Abstract
A study recording directly from the human brain shows that connectivity between the prefrontal cortex, parietal cortex and the medial temporal lobe across different frequency bands underlies successful memory retrieval.
Memory is central to everyday life and is disrupted in many neurological disorders. This makes understanding how humans remember one of the most salient problems in neuroscience. Abundant single-unit, field potential, lesion and neuroimaging evidence obtained from animals and humans supports the notion that memory is not a unitary process but instead is fractionated into several processes, each dependent on activity in distributed neural regions. Using electrocorticography in human subjects, a study published in this issue of Nature Neuroscience by Watrous et al.1 now shows that specific temporal versus spatial components of episodic memory recall are mediated by differential oscillatory coupling of prefrontal, parietal and parahippocampal cortices.
One widely accepted idea of memory partitioning distinguishes explicit memory supported by a cortical-hippocampal system from different types of implicit memory involving multiple parallel brain systems. However, each distinct memory system is subject to further neuroanatomical fractionation reflecting functional components that closely interact in the service of each form of memory. Given the immense neuroanatomical complexity of each system, how does a system rapidly and precisely organize information flow subserving a particular type of memory in real-time situations? For example, within the explicit memory system, a memorable scene has many subcomponents (such as location, color, time, emotional engagement), each processed by a functionally specific cortical area. How does the brain configure these processes in parallel? Despite a wealth of information about the functional components of explicit memory, we know little about the physiological mechanisms that underlie the coordination and integration of the streams of information that form a coherent memory.
In the new paper, Watrous et al.1 address this issue by targeting the neural substrates supporting two key aspects of human explicit memory; namely, memory for spatial location and temporal order. These two linchpins of explicit memory (where and when did each event occur?) have been linked to a common distributed neural system that includes prefrontal, parietal and parahippocampal/hippocampal regions2. The authors hypothesized that the parahippocampal region, and, by anatomical inference, the hippocampus proper, serves as a hub for network interactions in prefrontal and parietal sites and subserves explicit memory, including information about both spatial and temporal context. They further proposed that network interactions would be manifest as enhanced physiological metrics of inter-regional coherence that would predict correct versus incorrect memory performance at a single-trial level. Critically, they also posited that coherence in distinct frequency bands would link prefrontal, parietal and parahippocampal regions during either memory for spatial location or memory for temporal sequence. This ‘spectral fingerprint hypothesis’ proposes that frequency band–specific inter-regional coherence subserves many cognitive processes3. This concept is receiving increasing empirical support from both monkey and human studies4,5. Notably, the spectral fingerprint hypothesis does not state that information is being encoded in lower frequency oscillations. Rather, the idea is that setting two or more regions in phase coherence facilitates information transfer in single-unit volleys by local adjustments in the likelihood of spiking6.
To address these issues, the authors turned to direct cortical recording in humans with implanted subdural electrodes for surgical treatment of epilepsy, providing unprecedented spatiotemporal information obtained directly from the human cortex. The authors used a simple yet clever ‘taxi-driver’ task in which different stores were laid out on a grid in a virtual environment and subjects drove passengers to different stores in a specific order. In one condition, the subject had to recall where the stores were located, and in another, the order in which they were visited. The researchers then used phase coherence metrics and graph theoretic analysis to address each of their hypotheses.
First, they found increased low-frequency coherence for correctly recalled spatial or temporal trials evident between 1 and 10 Hz. This finding fits well with other data from animals and humans on the role of network coherence in behavior. Second, graph theory and coherence metrics provided evidence that the parahippocampal gyrus was the critical hub for both spatial as well as temporal memory. This finding also agrees nicely with the classic clinical observation that marked recall deficits only occur with medial temporal compromise2. Third, and perhaps most insightful for understanding multifaceted behavior inherent in human cognition and for providing support for the spectral fingerprint hypothesis, different low frequency bands supported recollection of spatial information (1–4 Hz) as opposed to temporal information (7–10 Hz). The data also provided evidence for differences in the networks for spatial (parahippocampal-prefrontal-precuneus) and temporal (parahippocampal-prefrontal-inferior parietal) memory recall that were associated with these different spectral bands (Fig. 1).
Figure 1.
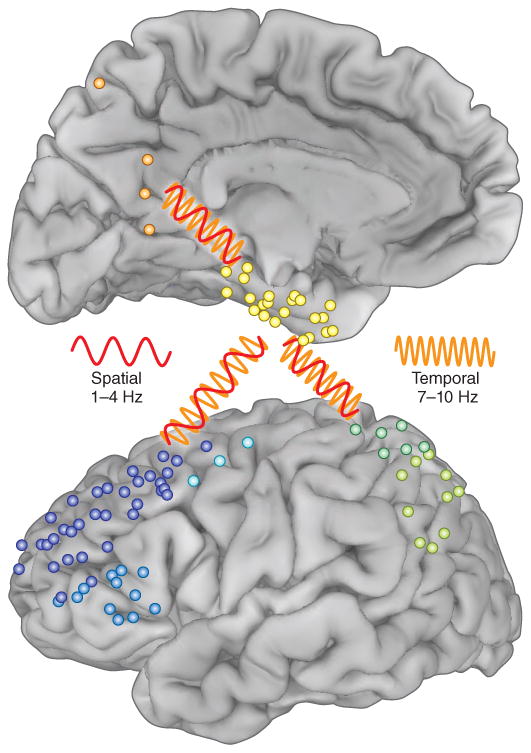
Individual subdural recording sites from the patients studied by Watrous et al.1 (blue, prefrontal; green, parietal; orange, precuneus; yellow, parahippocampal). The red oscillation (1–4 Hz) represents coherence between brain regions during spatial memory. The orange oscillation (7–10 Hz) represents coherence between these regions during temporal memory. Adapted from Watrous et al.1 with permission.
These results provide compelling support that neural oscillations support human memory and provide insights into how several contextual aspects of memory might be instantiated in the same general neural network. The work, however, also raises as many questions as it answers. For instance, what drives the parahippocampal hub? The authors excluded zero-lag phase coherence to avoid confounds from volume conduction of intracranial fields. However, recent work has shown that common input from the pulvinar can drive cortico-cortical phase coherence with zero lag7. Of course, one might then ask what drives the pulvinar: might it be decision-related activity in prefrontal regions? If we assume that coherence sets up networks to interact by spike volleys, what is the code transmitted on these frequency-dependent lines of communication? Recent work has shown that the high gamma response (70–250 Hz) observed in animal and human cortex is a likely surrogate for cortical spiking8. Other data have shown that the phase of low-frequency oscillations determines the probability of both spiking and high gamma response in motor, language and memory tasks in animals and humans9–12. Given that spatial and temporal coding in the hippocampus seem to be supported by the same principal neurons13,14, how do the multiplexed streams converge to influence activity of single hippocampal neurons, and then how do the distinct streams subsequently diverge on their way back out to modality-specific cortical areas? Might cross-frequency coupling metrics be useful in addressing coding principles?
A remaining issue is whether clear evidence exists that oscillations support behavior. Recent work disrupting specific hippocampal rhythms has begun to address this missing link in the puzzle15. This issue is being actively debated in the literature, and the resolution is critical to addressing how single-unit activity in widely distributed brain regions is integrated into organized behavior. Although causal manipulations were not included in the present study, the article by Watrous et al.1 provides an important stepping stone toward unraveling one of the jewels of cognition: how we remember and where in the brain this memory is encoded. The work also highlights the power of direct cortical recording in humans to bridge animal and human neurophysiology.
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
Contributor Information
Robert T Knight, Email: rtknight@berkeley.edu, Department of Psychology and the Helen Wills Neuroscience Institute, University of California at Berkeley, Berkeley, California, USA.
Howard Eichenbaum, Email: hbe@bu.edu, Department of Psychology and the Center for Memory and Brain, Boston University, Boston, Massachusetts, USA.
References
- 1.Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Nat Neurosci. 2013;13:349–356. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenbaum H, Yonelinas AR, Ranganath C. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel M, Donner TH, Engel AK. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 4.Colgin LL, et al. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 5.Fell J, Axmacher N. Nat Rev Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 6.Fries P. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardin JA, et al. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tort ABL, et al. Proc Natl Acad Sci USA. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 11.Canolty RT, et al. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axmacher N, et al. Proc Natl Acad Sci USA. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berzhanskaya J, Chernyy N, Gluckman BJ, Schiff SJ, Ascoli GA. J Comp Neurosci. 2012 Oct 7; doi: 10.1007/s10827-012-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


