Abstract
Relapse after achieving a prior response remains one of the most important obstacles to improving the outcome of patients with acute myeloid leukemia (AML). Although overall, the majority of patients with disease relapse do poorly, this is by no means uniform and a number of predictors of outcome have been identified. Previously, most trials of investigational agents in the setting of disease relapse in AML have accrued a wide range of patients with widely different patient and disease characteristics. With increased understanding of the biology of the neoplastic change in AML, and better identification of disease subsets based on their molecular characterization, target-specific novel agents are being developed that will hopefully lead to better strategies, not only for treating relapsed disease, but also for the initial induction treatment.
Keywords: Relapse, Acute myeloid leukemia, biology, targeted therapy
Introduction
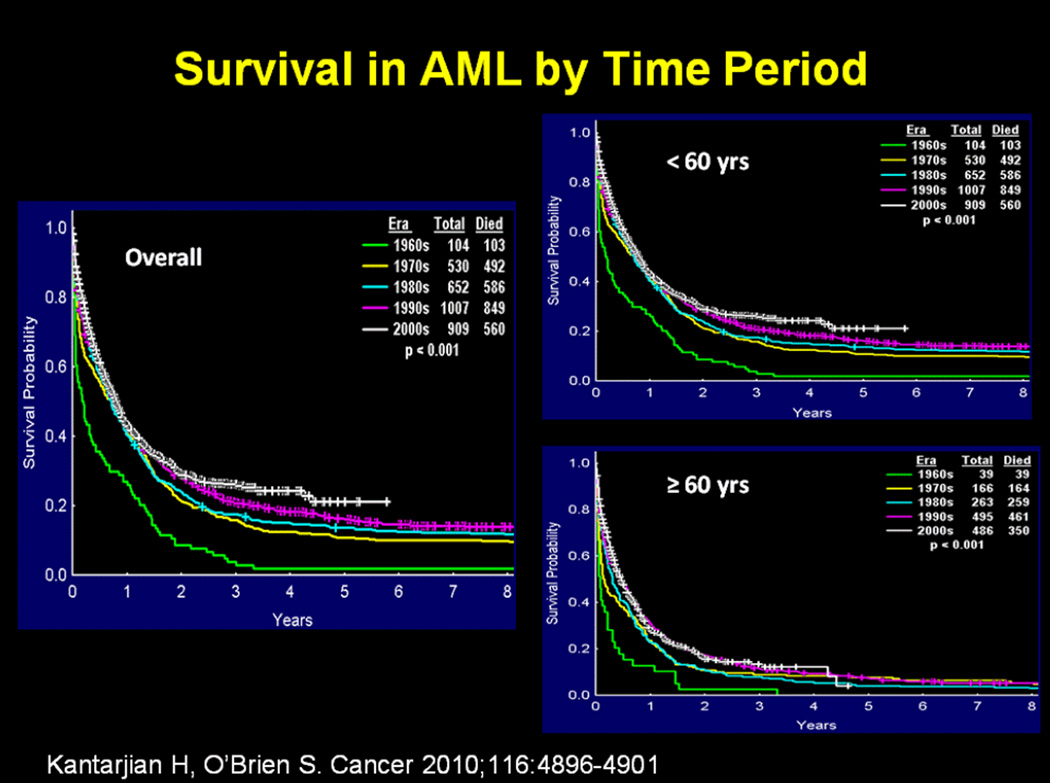
The treatment of adult patients with acute myeloid leukemia (AML) has not changed significantly over the past several decades, with cytarabine and anthracyclines remaining the most important agents used in the induction regimens M [1]. The modest improvement in the survival of patients over the past several decades can be attributed, at least in part, to improvements in supportive care, including better blood product support and better control of infections, as well as higher success in treating patients with better risk disease, such as those with core binding factor leukemias and acute promyelocytic leukemia [2]. This progress has been limited particularly in older patients who constitute the majority of the cases (Figure 1) [2].
Figure 1. Survival in AML by time period.
From: Kantarjian H, O’Brien S. Questions regarding frontline therapy of acute myeloid leukemia. Cancer 2010;116:4896–4901. Reprinted with permission of John Wiley and Sons. Copyright © 2010 American Cancer Society.
Relapse continues to remain a major obstacle to achieving cure after successful initial therapy of patients with AML. Rowe et al reported that among 362 patients over the age of 55 years who achieved a complete remission (CR) after the initial induction therapy in various frontline studies conducted by the Eastern Cooperative Oncology Group (ECOG), 237 (65%) relapsed and had a median survival of 4.7 months with only 6% of patients remaining alive at 5 years [3]. In the same report, the outcome of patients treated on the study E3489 who relapsed was better for patients who did undergo an allogeneic stem cell transplant in second CR compared to those who received continued chemotherapy alone (5-year survival 18% vs 0%, respectively).
Outcome predictors
A number of predictors of outcome in first relapse have been described. Keating et al demonstrated clearly that age per se is an important predictor of response to first salvage regimen [4]. Advancing age decreases the proportion of patients achieving CR and increases resistant disease [4]. Estey et al demonstrated that the duration of the first remission was an important predictor of survival in first relapse [5]. Patients whose first CR duration was less than 1 year had similar outcome whether they received high-dose cytarabine-based regimens or investigational agents on phase 1 trials, whereas those with a first CR duration longer than a year had significantly better outcomes when treated with cytarabine-based regimens [6].
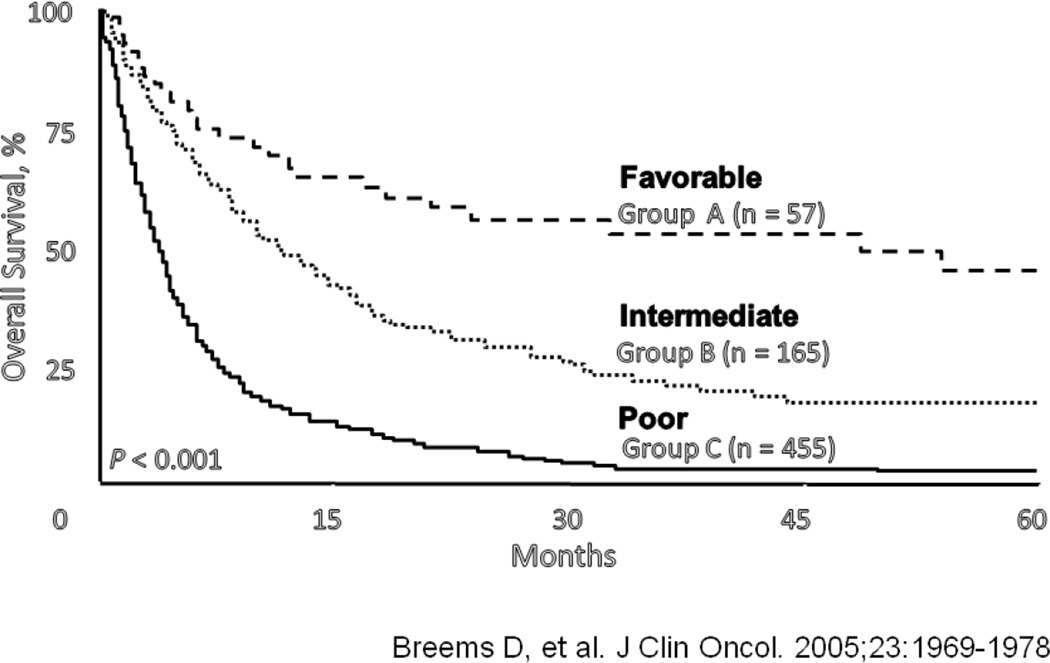
Breems et al evaluated the outcomes of 1540 patients treated on the HOVON/SAKK trials between 1987 and 2001. Six hundred sixty-seven (60%) of 1108 patients who had achieved CR and were alive, relapsed [7]. Using multivariate analysis, they identified four important prognostic indicators for survival, including cytogenetics at initial diagnosis, age at relapse, duration of first CR (CR1), and whether the patients had undergone an allogeneic stem cell transplant in CR1 before relapsing [7]. Using these factors, they were able to divide the population into 3 groups with favorable, intermediate, and unfavorable outcomes (Figure 2). Importantly, only 57 patients were in the favorable group with 5-year survival of 40%, and 455 patients were in the unfavorable group with 5-year survival of < 5% [7]. Similar prognostic factors have been reported by others. Kurosawa and colleagues reported that, on multivariate analysis, important predictors of outcome for Japanese patients with AML in first relapse included age, duration of first remission, number of courses of chemotherapy to achieve the first remission, and whether they achieved a second CR and underwent an allogeneic stem cell transplant as a part of their salvage therapy [8].
Figure 2.
Prognostic indicators for survival in AML in first relapse (Increasing age, short duration of first CR, adverse cytogenetics, prior hematopoietic stem cell transplant).
From [7]. Reprinted with permission. © 2005 American Society of Clinical Oncology. All rights reserved.
Length of first remission
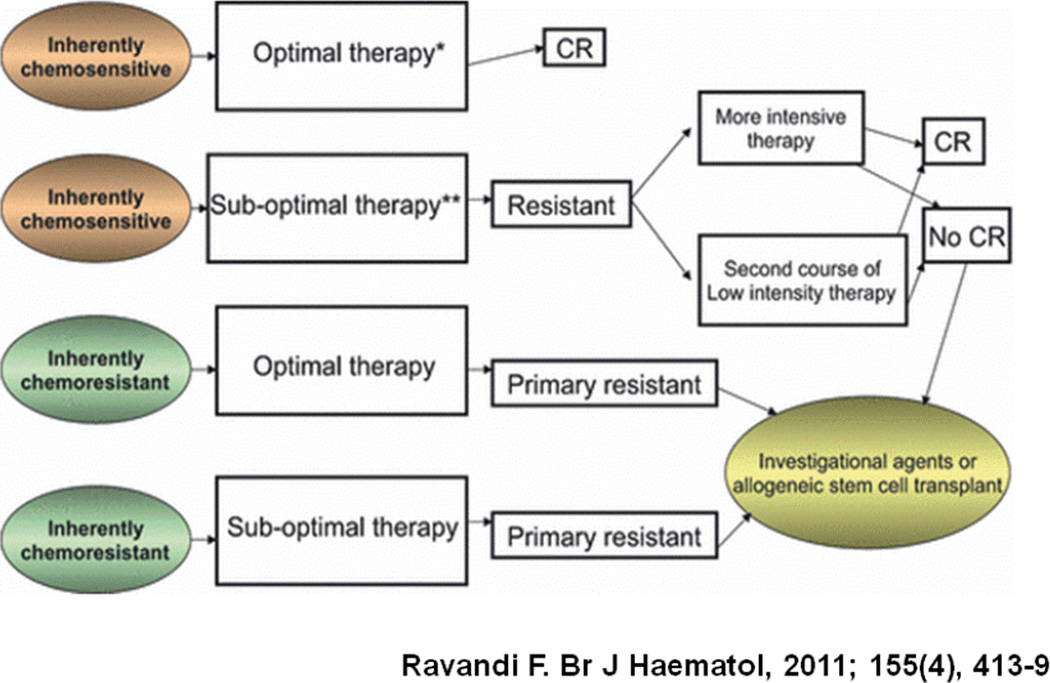
The length of the first remission is an important indicator of the sensitivity of the patients’ leukemic blasts to the induction chemotherapy, typically a combination of cytarabine and an anthracycline. The longer the first CR, the more sensitive the leukemic blasts to the chemotherapy used and the greater likelihood that the same or similar regimen will be effective [9]. At the extreme, there is primary refractory AML where the duration of the first CR is essentially zero. However, this is with the assumption that all patients receive the same treatment regimens. Clearly, responsiveness to therapy is highly dependent upon the form and intensity of therapy (Figure 3) [10]. For example, we previously reported that among 285 patients refractory to one cycle of high-dose ara-C-based induction, only 22% achieved a response with the salvage regimen, and only 7% were alive and in CR for at least 6 months [11].
Figure 3. Primary refractory AML by treatment and disease biology.
From [10]. Reprinted with permission of John Wiley and Sons. Copyright © 2011 Blackwell Publishing Ltd.
On the other hand, Rowe and colleagues reported that among 1980 patients treated on 6 consecutive studies conducted by the Eastern Cooperative Oncology Group (ECOG) between 1983 and 1993, 1272 (64%) achieved CR, including 74% who achieved CR after one cycle of standard-dose ara-C induction and 26% who achieved CR after 2 cycles [12]. The long-term outcome was similar between the patients who required 1 or 2 courses of chemotherapy to achieve CR [12]. Therefore, it appears that a patient failing high-dose ara-C induction is likely to have a worse outcome than a patient failing standard dose ara-C regimens who is more likely to be salvaged by a high-dose cytarabine-based salvage strategy [10]. These are important points to consider when designing clinical trials for salvage regimens in both younger and older patients, as clearly the response to the salvage regimen can be at least partly dependent on the actual treatment received during induction as well as the duration of first CR.
Salvage regimens
Clearly, the dose of ara-C used in salvage and the agents that are utilized as components of the regimen are of potential relevance. In a study by Kern and colleagues, patients with AML with relapsed or refractory disease were randomized to receive one of two regimens combining ara-C with mitoxantrone [13]. Younger patients (<60 years) received mitoxantrone with either ara-C given as a 3-hour infusion on days 1, 2, 8, and 9 at 3 g/m2 or 1 g/m2 per dose. Older patients (≥60 years) received the same regimen but with ara-C at 1 g/m2 or 0.5 g/m2 per dose [13]. In the younger patients, the higher dose level resulted in a significant reduction of resistant cases (12% vs 31%; P = 0.01) but also a higher rate of early death (32% vs 17%), thus leading to only a marginally higher CR rate (52% vs 45%).
Within the subgroup of patients with refractory AML, the tendency towards a higher CR rate after high dose ara-C was more pronounced (46% vs 26%; P = 0.045). In the older patients, corresponding differences were observed with a higher rate of resistance in the lower dose group (26% vs 16%) and early death occurring more frequently with the higher doses (36% vs. 26%). The authors concluded that high-dose ara-C has a significantly higher antileukemic efficacy, but this is not associated with an increase in CR rate due to a higher incidence of toxicity and early death, predominantly from infections [13]. Improvements in supportive care and therapy of infections over the past several years may mean that the ability to administer high-dose ara-C-based regimens has increased, thereby the likelihood of reaping the benefits is higher. The counter argument is based on elegant studies by Plunkett and colleagues who demonstrated the saturation of intracellular cytarabine triphosphate levels with increasing doses of ara-C, thereby providing the rationale for limiting the dose of ara-C to intermediate doses in the treatment of AML patients [14].
Experience with chemotherapy over the last several decades has taught us that, rather simplistically, AML can be divided into two major subgroups. There are those subtypes of AML sensitive to the actions of the conventional chemotherapeutic agents, in particular ara-C and anthracyclines. Clearly, dose escalation, when tolerated, will benefit the patients with these diseases, such as the use of repetitive courses of high-dose ara-C in consolidating core binding factor leukemias [15] and the advantage for a higher dose of daunorubicin in younger patients with AML without adverse features [16]. And the other subtype is the group of AML patients in whom traditional chemotherapeutic agents are significantly less active, such as those with adverse cytogenetics, or with mutated FLT3, or therapy-related AML, who generally are less likely to benefit from such dose intensification strategies. For this group, novel therapeutic strategies based on understanding the biology of the disease are likely to be the only way progress can be achieved.
The question then arises as to why none of the salvage regimens investigated over the last several decades has become established as the standard of care in adult patients with AML in first relapse. A number of randomized trials have investigated the superiority of one regimen over another, but unfortunately, in none of these studies has a clear advantage been demonstrated for one regimen over the others (Table 1). A number of potential explanations include the heterogeneity of the initial therapy, presence of comorbid factors complicating the response to therapy, and probably most importantly, heterogeneity of the disease biology in different patients receiving such combination cytotoxic regimens. For example, in one such trial conducted recently, 320 older patients with relapsed AML were randomized to receive ara-C alone or ara-C plus clofarabine. The initial induction therapy was not specified, but at least 3 months had to have elapsed from the last exposure to intermediate and high dose ara-C. Although the combination therapy was statistically superior to ara-C alone both for overall response rate and eventfree survival (EFS) (P< 0.01 for both), the overall survival (OS) was not different (median OS, 6.6 vs. 6.0 months, P=1.00) [17]. The induction mortality was higher for the combination arm (16%) compared to the ara-C arm (5%) [17]. An ongoing large, multicenter randomized trial is currently evaluating whether the addition of the novel, non-cardiotoxic topo-isomerase II inhibitor vosaroxin to intermediate dose ara-C can lead to an improved survival in patients with first relapsed or refractory AML. Design advantages of this trial may include the specific requirements for the doses of ara-C and daunorubicin during induction and the statistical design allowing expansion of the trial after the Data Safety Monitoring Board excludes futility and excessive toxicity attributable to either arms.
Table 1.
Selected randomized trials in relapsed/refractory AML.
| Treatment | N | 2nd CR, % | Median duration 2nd CR, mo |
Median OS, mo |
|---|---|---|---|---|
| HDAra-C vs HDAra-C +Mit | 162 | 32 vs 44 | 9 vs 5 | 8 vs 6 |
| EMA vs EMA + GM-CSF | 72 | 81 vs 89 | 4 vs 5 | 9 vs 10 |
| MAE vs MAE + G-CSF | 129 | 25 vs 17 | 9 vs 10 | 5 vs 4 |
| MEC vs MEC + lintuzumab | 191 | 23 vs 29 | NA | 8 vs 6 |
| HDAra-C vs HDAra-C + laromustine | 178 | 19 vs 35 | 332 vs 275 d | 177 vs 128 d |
| HDAra-C + Mit vs IDAC + Mit | 186 | 52 vs 45 | 5.3 vs 3.3 | 5 vs NA |
| HDAra-C + Amsa vs HDAra-C + Mit | 52 | 53 vs 60 | 11 vs 12 | 8 vs 11 |
| HDAra-C vs HDAra-C + Amsa | 36 | 14 vs 53 | NA | 2 vs 6 |
| HDAra-C vs HDAra-C + Eto | 131 | 40 vs 45 | 12 vs 25 | 5 vs 5 |
Karanes, et al. Leuk Res. 1999;23:787–794; Thomas, et al. Leukemia. 1999; 13: 1214–1220; Greenberg, et al. J Clin Oncol. 2004;22: 1078–1086; Feldman, et al. J Clin Oncol. 2005;23:4110–4116; Giles, et al. Blood (ASH Annual Meeting Abstracts). 2006; 108. Abstract 1970; Kern, et al. Leukemia. 2000; 14:226–231; Martiat, et al. Eur J Haematol. 1990;45: 164–167; Larson, et al. Br J Haematol. 1992;82:337–346; Vogler, et al. Leukemia. 1994;8: 1847–1853; Ohno, et al. Blood. 1994;83:2086–2092
Alternatively, future trials of salvage therapy in AML are likely to be based on the description of the biology of the disease, identification of target-specific drugs, and enriching the study group for the specific target. Marked progress in understanding the biology of AML has led to the identification of a number of recurring gene mutations in patients with the disease and these molecular aberrations are being used increasingly to predict outcome after initial therapy with the standard chemotherapy regimens [18–20]. Furthermore, such molecular events are frequently recognized as targets for development of specific therapeutic agents.
Molecular targets
One such target is the transmembrane protein product of the gene FMS-like tyrosine kinase 3 (FLT3) [21]. Internal tandem duplication mutations involving the juxtmembrane domain of the kinase have been described in over 25% of patients with AML and are associated with a shortened relapse-free and overall survival [22]. Their presence has also been associated with adverse outcome at first relapse [23,24]. In the study reported by Chevallier et al, presence of FLT3-ITD mutations, relapse less than 1 year after achieving remission (including primary refractory patients), as well as adverse cytogenetics were the strongest independent adverse prognostic factors for OS and EFS in a group of 138 patients with relapsed/refractory AML (median age, 55 years; range, 19–70) [24]. They then divided the patients into 3 subgroups with distinctly different survival depending on the presence of 0, 1, or ≥2 of these adverse features [24].
A number of inhibitors of FLT3 kinase activity are in clinical development, including lestaurtinib, midostaurin, sorafenib, and quizartinib [25]. Lestaurtinib was evaluated in a randomized trial where patients with first relapse of FLT3-mutataed AML were treated with chemotherapy with or without the kinase inhibitor [26]. Unfortunately, there was no significant difference in the response rate or survival for the two arms of the study [26]. However, the authors were able to show that 46 of 79 (58%) of patients achieved target FLT3 inhibition on day 15 and this was associated with a higher response rate (including CR and CRi) and a longer overall survival [26]. These data may suggest that the use of a more potent kinase inhibitor or combinations of FLT3 inhibitors with other agents may be associated with further improvement in outcomes.
Quizartinib (AC220) is a potent, orally administered, second generation, class III tyrosine kinase inhibitor with a distinct inhibition profile against mutant FLT3, PDGFRA, and KIT isoforms [27]. In a phase 2 study in patients with relapsed AML, two cohorts of patients were treated with quizartinib at 135 mg per day for men and at 90 mg per day for women [28]. The first cohort included patients with AML ≥ 60 years, in first relapse, or with primary refractory disease. A composite response rate of 46% including CR, CRi, and CR without platelet recovery (CRp) was reported in patients with FLT3-ITD. In the second cohort of patients older than 18 years, relapsed after or refractory to second-line therapy or hematopoietic stem cell transplant, a composite response rate of 53% was seen in FLT3-ITD positive patients. These responses were durable with a median overall survival of more than 6 months. Clearly, these results demonstrate that when a specific target is identified and a specific potent inhibitor is used, durable responses are possible even in the salvage setting.
With further understanding of the mechanism of action of FLT3 kinase inhibitors and the potential mechanisms of resistance, it may be possible to improve these outcomes further. One of the potential mechanisms of resistance to FLT3 kinase inhibitors is autocrine and/or paracrine stimulation of the receptor by FLT3 ligand (FL) released as a result of myelosuppressive chemotherapy [29,30]. Elevated levels of FL have been previously shown to interfere with the action of lestaurtinib [26]. Therefore, it may be possible to combine FLT3 kinase inhibitors with less myelosuppressive agents such as 5-azacytidine without jeopardizing their activity. We have recently reported the results of a phase 2 study combining sorafenib with 5-azacytidine in patients with FLT3-ITD mutated AML [31]. Among 37 patients with FLT3-ITD positive AML, mostly with relapsed or refractory disease, an overall response rate of 46% was noted with some of the responses being very durable [31].
Conclusion
In conclusion, results of salvage therapy in patients with AML are likely to improve only when specific subsets with well-defined molecular/cellular pathways are identified and strategies to overcome these aberrant processes are devised. Recent description of the genome of 200 patients with AML and identification of at least 1 nonsynonymous mutation in 1 of 9 categories of genes likely relevant to the pathogenesis of the disease, in most samples tested, suggests that we may be closer to the identification of specific subsets based on the constellations of aberrant genetic and epigenetic machinery [18]. The experience in acute promyelocytic leukemia has taught us that when a drug is highly effective and able to produce molecular remissions in the relapse setting, this can likely be translated to the frontline, thereby hopefully reducing the proportion patients requiring salvage therapy [32].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
Farhad Ravandi, MD
Consulting fees: Sunesis; Novartis; Teva; Contracted research: Celgene, Bayer; Onyx; BMS.
References
- 1.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, O'Brien S, Cortes J, Wierda W, Faderl S, Garcia-Manero G, et al. Therapeutic advances in leukemia and myelodysplastic syndrome over the past 40 years. Cancer. 2008;113:1933–1952. doi: 10.1002/cncr.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe JM, Li X, Cassileth PA, Appelbaum FR, Schiffer CA, Wiernik PH, et al. Very poor survival of patients with AML who relapse after achieving a first complete remission: The Eastern Cooperative Oncology Group Experience. Blood. 2005;106:162a. (abstr 546) [Google Scholar]
- 4.Keating MJ, Kantarjian H, Smith TL, Estey E, Walters R, Andersson B, et al. Response to salvage therapy and survival after relapse in acute myelogenous leukemia. J Clin Oncol. 1989;7:1071–1080. doi: 10.1200/JCO.1989.7.8.1071. [DOI] [PubMed] [Google Scholar]
- 5.Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M. A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood. 1996;88:756. [PubMed] [Google Scholar]
- 6.Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14:476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 7.Breems DA, van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, Sakura T, Kanamori H, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;95:1857–1864. doi: 10.3324/haematol.2010.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estey E. Treatment of refractory AML. Leukemia. 1996;10:932–936. [PubMed] [Google Scholar]
- 10.Ravandi F. Primary refractory acute myeloid leukaemia - in search of better definitions and therapies. Br J Haematol. 2011;155:413–419. doi: 10.1111/j.1365-2141.2011.08869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravandi F, Cortes J, Faderl S, O'Brien S, Garcia-Manero G, Verstovsek S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010;116:5818–5823. doi: 10.1182/blood-2010-07-296392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe JM, Kim HT, Cassileth PA, Lazarus HM, Litzow MR, Wiernik PH, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116:5012–5021. doi: 10.1002/cncr.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern W, Aul C, Maschmeyer G, Schonrock-Nabulsi R, Ludwig WD, Bartholomaus A, et al. Superiority of high-dose over intermediate-dose cytosine arabinoside in the treatment of patients with high-risk acute myeloid leukemia: results of an age-adjusted prospective randomized comparison. Leukemia. 1998;12:1049–1055. doi: 10.1038/sj.leu.2401066. [DOI] [PubMed] [Google Scholar]
- 14.Plunkett W, Liliemark JO, Estey E, Keating MJ. Saturation of ara-CTP accumulation during high-dose ara-C therapy: pharmacologic rationale for intermediate-dose ara-C. Semin Oncol. 1987;14:159–166. [PubMed] [Google Scholar]
- 15.Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179. [PubMed] [Google Scholar]
- 16.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30:2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrozek K, Bloomfield CD. Chromosome aberrations, gene mutations and expression changes, and prognosis in adult acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2006:169–177. doi: 10.1182/asheducation-2006.1.169. [DOI] [PubMed] [Google Scholar]
- 21.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 22.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 23.Ravandi F, Kantarjian H, Faderl S, Garcia-Manero G, O'Brien S, Koller C, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res. 2010;34:752–756. doi: 10.1016/j.leukres.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chevallier P, Labopin M, Turlure P, Prebet T, Pigneux A, Hunault M, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25:939–944. doi: 10.1038/leu.2011.25. [DOI] [PubMed] [Google Scholar]
- 25.Prescott H, Kantarjian H, Cortes J, Ravandi F. Emerging FMS-like tyrosine kinase 3 inhibitors for the treatment of acute myelogenous leukemia. Expert Opin Emerg Drugs. 2011;16:407–423. doi: 10.1517/14728214.2011.568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117:3294–3301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kampa-Schittenhelm KM, Heinrich MC, Akmut F, Dohner H, Dohner K, Schittenhelm MM. Quizartinib (AC220) is a potent second generation class III tyrosine kinase inhibitor that displays a distinct inhibition profile against mutant-FLT3, -PDGFRA and -KIT isoforms. Mol Cancer. 2013;12:19. doi: 10.1186/1476-4598-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levis MJ, Perl AE, Dombret H, Dohner H, Steffen B, Rousselot P, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. Blood (ASH Annual Meeting Abstracts) 2012;120 abstr 673. [Google Scholar]
- 29.Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–5102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- 30.Zheng R, Bailey E, Nguyen B, Yang X, Piloto O, Levis M, et al. Further activation of FLT3 mutants by FLT3 ligand. Oncogene. 2011;30:4004–4014. doi: 10.1038/onc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121:4655–4662. doi: 10.1182/blood-2013-01-480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo-Coco F, Avvisati G, Orlando SM, Ferrara F, Vignetti M, Fazi P, et al. ATRA and arsenic trioxide (ATO) versus ATRA and idarubicin (AIDA) for newly diagnosed, non high-risk acute promyelocytic leukemia (APL): Results of the phase III, prospective, randomized, Intergroup APL0406 study by the Italian-German Cooperative Groups Gimema-SAL-AMLSG. Blood (ASH Annual Meeting Abstracts) 2012;120 abstr 6. [Google Scholar]