Summary
The definition of primary refractory acute myeloid leukaemia is the failure to achieve a response after one or two cycles of induction. Given that there are many different strategies involving different doses of cytarabine and anthracyclines, which may or may not be equivalent, and as this is an area of unmet need with the potential for the development of new agents and strategies, uniform criteria for response have been described that need to be adhered to. The outcome of patients with chemoresistant disease is poor with only a proportion of patients salvaged by allogeneic stem cell transplantation. Progress in supportive care strategies and donor identification has enabled more of these patients to undergo unrelated donor transplantation. Novel strategies and new agents directed at the biology of the disease and the mechanisms of resistance are needed.
Keywords: AML, primary refractory, chemosensitive, chemoresistant
The importance of achieving complete remission
Historically, the achievement of complete remission (CR) after receiving induction chemotherapy has been considered as essential for improving survival in patients with acute myeloid leukaemia (AML) (Freireich, et al 1961). In a recent study of over 6,000 patients with AML treated by the Eastern Cooperative Oncology Group (ECOG), the Southwest Oncology Group (SWOG), or at the M. D. Anderson Cancer Center (MDACC), approximately 90% of the patients who were alive 3 or 5 years after induction had achieved CR after their initial therapy (Walter, et al 2010). This was independent of the cytarabine dose and age of the patients. Furthermore, among the few patients who received non-cytarabine containing regimens and were alive at 3 years, the majority had achieved CR with the initial therapy.(Walter, et al 2010) Therefore, although this notion has been recently challenged in trials of older patients receiving less intensive treatment strategies, such as hypomethylating agents (Fenaux, et al 2010), the achievement of CR has been considered as a requisite first step for achieving long-term survival in patients with AML.(Walter, et al 2010)
Of course, CR is an arbitrarily defined state of reduced disease burden based on morphological assessment of the bone marrow and peripheral blood and is by no means sufficient for achieving cure, as shown by studies demonstrating the need for post-remission therapy in both younger and older patients with AML.(Stone 2010) However, achieving CR and perhaps other definitions of lesser response, such as CR with incomplete platelet recovery (commonly denoted as CRp), serve to identify patients that are sensitive to the standard cytotoxic chemotherapeutic agents, particularly cytarabine and the anthracyclines. It is clear, both in leukaemia and in cancer therapy, that morphological CR is not equivalent to complete eradication of all neoplastic cells and new and more sensitive techniques of identifying minimal residual disease (MRD) have demonstrated the persistence of cells responsible for relapse in most patients achieving CR. (Grimwade, et al 2010) So, it is likely that, with the development of new and effective targeted strategies with the potential for eradicating MRD, our concepts and definitions of CR, may evolve further in the future.(Hokland & Ommen, 2011)
At the present time, however, achievement of morphological CR will remain the gold standard for assessing the sensitivity of an individual patient's leukaemic cells to cytotoxic chemotherapy while other indicators, such as lesser responses and the degree of clearance of leukaemic cells from the bone marrow on day 14 or 21, may also be useful to assess and grade chemosensitivity.
Definition of complete remission in AML and need for uniformity of its application
The original definitions of response as devised in a report by a group of international investigators interested in conducting clinical trials in patients with AML, was revised in 2003 to include updated endpoints of clinical relevance.(Cheson, et al 2003, Cheson, et al 1990) The definition of morphological CR requires a morphological leukaemia-free state with less than 5% blasts in the bone marrow aspirate sample (including marrow spicules and at least 200 nucleated cells), together with an absolute neutrophil count ≥ 1.0 × 109/l and platelet count ≥ 100 × 109/l in the absence of any evidence of extramedullary disease.(Cheson, et al 2003) Frequently, 1 to 5% circulating blasts may be identified at the time of CR and at least one study has suggested that these low numbers of persistent peripheral blasts at CR have no effect on the subsequent outcome.(Estey, et al 2003) However, in general, and particularly in clinical research, circulating blasts are considered to be evidence of resistant disease unless they disappear on subsequent evaluations. Another issue is the time of defining CR in relation to the induction treatment. Frequently, a regenerating bone marrow can be mistaken for persistent leukaemia, when repeating a bone marrow examination a week later can establish the achievement of CR. Clearly, in practice and in clinical research these definitions and practices need to be closely adhered to in order to achieve uniformity in studies of agents for primary resistant disease. Some definitions, such as CR with incomplete recovery of peripheral counts (CRi) or CRp remain problematic, as they do not specify the timing of the platelet or neutrophil count in relation to the bone marrow examination and or indicate if there is a leeway in the number of days allowed before this recovery occurs (i.e. a week after the bone marrow examination or longer?).
Definitions of induction failure
Despite the significant advances in the treatment of AML in the recent years, a significant proportion of patients fail to achieve CR with the initial induction regimen. Although inherent resistance of the leukaemia cells to the cytotoxic drugs is responsible for a proportion of these failures, other events, such as death as a result of infections and other complications of therapy, account for a proportion of patients failing to achieve a response. Clearly, there is an interaction between inherent resistance and the complications, as those patients who do not recover normal haematopoiesis within about a month of induction are more susceptible to the complications of prolonged apalsia related to persistent disease. Therefore, it is possible to further segregate these patients depending on the time of infectious death (beyond 4 weeks, this is more likely due, at least in part, to true resistance). Alternatively, induction death can be considered a separate entity as it would not be clear whether the patient would have achieved CR had it not been for an early death due to complications. Patients with chemotherapy-resistant disease would then be considered to be those who are alive after the induction but fail to fulfill the criteria for CR or CRi, coined by the International Working Group and more recently the European LeukaemiaNet (Cheson, et al 2003, Dohner, et al 2010) (Table I). Clearly, the patient will need to be alive for a minimum number of days to have the chance of demonstrating their ability to reach a response. The definition of resistant disease determined by the International Working Group and European LeukaemiaNet requires the patients to be alive for only 7 or more days after the completion of the first course of chemotherapy and have persistent leukaemia cells in the peripheral blood or bone marrow at this time (Cheson, et al 2003, Dohner, et al 2010). Clearly, with this definition, we are likely to encounter cases that would have been classed as resistant but, with further follow up, may actually achieve a CR. The early assessment proposed by the International Working Group, i.e., an evaluation at 7 to 10 days after completing the last dose of the initial course of treatment, serves to provide an early indication of the anti-leukaemic efficacy of the regimen.
Table I. Response criteria in acute myeloid leukaemia as reported by the International Working Group and the European LeukaemiaNet.
| Category | Definition |
|---|---|
| Complete remission (CR)* | Bone marrow blasts < 5%; absence of blasts with Auer rods; absence of extramedullary disease; absolute neutrophil count > 1.0 × 109/l; platelet count > 100 × 109/l; independence of red cell transfusions |
| CR with incomplete recovery (CRi) | All CR criteria except for residual neutropenia (< 1.0 × 109/l) or thrombocytopenia (< 100 × 109/l) |
| Morphological leukaemia-free state | Bone marrow blasts < 5%; absence of blasts with Auer rods; absence of extramedullary disease; no haematological recovery required |
| Partial remission (PR) | Relevant in the setting of Phase 1 and 2 clinical trials only; all haematological criteria of CR; decrease of bone marrow blast percentage to 5% to 25%; and decrease of pretreatment bone marrow blast percentage by at least 50% |
| Cytogenetic CR (CRc) | Reversion to a normal karyotype at the time of morphological CR (or CRi) in cases with an abnormal karyotype at the time of diagnosis; based on the evaluation of 20 metaphase cells from bone marrow |
| Resistant disease (RD) | Failure to achieve CR or CRi (general practice; Phase 2/3 trials), or failure to achieve CR, CRi, or PR (Phase 1 trials); only includes patients surviving ≥ 7 days following completion of initial treatment, with evidence of persistent leukaemia by blood and/or bone marrow examination |
| Death in aplasia | Death occurring ≥ 7 days following completion of initial treatment while cytopenic; with an aplastic or hypoplastic bone marrow obtained within 7 days of death, without evidence of persistent leukaemia |
| Death from indeterminate cause | Death occurring before completion of therapy, or < 7 days following its completion; or deaths occurring ≥ 7 days following completion of initial therapy with no blasts in the blood, but no bone marrow examination available |
| Relapse | Bone marrow blasts ≥ 5%; or reappearance of blasts in the blood; or development of extramedullary disease |
This leads to the point of whether early clearance of the bone marrow and blood from blast cells is an important goal of therapy. Previous studies have indicated this to be the case. In a of 449 patients with AML aged 16 to 76 years of age, lower marrow blasts count at day 16 was associated with a better CR rate (p < 0.0001), and higher event-free, disease-free and overall survival (p < 0.0001 for all endpoints) on multivariate analysis.(Kern, et al 2003) When applied to the “3+7” regimens this observation is very valid. However, one would expect this to be a function of the intensity of the initial therapy, at least in chemo-sensitive disease, with more intensive regimens more likely to produce hypoplasia in the majority of the patients.
Is achieving CR after one course similar to achieving it after 2 courses? What about doses of cytarabine and anthracyclines?
Recently, Lowenberg et al (2011) reported the results from a randomized study comparing two induction regimens in patients with mostly de novo AML, aged 18 to 60 years of age. This included 431 patients who received an intermediate dose cytarabine regimen consisting of 2 cycles, with the first cycle including cytarabine 200 mg/m2 administered as a continuous infusion for 7 days (total of 1.4 g/m2) together with idarubicin for 3 days. The second cycle consisted of cytarabine 1000 mg/m2 given intravenously for 3 h twice daily for 6 days (total of 12 g/m2) together with amascarine. The 429 patients on the high dose cytarabine arm of the study received cytarabine 1000 mg/m2 twice daily for 5 days (total of 10 g/m2) plus the same dose of idarubicin as above followed by a second cycle of amascarine as above together with cytarabine 2000 mg/m2 twice daily for 4 days. They demonstrated no benefit for the more intensive regimen with respect to CR rate, probability of relapse, event-free survival at 5 years or overall survival and concluded that induction therapy with cytarabine at the lower dose produced maximal anti-leukaemic activity, suggesting that the first regimen produced a plateau level above which further dose intensification with cytarabine was unlikely to be beneficial.(Lowenberg, et al 2011) Earlier studies demonstrated the saturation of intracellular cytarabine triphosphate accumulation with increasing doses of cytarabine, thereby providing the rationale for the use of intermediate dose cytarabine in the treatment of AML patients (Plunkett, et al 1987).
Although accepting the conclusions of the study by Lowenberg et al (2011), there are dangers in the potential ways these data can be interpreted and clarification of the exact regimens being compared is crucial. A common practice by the United States cooperative groups is to use an induction regimen of cytarabine 100 mg/m2 by continuous intravenous infusion for 7 days (total 0.7 g/m2) together with an anthracycline, typically daunorubicin, at doses of 45 to 90 mg/m2 daily for 3 days (Baer, et al 2011, Burnett, et al 2009, Fernandez, et al 2009). A bone marrow examination performed on approximately day 14 determines the need for a second cycle of induction of similar intensity, with 5 to 7 days of cytarabine at 100 mg/m2 (total 0.5 to 0.7 g/m2) and 2 to 3 days of an anthracycline (typically the need for second course is defined by the presence of ≥5% blasts in the aspirate and ≥20% cellularity in the biopsy on day 14 following initiation of induction therapy). Response is determined at the completion of the first, and if needed, the second cycle of the treatment, with a recent ECOG report suggesting a similar outcome for all the patients achieving CR irrespective of whether they needed one or two cycles of induction (Rowe, et al 2010). A report from the UK Medical Research Council (MRC) has also shown that a reduction of bone marrow blasts to 5% -15% after the first cycle of induction is associated with a high chance of achieving CR and, although the overall survival was worse for patients achieving partial remission, there was no difference in relapse rate whether CR was achieved with one or two cycles (Wheatley, et al 1999). Clearly, extrapolation of the data from the study reported by Lowenberg et al (2011) to such regimens is problematic as, although the first cycle of the lower dose regimen in that study is comparable to the induction regimens used by the MRC and the US cooperative groups, the second cycle is clearly more intensive. This further highlights the fact that the definition of primary resistant disease is highly dependent on the dose of the drugs used, at least in patients with inherently chemosensitive disease, such as those with more favourable cytogenetics and molecular features.
A meta-analysis of 3 randomized trials including approximately 1700 patients with de novo AML, aged 15 to 60 years, who received either a high dose (≥ 1 g/m2 per dose) or a standard dose (≤ 0.2 g/m2 per dose) cytarabine-based induction concluded that high dose cytarabine-based induction was associated with an improvement in event-free and overall survival in these patients with no difference in the rate of CR or early death between the two strategies (Kern and Estey 2006). This has led to the current practice by our group of using cytarabine 1500 mg/m2 by continuous intravenous infusion daily for 4 days (or for 3 days in patients 60 to 65 years) (total 4.5 to 6 g/m2) with a CR rate of 75% to 80%. (Ravandi, et al 2010a) The advantage of high dose cytarabine regimens in improving CR duration and event-free survival may be limited by higher induction toxicity associated with these regimens, which can potentially be overcome by improvements in supportive care.(Bishop, et al 1996)
An important question remains as to whether the total dose of cytarabine in the initial induction attempts (cycles 1 and 2) needs to be a total of around 1 g/m2, as is the current practice among the US cooperative groups and most European centres, or whether it needs to be escalated to 4.5 - 6 g/m2 (as practiced by our group). The study by Lowenberg et al (2011) does not provide guidance in this area as their intermediate risk group received an initial total dose of 1.4 g/m2 and could be “rescued” with a high dose of cytarabine (12 g/m2) in the second cycle; this is not a common strategy employed by the US and most European cooperative groups. Clearly, the higher dose group with the potential 26 g/m2 total cytarabine dose for cycles 1 and 2 did not benefit from this strategy, but the question still remains whether the traditional regimens are inferior to intermediate dose regimens of cytarabine (5 - 10 g/m2) for induction.
We have shown previously that failing one cycle of high dose cytarabine-based induction is associated with a very poor outcome and a very low likelihood of long-term survival (Ravandi, et al 2010b). It has also been shown that patients who are unresponsive to one cycle of “standard’ dose cytarabine induction can achieve a CR with a second cycle and do as well (Rowe, et al 2010). It is likely that patients who fail these higher dose cytarabine induction regimens have a disease that is inherently resistant to cytotoxic chemotherapy and are unlikely to benefit from similar chemotherapy-based interventions in their subsequent treatment. This has also been demonstrated in studies comparing “standard” versus escalated dose anthracyclines, where the younger patients with more favourable risk AML and without adverse cytogenetics or molecular aberrations benefited from increasing the dose of daunorubicin from 45 to 90 mg/m2 but those with these adverse features did not (Fernandez, et al 2009, Lowenberg, et al 2009). It is likely that such patients with inherently “chemotherapy-resistant” AML would benefit from alternate strategies, such as allogeneic stem cell transplantation or novel investigational agents directed at the molecular aberrations within their leukaemic cells. However, in patients with chemosensitive disease, the daunorubicin dose of 45 mg/m2 is clearly inadequate. Whether 80 mg/m2 is necessary or similar outcome can be obtained using 60 mg/m2 remains unclear.
In summary, resistance is a function of inherent disease biological characteristics as well as the dose and type of the chemotherapeutic agents used in induction. Patients with inherently sensitive disease who demonstrate a reduction of disease burden using various parameters, such as early blast clearance, can be “rescued” by a second course of similar or higher intensity if not in CR after the first course. However, those with inherent resistance to the commonly used induction drugs are unlikely to benefit from further dose intensification. Clearly, the age of the patient is an important determinant of the disease biology, impacting the likelihood of response to induction as demonstrated by numerous studies regarding the importance of age in the outcome of AML patients (Appelbaum, et al 2006, Kantarjian, et al 2006).
What is the importance of minimal residual leukaemia
The identification and eradication of MRD is increasingly important in cancer therapy due to the development and refinement of progressively more sophisticated techniques for MRD detection.(Grimwade, et al 2010, Kern, et al 2010) Quantitative polymerase chain reaction (QT-PCR) and multi-parameter flow cytometry (MFC) are increasingly used to detect MRD in various lymphoid and myeloid leukaemias and multiple studies have established the association of persistent MRD with a worse outcome (Campana 2010, Cilloni, et al 2009, Grimwade, et al 2009, Schnittger, et al 2009). This will obviously influence our concepts and definitions of sensitive and resistant disease. For example, a patient remaining positive by PCR for the PML-RARA fusion gene after consolidation therapy for acute promyelocytic leukaemia (APL) is clearly at a high risk of relapse and therefore such a patient must be considered as being resistant to the regimen he or she received and offered pre-emptive treatment with arsenic trioxide (Grimwade, et al 2009).We and others have reported that persistence of cytogenetically abnormal metaphases at the time of CR is associated with a significantly shorter relapse-free and overall survival in AML (Chen, et al 2011, Marcucci, et al 2004). As such, patients with cytogenetically persistent disease should be considered for intensification of post-remission therapy or alternative strategies and perhaps be considered as having primarily resistant disease (Chen, et al 2011).
A number of molecular markers are candidates for MRD monitoring in AML, including translocation fusion products in core-binding factor leukaemias, as well as newly described molecular markers such as WTI and NPM1. (Cilloni, et al 2009, Grimwade, et al 2010, Kronke, et al 2011) These studies have clearly demonstrated the feasibility of establishing time points when persistent MRD is associated with a higher risk of relapse, providing opportunity for disease intensification or development of novel immunotherapeutic approaches. Undoubtedly, the usefulness of such definitions is highly dependent on the availability of effective and non-toxic strategies to eradicate the MRD and achieve “cure”. How the definitions of disease response and resistance will evolve in this era remain to be seen.
Is there a role for chemotherapy in primary refractory AML?
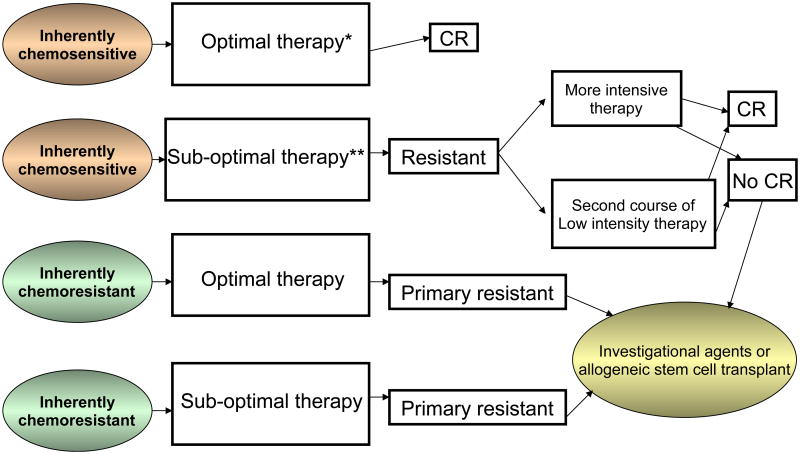
The question of efficacy of further chemotherapy in primary refractory AML is highly dependent on the definitions used and the initial treatment received. Patients with inherently chemosensitive leukaemia who receive less intensive therapy (standard 3+7 regimens) and do not achieve a CR may be “rescued” with a second course of similar or more intensive chemotherapy with a reasonable likelihood of response, whereas patients with an inherently chemo-resistant disease who receive intensive chemotherapy (cytarabine dose of at least about 5 g/m2 for induction together with either idarubicin or higher dose of daunorubicin) and who do not achieve a response are unlikely to benefit from further chemotherapy and should be considered for alternative strategies, such as allogeneic stem cell transplant or investigational agents targeted at the biology of the disease (Figure 1). These distinctions are important to consider when designing clinical trials specific for patients with primary refractory AML. It can be argued that true definitions of resistant and sensitive disease are probably based on disease biology. However, although biological predictors of response to chemotherapy, such as cytogenetic abnormalities, molecular aberrations (such as NPM1 and KIT mutations) and the existence of efflux pumps do exist, their predictive value is not universally established and they are not used in clinical practice to select initial therapy (Leith, et al 1999, Smith, et al 2011). It is also important to note that chemosensitivity should be viewed in the context of particular medications and that the same patient may be chemoresistant to one regimen and chemosensitive to another, particularly with identification of new specific targeted agents directed towards these molecular aberrations. In patients with truly chemoresistant disease, various chemotherapy strategies have been associated with significantly lower response rates compared to those with relapse following a prior response.
Figure 1.
A potential decision tree for patients with primary resistant acute myeloid leukaemia.
* e.g., may include higher dose anthracycline regimens or higher dose cytarabine regimens
** e.g., may include the 3+7 regimen with lower dose daunorubicin (45 mg/m2/dose)
Allogeneic stem cell transplant has been considered as the only strategy capable of producing meaningful responses and achieving long term disease-free survival in a proportion of these patients.(Biggs, et al 1992, Fung, et al 2003) Its role in this setting has been better delineated by a recent publication defining the predictors of outcome (Duval, et al 2010). This large study of over 1600 patients with AML who were not in CR at the time of transplant included 636 patients considered to be primary induction failures. The factors predicting the outcome in the overall population included poor risk cytogenetics, presence of circulating blasts, poor performance status, donors other than a human leucocyte antigen-identical sibling, and first CR duration less than 6 months, with patients with primary refractory disease having the worst survival (Duval, et al 2010). In another recent report, 22% of 168 patients with primary refractory AML who underwent an unrelated donor stem cell transplant were alive at 5 years, demonstrating the feasibility of this approach.(Craddock, et al 2011) Primary refractory disease was defined as failure to achieve a morphological CR in the bone marrow after 2 or more courses of induction without specifying the drugs used or their doses. On multivariate analysis, lower percentage of bone marrow blasts, fewer than 3 courses of induction, and cytomegalovirus seropositivity were associated with better patient survival, with survival differing from 44% to 0% based on the absence or presence of these factors.
Therefore, allogeneic stem cell transplant, both from related and unrelated donors, has the potential for achieving long-term survival at least in a proportion of these patients who otherwise have a very poor outcome. In our report of patients with primary refractory AML after receiving a high dose cytarabine-based induction, about half of the patients who were alive and in CR for at least 6 months had undergone an allogeneic stem cell transplant (Ravandi, et al 2010b). Unfortunately, these are only a minority of such patients. Novel treatment strategies, such as sequential therapy with chemotherapy followed by reduced-intensity allogeneic stem cell transplantation should be further evaluated (Schmid, et al 2006). However, the best prospect for improving the outcome of these patients probably involves a better understanding of the biology of the disease in these patients and the mechanisms that predispose to their insensitivity to cytotoxic agents.
Conclusions
Despite the availability of standard criteria for response and resistant disease in AML, the variability of dose and the number of drugs used by different groups in induction therapy and the existence of different strategies regarding the timing of the second induction course, has inevitably led to inconsistencies in defining patients with primary disease resistance. More precise definitions, taking into account the dose of drugs, the timing of the assessments to establish resistance, and perhaps incorporating biological characteristics of the disease, may provide us with a clearer picture of truly resistant disease, hence enabling a better comparison of data from various clinical trials of novel agents in this setting and enabling the development of better strategies for managing these patients.
References
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer MR, George SL, Sanford BL, Mrozek K, Kolitz JE, Moore JO, Stone RM, Powell BL, Caligiuri MA, Bloomfield CD, Larson RA. Escalation of daunorubicin and addition of etoposide in the ADE regimen in acute myeloid leukemia patients aged 60 years and older: Cancer and Leukemia Group B Study 9720. Leukemia. 2011;25:800–807. doi: 10.1038/leu.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs JC, Horowitz MM, Gale RP, Ash RC, Atkinson K, Helbig W, Jacobsen N, Phillips GL, Rimm AA, Ringden O, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–1093. [PubMed] [Google Scholar]
- Bishop JF, Matthews JP, Young GA, Szer J, Gillett A, Joshua D, Bradstock K, Enno A, Wolf MM, Fox R, Cobcroft R, Herrmann R, Van Der Weyden M, Lowenthal RM, Page F, Garson OM, Juneja S. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710–1717. [PubMed] [Google Scholar]
- Burnett AK, Hills RK, Milligan DW, Goldstone AH, Prentice AG, McMullin MF, Duncombe A, Gibson B, Wheatley K. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2009;28:586–595. doi: 10.1200/JCO.2009.22.9088. [DOI] [PubMed] [Google Scholar]
- Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:7–12. doi: 10.1182/asheducation-2010.1.7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cortes J, Estrov Z, Faderl S, Qiao W, Abruzzo L, Garcia-Manero G, Pierce S, Huang X, Kebriaei P, Kadia T, De Lima M, Kantarjian H, Ravandi F. Persistence of cytogenetic abnormalities at complete remission after induction in patients with acute myeloid leukemia: Prognostic significance and the potential role of allogeneic stem cell transplantation. J Clin Oncol. 2011;29:2507–2513. doi: 10.1200/JCO.2010.34.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, Brunning R, Gale RP, Grever MR, Keating MJ, Sawitsky A, Stass S, Weinstein H, Woods WG. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, Gottardi E, Fava M, Schnittger S, Weiss T, Izzo B, Nomdedeu J, van der Heijden A, van der Reijden BA, Jansen JH, van der Velden VH, Ommen H, Preudhomme C, Saglio G, Grimwade D. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195–5201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H, Ehninger G, Steckel NK, Zander AR, Schwerdtfeger R, Buchholz S, Kolb HJ, Volin L, Fauser A, Polge E, Schmid C, Mohty M, Rocha V. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25:808–813. doi: 10.1038/leu.2011.13. [DOI] [PubMed] [Google Scholar]
- Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Lowenberg B, Bloomfield CD. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, Kamble R, Copelan E, de Lima M, Gupta V, Keating A, Lazarus HM, Litzow MR, Marks DI, Maziarz RT, Rizzieri DA, Schiller G, Schultz KR, Tallman MS, Weisdorf D. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estey EH, Thall PF, Wang X, Verstovsek S, Cortes J, Kantarjian HM. Effect of circulating blasts at time of complete remission on subsequent relapse-free survival time in newly diagnosed AML. Blood. 2003;102:3097–3099. doi: 10.1182/blood-2003-04-1309. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM, Rowe JM, Lazarus HM, Tallman MS. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freireich EJ, Gehan EA, Sulman D, Boggs DR, Frei E., 3rd The effect of chemotherapy on acute leukemia in the human. J Chronic Dis. 1961;14:593–608. doi: 10.1016/0021-9681(61)90118-7. [DOI] [PubMed] [Google Scholar]
- Fung HC, Stein A, Slovak M, O'Donnell MR, Snyder DS, Cohen S, Smith D, Krishnan A, Spielberger R, Bhatia R, Bhatia S, Falk P, Molina A, Nademanee A, Parker P, Rodriguez R, Rosenthal J, Sweetman R, Kogut N, Sahebi F, Popplewell L, Vora N, Somlo G, Margolin K, Chow W, Smith E, Forman SJ. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant. 2003;9:766–771. doi: 10.1016/j.bbmt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, Diverio D, Jones K, Aslett H, Batson E, Rennie K, Angell R, Clark RE, Solomon E, Lo-Coco F, Wheatley K, Burnett AK. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27:3650–3658. doi: 10.1200/JCO.2008.20.1533. [DOI] [PubMed] [Google Scholar]
- Grimwade D, Vyas P, Freeman S. Assessment of minimal residual disease in acute myeloid leukemia. Curr Opin Oncol. 2010;22:656–663. doi: 10.1097/CCO.0b013e32833ed831. [DOI] [PubMed] [Google Scholar]
- Hokland P, Ommen HB. Towards individualized follow-up in adult acute myeloid leukemia in remission. Blood. 2011;117:2577–2584. doi: 10.1182/blood-2010-09-303685. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, O'Brien S, Cortes J, Giles F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J, Estey E. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- Kern W, Estey EH. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials. Cancer. 2006;107:116–124. doi: 10.1002/cncr.21543. [DOI] [PubMed] [Google Scholar]
- Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, Sauerland MC, Berdel W, Buchner T, Hiddemann W. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101:64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- Kern W, Bacher U, Haferlach C, Schnittger S, Haferlach T. The role of multiparameter flow cytometry for disease monitoring in AML. Best Pract Res Clin Haematol. 2010;23:379–390. doi: 10.1016/j.beha.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Kronke J, Schlenk RF, Jensen KO, Tschurtz F, Corbacioglu A, Gaidzik VI, Paschka P, Onken S, Eiwen K, Habdank M, Spath D, Lubbert M, Wattad M, Kindler T, Salih HR, Held G, Nachbaur D, von Lilienfeld-Toal M, Germing U, Haase D, Mergenthaler HG, Krauter J, Ganser A, Gohring G, Schlegelberger B, Dohner H, Dohner K. Monitoring of Minimal Residual Disease in NPM1-Mutated Acute Myeloid Leukemia: A Study From the German-Austrian Acute Myeloid Leukemia Study Group. J Clin Oncol. 2011;29:2709–2716. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnell TS, Head DR, Weick J, Grever MR, Appelbaum FR, Willman CL. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood. 1999;94:1086–1099. [PubMed] [Google Scholar]
- Lowenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Maertens J, Jongen-Lavrencic M, von Lilienfeld-Toal M, Biemond BJ, Vellenga E, van Marwijk Kooy M, Verdonck LF, Beck J, Dohner H, Gratwohl A, Pabst T, Verhoef G. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Biemond BJ, Gratwohl A, de Greef GE, Verdonck LF, Schaafsma MR, Gregor M, Theobald M, Schanz U, Maertens J, Ossenkoppele GJ. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364:1027–1036. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- Marcucci G, Mrozek K, Ruppert AS, Archer KJ, Pettenati MJ, Heerema NA, Carroll AJ, Koduru PR, Kolitz JE, Sterling LJ, Edwards CG, Anastasi J, Larson RA, Bloomfield CD. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol. 2004;22:2410–2418. doi: 10.1200/JCO.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Plunkett W, Liliemark JO, Estey E, Keating MJ. Saturation of ara-CTP accumulation during high-dose ara-C therapy: pharmacologic rationale for intermediate-dose ara-C. Semin Oncol. 1987;14:159–166. [PubMed] [Google Scholar]
- Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, O'Brien S, Estrov Z, Borthakur G, Thomas D, Pierce SR, Brandt M, Byrd A, Bekele BN, Pratz K, Luthra R, Levis M, Andreeff M, Kantarjian HM. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010a;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F, Cortes J, Faderl S, O'Brien S, Garcia-Manero G, Verstovsek S, Santos FP, Shan J, Brandt M, de Lima M, Pierce S, Kantarjian H. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010b;116:5818–5823. doi: 10.1182/blood-2010-07-296392. quiz 6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JM, Kim HT, Cassileth PA, Lazarus HM, Litzow MR, Wiernik PH, Tallman MS. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116:5012–5021. doi: 10.1002/cncr.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, Harsdorf SV, Scheid C, Holtick U, Greinix H, Keil F, Schneider B, Sandherr M, Bug G, Tischer J, Ledderose G, Hallek M, Hiddemann W, Kolb HJ. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B, Haferlach C, Haferlach T. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood. 2009;114:2220–2231. doi: 10.1182/blood-2009-03-213389. [DOI] [PubMed] [Google Scholar]
- Smith ML, Hills RK, Grimwade D. Independent prognostic variables in acute myeloid leukaemia. Blood Rev. 2011;25:39–51. doi: 10.1016/j.blre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Stone RM. New agents in post-remission therapy. Best Pract Res Clin Haematol. 2010;23:475–479. doi: 10.1016/j.beha.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Walter RB, Kantarjian HM, Huang X, Pierce SA, Sun Z, Gundacker HM, Ravandi F, Faderl SH, Tallman MS, Appelbaum FR, Estey EH. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol. 2010;28:1766–1771. doi: 10.1200/JCO.2009.25.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, Rees JK, Stevens RF, Walker H. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]