in the presence or absence of co-culture with NEHUCB-CD34+ cells (1:1 ratio, NEHUCB-CD34+ cells removed after co-culture) for 1 h, 6 h, or 24 h. C. IL-10 protein levels in the culture supernatant of NEHUCB-CD34+ cells were analyzed by ELISA in serum-free media collected after 24 h E. IL-10 ELISA in the supernatant of human dermal fibroblasts in the presence or absence of TNF-α (10 ng/ml) and NEHUCB-CD34+ cells (1:1 ratio) collected after 24 h.
A further experiment was performed to confirm whether the NEHUCB-CD34+ cells express IL-10 in the presence of dermal fibroblasts and stimulus. Indeed, NEHUCB-CD34+ cells co-cultured with dermal fibroblasts express IL-10 mRNA at significantly (p b 0.05) higher levels after TNF-α stimula-tion compared to fibroblasts stimulated with TNF-α alone at 1 h, 6 h or 24 h time points (Fig. 6B). We observe that fibroblast cells also express IL-10 at the 24 h time point following stimulation with TNF-α. These results indicate that IL-10 expression can originate both from NEHUCB-CD34+ cells and fibroblast cells. To further investigate whether the increased IL-10 expression in NEHUCB-CD34+ cells translates to the protein levels, an IL-10 specific ELISA was performed using cell culture supernatants. ELISA analysis revealed that NEHUCB-CD34+ cells secreted a lower amount of IL-10 (1.91 pg/ml) during the nanofiber-expansion (day 10), however, when cultured in serum-free media (without growth factors) for 24 h, NEHUCB-CD34+ cells secreted a significant amount of IL-10 (20.3 pg/ml) (Fig. 6C). Similarly, CD34+ cells co-cultured with dermal fibroblasts secreted an almost 10-fold higher amount (11.7 pg/ml) of IL-10 protein upon TNF-α stimulation, compared to their counterparts stimu-lated with TNF-α alone (1.2 pg/ml of IL-10). This finding indicates that the significant amount of IL-10 protein present in the media is contributed by NEHUCB-CD34+ cells rather than by the fibroblast cells (Fig. 6E). In addition, NEHUCB-CD34+ cells co-cultured with dermal fibroblast without stimulus also secreted almost 12.5-fold higher (15.13 pg/ml) amount of IL-10 compared to the control (Fig. 6E), indicating that NEHUCB-CD34+ cells have the potential to secrete IL-10 protein in both inflammatory and non-inflammatory environments.
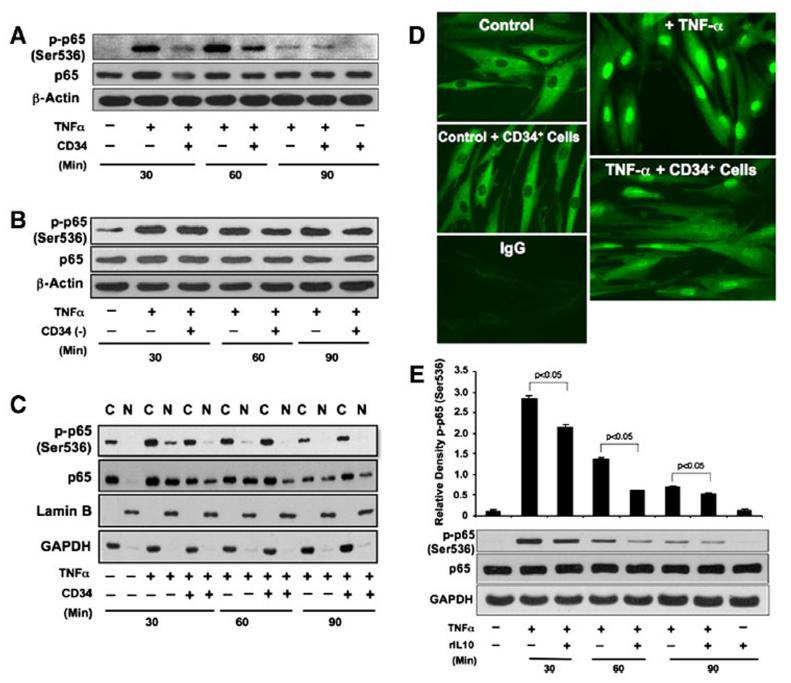
NEHUCB-CD34+ cells suppress TNFα-dependent NF-κB activation in fibroblasts As NF-κB plays a central role in regulating the transcription of most of inflammatory factors (such as IL-1β, IL-6, TNF-α and NOS2A), we next investigated whether NEHUCB-CD34+ cells have any impact on activation of the NF-κB molecule. We assessed the levels of activated and total NF-κB-p65 in dermal fibroblasts by WB assay. NF-κB-p65 phosphoryla-tion at serine 536 is essential for its activation and trans-criptional activity (Jiang et al., 2003; Sakurai et al., 1999, 2003). Therefore, we investigated whether the presence of NEHUCB-CD34+ cells inhibits the phosphorylation of p65 to regulate TNF-α-dependent activation in fibroblast cells. Western blot analysis revealed that stimulation with TNF-α alone resulted in rapid and sustained p65 phosphorylation at serine 536 after 30 and 60 min, and that activation was saturated at 90 min, correlating closely with earlier findings (Sakurai et al., 1999, 2003). On the other hand, the presence of NEHUCB-CD34+ cells strongly inhibited TNFα-induced NF-κB activation in dermal fibroblasts at the 30 and 60 min time points (Fig. 7A). There was no significant change in total p65 levels after addition of NEHUCB-CD34+ cells except at the 30 min time point, where a slight decrease in level was observed. These data suggest that NEHUCB-CD34+ cells inhibit NF-κB-p65 activation in dermal fibroblasts in an inflammatory environment. To confirm the specificity of this effect, a similar experiment was performed using NEHUCB-CD34 (−) cells. We did not observe any suppression of NF-κB activation in dermal fibroblasts co-cultured with NEHUCB-CD34 (−) cells at any time points studied (Fig. 7B).
Figure 7.
NEHUCB-CD34+ cells or IL-10 limit TNF-α-induced NF-κB activation and nuclear translocation. A. Western blot (WB) analysis was performed for p-p65, p65 and β-actin using total protein collected from human primary dermal fibroblast cells cultured in the presence or absence of NEHUCB-CD34+ cells (1:1 ratio, NEHUCB-CD34+ cells removed after co-culture) and with or without TNF-α (10 ng/ml) for 30, 60, or 90 min. B. Similar analysis was performed for p-p65, p65 and β-actin using total protein collected from human primary dermal fibroblast cells cultured in presence or absence of NEHUCB-CD34 negative (−) cells (1:1 ratio and NEHUCB-CD34 (−) cells were removed after co-culture) and with or without TNF-α (10 ng/ml) for 30, 60, or 90 min. C. WB analysis was performed for p-p65, p65, Lamin B (nuclear control) and GAPDH (cytoplasmic control) using nuclear and cytoplasmic fractions collected from human primary dermal fibroblast cells cultured in presence or absence of NEHUCB-CD34+ cells (1:1 ratio and NEHUCB-CD34+ cells were removed after culture) and with or without TNF-α (10 ng/ml) for 30, 60, or 90 min. D. Immunofluorescence staining for p65 (green) was performed in human primary dermal fibroblast cells cultured in the presence or absence of NEHUCB-CD34+ cells (1:1 ratio and NEHUCB-CD34+ cells were removed after culture) and with or without TNF-α (10 ng/ml) for 60 min. E. WB analysis was performed for p-p65, p65 and GAPDH using total protein collected from human primary dermal fibroblast cells cultured alone or in presence or absence of TNF-α (10 ng/ml) and with or without IL-10 (10 ng/ml) for 30, 60, or 90 min. Densitometry analysis of the targeted protein bands were performed and graphically presented relative to GAPDH amount.
NEHUCB-CD34+ cells suppress TNF-α-dependent nuclear translocation of NF-κB
To further investigate whether suppression of NF-κB activa-tion by NEHUCB-CD34+ cells also impaired nuclear transloca-tion of NF-κB signaling, we isolated nuclear and cytoplasmic fraction of proteins from fibroblast cells after co-culture with NEHUCB-CD34+ cells in the presence or absence of stimulus. Nuclear abundance of phosphorylated (Ser 536) and total NF-κB -p65 was assessed using WB and immunofluorescence techniques. WB analysis revealed a higher abundance of phosphorylated p65 and total p65 in the nuclear fraction of TNF-α-induced fibroblast cells at both 30 and 60 min (Fig. 7C; with a 10.2 and 2.4 fold increase in phosphorylated p65 at 30 min and 60 min, respectively, and 4.6- and 4.9 fold increases in total p65 at 30 min and 60 min, respectively, compared to controls without stimulus). As expected, the nuclear fractions of phosphorylated p65 and total p65 were markedly reduced at both 30 and 60 min after co-culture with NEHUCB-CD34+ cells [Fig. 7C; with a decrease of phosphorylated p65 from 10.2-fold to 2.0-fold (30 min), and 2.4 fold to 0.6 fold (60 min) and total p65 4.6-fold to 3.1-fold (30 min), 4.9-fold to 2.2-fold (60 min)]. To confirm this important finding, immunocytochemistry was performed using the above-mentioned protocols. Immunofluorescence analysis of the nuclear translocation of p65 revealed that there were less p65 molecules in the nucleus of fibroblast cells after co-culture with NEHUCB-CD34+ cells in presence of TNF-α (Fig. 7D). These data confirm that NEHUCB-CD34+ cells suppress the nuclear translocation of NF-κB -p65 molecules in fibroblast cells in an inflammatory milieu.
IL-10 suppresses TNFα-dependent NF-κB activation in dermal fibroblast
As IL-10 was the only predominantly immunosuppressive molecules secreted from NEHUCB-CD34+ cells, we wanted to assess whether IL-10 was the molecule, which played a role in the suppression of NF-κB activation and translocation. To verify this hypothesis, we added recombinant IL-10 to dermal fibroblasts in the presence or absence of inflammatory stimulus, and assessed the activation of NF-κB molecule using WB techniques. WB analysis revealed that phosphorylation of p65 at serine 536 was significantly suppressed in the presence of rIL-10 at the 30 and 60 min time points in the presence of TNF-α stimulus (Fig. 7E). These data indicate that IL-10 suppresses NF-κB-p65 activation by inhibiting its phosphoryla-tion at serine 536.
Discussion
Human umbilical cord blood as a whole has been used in the treatment of multiple hematological disorders for more than five decades (Mimeault and Batra, 2006). However, the number of progenitor cells obtained from a single unit of cord blood is not sufficient for routine clinical applications and combining units enhances the possibility of immune rejection (Weissman, 2000). Thus, ex-vivo expansion of progenitor cells has become recognized as a critically important step in translation to the clinic. Our ex vivo expansion technology provides a sufficient number of progenitor cells for preclinical evaluations, and expanded cells have been found to be biologically functional in various ischemic and degenerative models (Aggarwal et al., 2012; Das et al., 2009a,b). However, their potential uses for wound healing and their underlying mechanisms of action have yet to be established.
Herein, we show that NEHUCB-CD34+ cell therapy accel-erates wound closure in NOD/SCID mice, consistent with previous findings (Barcelos et al., 2009; Kim et al., 2010; Sivan-Loukianova et al., 2003). We used immunocompromised NOD/SCID mice for this study, as this mouse model is often used for human cell transplantation due to lower allograft rejection. This unique feature has led to these mice to become a well-established preclinical model for studying human cell therapeutic efficacy for various disease states including cutaneous wounds (Aggarwal et al., 2012; Grunewald et al., 2006; Lu et al., 2013; Shultz et al., 2007). Although the conditions generated in NOD/SCID mice do not recapitulate the human condition exactly, this murine model has become an important preclinical tool to avoid allograft rejection (Monk et al., 2006). Cell-based therapeutic mechanisms proposed in previous wound healing studies have primarily focused on angiogenesis (Barcelos et al., 2009; Sivan-Loukianova et al., 2003). We also find that NEHUCB-CD34+ cells enhance angiogenesis (Fig. 3) to facilitate faster wound closure. This enhanced angiogenesis might be associated with various secretory molecules, as it is evident that stem cells secrete various growth factors such as VEGF, PDGF, and IGF, which trigger angiogenesis (Barcelos et al., 2009; Duran et al., 2013; Gnecchi et al., 2008). In addition, NEHUCBCD34+ cells have the ability to differentiate into endothelial cells which also further facilitate angiogenesis (Das et al., 2009a). Angiogenesis helps in bringing oxygen and nutrients to the wound bed to facilitate the healing process. Thus, NEHUCB-CD34+ cell therapy mediates granular tissue forma-tion and consequent re-epithelialization, consistent with previous studies (Gurtner et al., 2008).
Wound healing is enabled by efficient homing of NEHUCB-CD34+ cells to the wound bed (Figs. 4A, B), which correlates with our previous observations (Aggarwal et al., 2012; Das et al., 2009a). The presence of a substantial number of GFP + CD34+ cells (Fig. 4B) at the wound bed as early as 3 h post-transplantation indicates an efficient homing effect, which is mediated by the constitutive expression of a high level of CXCR4 on NEHUCB-CD34+ cells after nanofiber-expansion (Das et al., 2009a). In addition, the subsequent decrease over time in the number of NEHUCB-CD34+ cells observed in the wound bed indicates that cells do not undergo in situ cell division.
Recruitment of immune cells and initiation of inflammation are important steps in effective wound healing (Eming et al., 2007). However, persistent inflammation actually inhibits the healing process. Higher levels of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α are found in human chronic wounds (Cooney et al., 1997; Elias et al., 1987; Maish et al., 1998). Thus, managing persistent inflammation is critical to achieving faster wound healing. In our current study, we observe that NEHUCB-CD34+ cell therapy decreases expression of various pro-inflammatory cytokines in the wound bed compared to controls, which correlated well with the increased rate of wound closure (Fig. 5). This decline in cytokine levels correlates with the previous report that cord blood-mesenchymal stem cells (CB-MSC) treatment reduced the production of inflammatory cytokines, IL-1β and IL-6 in a lung injury model (Chang et al., 2009). Additionally, CB-MSC therapy has also shown immunological and anti-inflammatory properties in various in vivo models (Fiorina et al., 2011; Francese and Fiorina, 2010). On the other hand, IL-10, one of the best-known and -studied anti-inflammatory cytokines, is required to negatively regulate inflammation. IL-10 targets multiple pro-inflammatory mediators such as TNF-α, IL-6, IL-1α, IL-1β, and chemokines (Lang et al., 2002). Additionally, we have found that NEHUCB-CD34+ cell therapy significantly increases expression of IL-10 in wound tissue, and might contribute to reduced inflammation, which correlated with earlier report where IL-10 overexpression contributed to decreased inflammatory response and promoted regenerative healing in murine wounds (Peranteau et al., 2008). Moreover, our current study provides evidence that NEHUCB-CD34+ cells express and secrete a higher amount of IL-10 (Figs. 6A & C). Higher levels of IL-10 (Figs. 6B & D) are also observed when NEHUCB-CD34+ cells are co-cultured with dermal fibroblasts in the inflammatory milieu. These observations strongly suggest that NEHUCB-CD34+ cell therapy induces IL-10 secretion, which has an inhibitory effect on the expression of multiple pro-inflammatory mediators such as TNF-α, IL-6 and IL-1β (Bogdan et al., 1992; Fiorentino et al., 1991; Wang et al., 1994).
In the context of inflammation, NF-κB (p65) activation plays a central role in the regulation of pro-inflammatory cytokines (Klement et al., 1996). Elevated levels of TNF-α contributed to deficient healing as TNF-α upregulates its own synthesis and induces the production of IL-1β and IL-6 in fibroblast cells (Henry and Garner, 2003; Le et al., 1987; Mast and Schultz, 1996). We show that NEHUCB-CD34+ cell therapy suppresses NF-κB activation (Fig. 7A) and nuclear-translocation (Figs. 7C & D) in dermal fibroblasts, which correlates with the transcrip-tional down-regulation of pro-inflammatory cytokines (Fig. 5) in the wound bed. These observations are consistent with previous findings of NF-κB-dependent regulation of the pro-inflammatory cytokines (Baldwin, 1996; Wang et al., 1995). In addition, rIL-10 significantly suppresses activation of NF-κB (Fig. 7E), which not only correlates well with the previous studies (Dhingra et al., 2009; Smallie et al., 2010), but also suggests that suppression of NF-κB activation by NEHUCB-CD34+ cells (Fig. 7A) is part of the mechanism by which they accelerate wound healing. These observations also correlate well with earlier reports in which cord blood mesenchymal stem cells displayed several in vitro anti-inflammatory capabilities (Fiorina et al., 2011; Francese and Fiorina, 2010).
In summary, herein we show that NEHUCB-CD34+ cell therapy accelerates cutaneous wound closure in NOD/SCID mice, providing further evidence that ex vivo expansion of NEHUCB-CD34+ cells preserves their biological functionality. In addressing the mechanisms by which NEHUCB-CD34+ cells mediate wound healing, we find that NEHUCB-CD34+ cells home to the wound site, enhance angiogenesis, down regulate pro-inflammatory factors such as IL-1β, IL-6 and TNF-α, and upregulate expression of the anti-inflammatory molecule IL-10. NEHUCB-CD34+ cells suppress NF-κB activation and nuclear translocation in dermal fibroblasts. Collectively, these data provide the first mechanistic evidence into the inflam-matory pathway by which NEHUCBCD34+ cells mediate efficient wound healing.
Acknowledgments
This work was part of the PhD dissertation thesis of Suman Kanji. This work was supported in part by the National Institutes of Health grants, K01 AR054114 (NIAMS), SBIR R44 HL092706-01 (NHLBI) and R01 DK098045 (SB), Pelotonia IDEA Award, and The Ohio State University start-up fund. Nanofiber-coated plates were a kind gift from Hai-Quan Mao (John’s Hopkins University, MD). Human primary skin fibrobalst cells were a kind gift from Heather M. Powell, The Ohio State University. The authors are thankful to Drs. Mukesh K. Jain (Case Western Reserve University) and Jack F. Bukowski (Brigham and Women’s Hospital, Harvard Medical School) for their critical reading of the manuscript and thoughtful suggestions.
References
- Aggarwal R, Lu J, Kanji S, Joseph M, Das M, Noble GJ, et al. Human umbilical cord blood-derived CD34+ cells reverse osteoporosis in NOD/SCID mice by altering osteoblastic and osteoclastic activities. PLoS One. 2012;7(6):e39365. doi: 10.1371/journal.pone.0039365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 1997;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ. Res. 2009;104(9):1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone EJ, Yager DR, Pozez AL, Olutoye OO, Crossland MC, Diegelmann RF, Cohen IK. Interleukin-1alpha and collagenase activity are elevated in chronic wounds. Plast. Reconstr. Surg. 1998;102(4):1023–1027. (discussion 1028–1029) [PubMed] [Google Scholar]
- Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J. Biol. Chem. 1992;267(32):23301–23308. [PubMed] [Google Scholar]
- Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast. Reconstr. Surg. 2006;117(7 Suppl):193S–209S. doi: 10.1097/01.prs.0000225459.93750.29. (discussion 210S-211S) [DOI] [PubMed] [Google Scholar]
- Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18(8):869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- Cooney R, Iocono J, Maish G, Smith JS, Ehrlich P. Tumor necrosis factor mediates impaired wound healing in chronic abdominal sepsis. J. Trauma. 1997;42(3):415–420. doi: 10.1097/00005373-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Das H, Abdulhameed N, Joseph M, Sakthivel R, Mao HQ, Pompili VJ. Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell Transplant. 2009a;18(3):305–318. doi: 10.3727/096368909788534870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das H, George JC, Joseph M, Das M, Abdulhameed N, Blitz A, et al. Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model. PLoS One. 2009b;4(10):e7325. doi: 10.1371/journal.pone.0007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK. IL-10 attenuates TNF-alpha-induced NF kappaB pathway activation and cardiomyocyte apoptosis. Cardiovasc. Res. 2009;82(1):59–66. doi: 10.1093/cvr/cvp040. [DOI] [PubMed] [Google Scholar]
- Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ. Res. 2013;113(5):539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JA, Gustilo K, Baeder W, Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J. Immunol. 1987;138(11):3812–3816. [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147(11):3815–3822. [PubMed] [Google Scholar]
- Fiorina P, Pietramaggiori G, Scherer SS, Jurewicz M, Mathews JC, Vergani A, et al. The mobilization and effect of endogenous bone marrow progenitor cells in diabetic wound healing. Cell Transplant. 2010;19(11):1369–1381. doi: 10.3727/096368910X514288. [DOI] [PubMed] [Google Scholar]
- Fiorina P, Voltarelli J, Zavazava N. Immunological applica-tions of stem cells in type 1 diabetes. Endocr. Rev. 2011;32(6):725–754. doi: 10.1210/er.2011-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francese R, Fiorina P. Immunological and regenerative properties of cord blood stem cells. Clin. Immunol. 2010;136(3):309–322. doi: 10.1016/j.clim.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Henry G, Garner WL. Inflammatory mediators in wound healing. Surg. Clin. North Am. 2003;83(3):483–507. doi: 10.1016/S0039-6109(02)00200-1. [DOI] [PubMed] [Google Scholar]
- Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T. The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 2003;278(2):919–926. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J. Clin. Invest. 2000;105(1):101–111. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Song SH, Kim KL, Ko JJ, Im JE, Yie SW, Ahn YK, Kim DK, Suh W. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19(12):1635–1644. doi: 10.3727/096368910X516637. [DOI] [PubMed] [Google Scholar]
- Kleinert H, Euchenhofer C, Ihrig-Biedert I, Forstermann U. In murine 3T3 fibroblasts, different second messenger pathways resulting in the induction of NO synthase II (iNOS) converge in the activation of transcription factor NF-kappaB. J. Biol. Chem. 1996;271(11):6039–6044. doi: 10.1074/jbc.271.11.6039. [DOI] [PubMed] [Google Scholar]
- Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, Chen CH, Rosen CA, Stewart CL. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Mol. Cell. Biol. 1996;16(5):2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 2002;169(5):2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- Le JM, Weinstein D, Gubler U, Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J. Immunol. 1987;138(7):2137–2142. [PubMed] [Google Scholar]
- Lu J, Kanji S, Aggarwal R, Das M, Joseph M, Wu LC, Mao HQ, Pompili VJ, Hadjiconstantinou M, Das H. Hematopoietic stem cells improve dopaminergic neuron in the MPTP-mice. Front. Biosci. 2013;18(970-981) doi: 10.2741/4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maish GO, III, Shumate ML, Ehrlich HP, Cooney RN. Tumor necrosis factor binding protein improves incisional wound healing in sepsis. J. Surg. Res. 1998;78(2):108–117. doi: 10.1006/jsre.1998.5315. [DOI] [PubMed] [Google Scholar]
- Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J. Clin. Invest. 2010;120(12):4207–4219. doi: 10.1172/JCI36858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing—aiming for perfect skin regener-ation. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4(4):411–420. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin. Dermatol. 2007;25(1):19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24(11):2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90(12):5013–5021. [PubMed] [Google Scholar]
- Monk NJ, Hargreaves RE, Simpson E, Dyson JP, Jurcevic S. Transplant tolerance: models, concepts and facts. J. Mol. Med. (Berl) 2006;84(4):295–304. doi: 10.1007/s00109-005-0006-4. [DOI] [PubMed] [Google Scholar]
- Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, Liechty KW. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J. Invest. Dermatol. 2008;128(7):1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- Potgens AJ, Gaus G, Frank HG, Kaufmann P. Character-ization of trophoblast cell isolations by a modified flow cytometry assay. Placenta. 2001;22(2–3):251–255. doi: 10.1053/plac.2000.0597. [DOI] [PubMed] [Google Scholar]
- Powell HM, Boyce ST. Wound closure with EDC cross-linked cultured skin substitutes grafted to athymic mice. Biomaterials. 2007;28(6):1084–1092. doi: 10.1016/j.biomaterials.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 1999;274(43):30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 2003;278(38):36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7(2):118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N. Engl. J. Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vasculariza-tion and healing of diabetic mouse skin wounds. J. Vasc. Res. 2003;40(4):368–377. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- Smallie T, Ricchetti G, Horwood NJ, Feldmann M, Clark AR, Williams LM. IL-10 inhibits transcription elongation of the human TNF gene in primary macrophages. J. Exp. Med. 2010;207(10):2081–2088. doi: 10.1084/jem.20100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998;176(2A Suppl):26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/ macrophages and neovascularization. Stem Cells. 2005;23(10):1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4(3):321–325. doi: 10.1046/j.1524-475X.1996.40307.x. [DOI] [PubMed] [Google Scholar]
- Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mito-genic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8(1):13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J. Immunol. 1994;153(2):811–816. [PubMed] [Google Scholar]
- Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Inter-leukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 1995;270(16):9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287(5457):1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269(7):4705–4708. [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- Zhou LJ, Matsui R, Ono I. Development of a chronic skin defect model and a study of cytokine secretion using the model. Wound Repair Regen. 2000;8(4):304–318. doi: 10.1046/j.1524-475x.2000.00304.x. [DOI] [PubMed] [Google Scholar]