Abstract
In this issue of Neuron, Suh et al. (2013) describe two rare ADAM10 prodomain mutations that cause late-onset Alzheimer’s disease by impairing prodomain chaperone function, attenuating α-secretase activity, and reducing adult hippocampal neurogenesis. These results support both ADAM10 as a therapeutic target and the amyloid hypothesis of Alzheimer’s disease.
Alzheimer’s disease (AD) is characterized by the cerebral accumulation of β-amyloid (Aβ), 38–43 amino acid peptides that self-aggregate into fibrils that comprise hallmark AD lesions called amyloid plaques. Evidence abounds that Aβ accumulation is a critical early AD event that starts a pathogenic cascade ultimately leading to synaptic loss, neuronal death, and dementia (Hardy and Selkoe, 2002). Biochemical, cellular, and animal-model studies suggest that Aβ is neurotoxic and disrupts neuronal function at multiple levels. Perhaps the most compelling evidence implicating Aβ in the etiology of AD comes from human genetic studies linking fully penetrant autosomal-dominant mutations in amyloid beta (A4) precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) to the occurrence of early-onset familial AD (EO-FAD) (Tanzi, 2012). These rare genetic cases of the disorder are very aggressive, resulting in AD that typically begins before the age of 60 years. In contrast, late-onset AD (LOAD), the most common form of the disease, appears after 60 years of age. The genetic lesions that cause EO-FAD total well over 200 in number and are mostly missense mutations in APP, PS1, and PS2. Without exception, these EO-FAD mutations either increase the ratio of the 42 amino acid Aβ isoform (Aβ42) to the 40 amino acid isoform (Aβ40) or increase the production of all lengths of Aβ (total Aβ). APP duplication in trisomy 21 (Down syndrome) or rare duplications limited to small chromosomal regions that include the APP locus also cause EO-FAD by raising total Aβ production via increased APP dosage. Importantly, a novel missense mutation that protects against AD by reducing total Aβ generation was recently discovered in APP (Jonsson et al., 2012), thus underscoring the critical role of Aβ in the pathophysiology of AD. Together, the human genetic evidence strongly suggests that Aβ is centrally involved in the etiology of EO-FAD. However, a definitive role for Aβ in the pathogenesis of LOAD has been controversial. Although the major genetic risk factor for LOAD, the apolipoprotein E ε4 variant (ApoE4), is associated with increased accumulation of cerebral Aβ, the mechanism by which ApoE4 causes increased amyloid is not fully understood (Holtzman et al., 2012). In addition, in the brain, ApoE4 may exert Aβ-independent effects that contribute to AD pathogenesis and cognitive decline. Thus, it has been argued that although Aβ accumulation may cause EO-FAD, its role in LOAD has not yet been firmly established.
Aβ is generated by the sequential proteolytic processing of APP via the action of two aspartic proteases, the β-secretase and γ-secretase enzymes (De Strooper et al., 2010). β-secretase, also called β-site APP-cleaving enzyme 1 (BACE1), cleaves APP first to generate the N terminus of Aβ (Figure 1A, right). The resulting membrane-bound APP C-terminal fragment (CTFβ) is then cut by γ-secretase (a complex of presenilin and other proteins), thus creating the C terminus of Aβ and causing the liberation and subsequent secretion of the Aβ peptide from the neuron. Accumulation of Aβ in the extracellular milieu of the brain ultimately leads to the formation of amyloid plaques and other downstream pathophysiological changes in AD. In an alternative, nonamyloidogenic pathway, a third enzyme called α-secretase cleaves APP within the Aβ domain, thus precluding Aβ generation (Figure 1A, left). In a process called ectodomain shedding, cleavage by α-secretase causes the secretion of an APP extracellular fragment, sAPPα, which has been reported to exhibit neuroprotective, neurotrophic, and neurogenic properties (Caillé et al., 2004; Mattson et al., 1993; Ring et al., 2007). Several enzymes in the “a disintegrin and metalloprotease” (ADAM) family, including ADAM9, ADAM10, and ADAM17, have α-secretase activity in vitro, although recent studies have demonstrated that ADAM10 is the major α-secretase that catalyzes APP ectodomain shedding in the brain (Kuhn et al., 2010). BACE1 competes with ADAM10 for cleavage of APP substrate, such that increased BACE1 activity causes decreased α-secretase processing of APP and vice versa. Importantly, the same principle applies for ADAM10, namely that increased ADAM10 activity leads to a reduction of β-secretase cleavage of APP and Aβ generation (Postina et al., 2004). This observation has two critical implications: (1) therapeutic strategies that increase ADAM10 activity should prove efficacious in lowering cerebral Aβ levels for AD, and (2) decreased ADAM10 activity would be expected to increase Aβ production and AD pathogenesis.
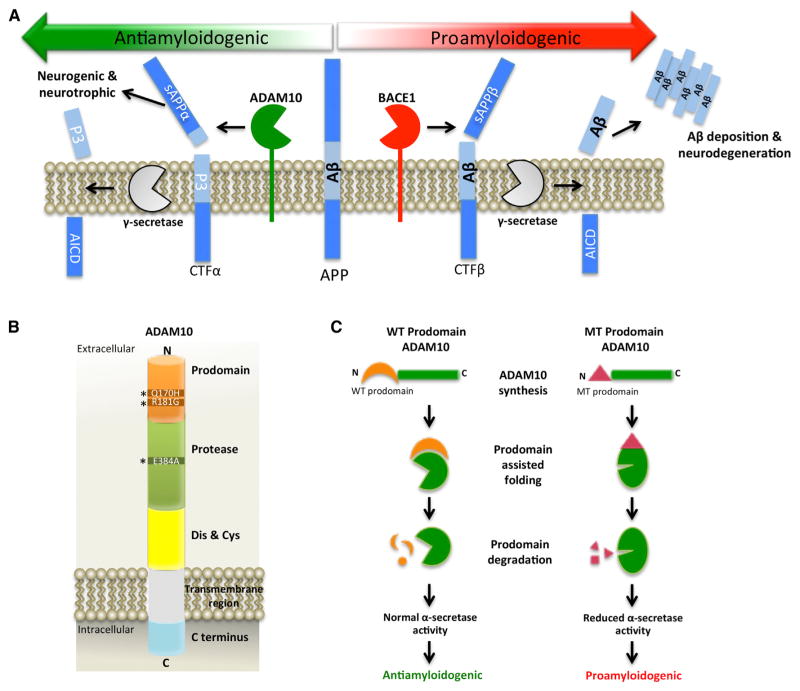
Figure 1. The Role of ADAM10 Prodomain Mutations in LOAD.
(A) In the proamyloidogenic pathway (right side), BACE1 cleavage of APP generates the N terminus of Aβ, thus creating the secreted sAPPβ ectodomain and membrane-bound APP CTFβ fragments. Next, γ-secretase cuts CTFβ to produce the C terminus of Aβ and the APP intracellular domain (AICD). Aβ is then secreted from the neuron, where it self-aggregates into extracellular amyloid plaques and causes neurodegeneration. In contrast, the antiamyloidogenic pathway (left side) involves cleavage of APP by ADAM10, leading to the generation of membrane-bound APP CTFα and the secreted sAPPα ectodomain, the latter of which has neurogenic and neurotrophic properties. γ-secretase then cuts CTFα to produce the nonamyloidogenic p3 fragment and the AICD. ADAM10 and BACE1 compete with each other to cut APP, such that changes in the amount of ADAM10 cleavage result in opposite effects on BACE1 processing of APP and vice versa.
(B) ADAM10 is a 748 amino acid type I membrane protein. Upon transit through the trans-Golgi network, the prodomain of ADAM10 is removed by furin or PC7. The mature enzyme is then trafficked to the plasma membrane, where it performs ectodomain shedding of diverse membrane protein substrates. The asterisks denote the positions of the two prodomain mutations that cause LOAD; the position of the dominant-negative artificial mutation in the protease domain is also indicated. The following abbreviation is used: Dis & Cys: disintegrin and cysteine domain.
(C) During synthesis of the enzyme, the wild-type (WT) ADAM10 prodomain serves as an intramolecular chaperone that facilitates correct protein folding of ADAM10 (left side). After prodomain removal and degradation, mature ADAM10 exhibits full α-secretase activity and performs antiamyloidogenic processing of APP. In contrast, the mutant (MT) ADAM10 prodomain has impaired intramolecular chaperone function, which results in improper ADAM10 protein folding and reduced α-secretase activity, thus leading to increased proamyloidogenic cleavage of APP by BACE1 (right side).
Previous studies have demonstrated that ADAM10 function is essential for neurogenesis and development of the embryonic brain. Constitutive and conditional Adam10-knockout mice both exhibit embryonic lethality at early stages (Hartmann et al., 2002; Jorissen et al., 2010), most likely as a result of deficient ADAM10 processing of Notch and its ligands. ADAM10 is a type I membrane protein synthesized as an inactive proenzyme and has an N-terminal prodomain that is removed by furin or proprotein convertase 7 (PC7) in the trans-Golgi network in order for the protease to become active (Figure 1B). Mature ADAM10 resides on the cell surface, where it performs ectodomain shedding of diverse membrane protein substrates, including APP. Although a major function of the ADAM10 prodomain is to maintain the enzyme in an inactive state during synthesis and maturation, the prodomain also functions as an intramolecular chaperone that assists in the correct folding of the enzyme’s various domains. The importance of prodomain chaperone function is underscored by the observation that expression of a prodomain-deleted ADAM10 construct results in a proteolytically inactive enzyme, whereas coexpression in trans of the prodomain with prodomain-deleted ADAM10 rescues enzyme activity (Anders et al., 2001).
Given the role of ADAM10 as the major APP α-secretase in the brain, Rudy Tanzi and colleagues at Massachusetts General Hospital and Harvard University assessed the candidacy of ADAM10 as a LOAD susceptibility gene. In a previous study, the group genotyped 30 SNPs that spanned ADAM10 and then performed targeted resequencing of the gene. This investigation identified two rare highly penetrant nonsynonymous mutations (Q170H and R181G) associated with LOAD in the prodomain of ADAM10 (Kim et al., 2009). These mutations occurred in 11 of 16 affected individuals from seven LOAD-affected families. In cell-culture experiments, ADAM10 with either the Q170H or the R181G prodomain mutation exhibited α-secretase activity that was reduced by greater than 70%. In addition, in cells coexpressing the prodomain mutants with APP, Aβ production was increased 1.5- to 3.5-fold. These results indicate that ADAM10 is indeed a LOAD susceptibility gene and suggest the intriguing possibility that the ADAM10 prodomain mutations reduce proteolytic activity, even though they are located far from the active site of the enzyme.
In their article in this issue of Neuron, the Tanzi group tested the role of the ADAM10 prodomain mutations in AD pathogenesis by generating transgenic mouse lines that express ADAM10 harboring the Q170H or R181G mutations in the brain (Suh et al., 2013). They also made control mouse lines expressing an artificial dominant-negative (DN) mutation, E384A, or wild-type (WT) ADAM10. Multiple lines of each transgenic construct were created, and expression levels across the various transgenes were matched. In addition, the team crossed the different ADAM10 transgenic lines with the well-characterized APP transgenic mouse, Tg2576, to determine the effects of the ADAM10 prodomain mutations on Aβ generation and amyloid deposition in the brain. They found that compared to control transgenic mice expressing WT ADAM10, transgenic mice expressing ADAM10 with the Q170H or R181G mutation exhibited reduced α-secretase cleavage of both endogenous mouse and transgenic human APP. In contrast, β-secretase processing of APP was concomitantly increased in ADAM10 prodomain mutant transgenic mice compared to in ADAM10-WT mice. ADAM10-DN transgenic mice exhibited even greater decreases and increases of α-secretase and β-secretase processing of APP, respectively, than did transgenic mice expressing either ADAM10-Q170H or ADAM10-R181G, indicating that the prodomain mutations attenuated, but did not eliminate, α-secretase activity.
Next, the team investigated whether expression of the LOAD ADAM10 prodomain mutations could cause elevated cerebral amyloid deposition. For these experiments, they crossed the ADAM10-Q170H transgenic line, which had the highest APP-CTFβ level, with Tg2576 mice and aged the bigenic mice to 3, 12, and 20 months. Importantly, both endogenous soluble and Tg2576 transgenic soluble and insoluble Aβ40 and Aβ42 levels were dramatically higher in the brains of ADAM10-Q170H/Tg2576 bigenic mice than in those of the ADAM10-WT/ Tg2576 mice, especially by 12 months of age. At 20 months of age, both amyloid plaque count and covered area were significantly increased in the brains of ADAM10-Q170H/Tg2576 mice relative to ADAM10-WT/Tg2576 mice. Interestingly, 20 month-old ADAM10-WT/Tg2576 mice were nearly devoid of amyloid plaques, whereas age-matched ADAM10-DN/ Tg2576 mice displayed an enormous plaque burden that was much greater than that in Tg2576 monogenic mice. These latter observations provide proof of concept that increased α-secretase activity should be an efficacious therapeutic strategy for lowering cerebral Aβ accumulation in AD. In addition, aged ADAM10-Q170H/Tg2576 mice exhibited greater levels of microgliosis and astrogliosis than did ADAM10-WT/Tg2576 bigenic mice. Taken together, these results demonstrate that the ADAM10 prodomain mutations promote cerebral amyloid pathology via attenuated α-secretase processing of APP, thus providing a mechanism for the genetic association between LOAD and the ADAM10 Q170H and R181G mutations.
Because previous studies suggested that sAPPα and ADAM10 play roles in neurogenesis, Tanzi and colleagues next investigated whether the ADAM10 prodomain mutations affect neurogenesis in the adult hippocampus. Interestingly, they found that proliferation of dentate gyrus neural precursor cells (NPCs) was significantly greater in 4-month-old ADAM10-WT transgenic mice than in nontransgenic mice. In contrast, NPC proliferation in ADAM10-Q170H, ADAM10-R181G, and ADAM10-DN mice was similar to that observed in nontransgenic mice. Importantly, the dentate gyrus in ADAM10-WT transgenic mice also displayed ~50% more BrdU:NeuN double-positive neurons than did the dentate gyrus in nontransgenic mice, whereas the dentate gyrus in ADAM10-Q170H mice exhibited a smaller neuronal increase. In addition, both the total number and the number with projecting apical dendrites of doublecortin (DCX)-positive immature neurons were significantly higher in the ADAM10-WT dentate gyrus than in the nontransgenic dentate gyrus. In contrast, the number of DCX-positive neurons was lower in the ADAM10-DN dentate gyrus than in the non-transgenic dentate gyrus, whereas the ADAM10-Q170H dentate gyrus had intermediate values between the WT and DN DCX-positive neuron numbers. Together, the results of these experiments indicate that ADAM10 regulates adult neurogenesis and that the LOAD prodomain mutations impair the neurogenic function of ADAM10.
Finally, Tanzi and colleagues endeavored to elucidate the mechanism by which the prodomain mutations had attenuated ADAM10 activity. Extensive cell biological analyses, including subcellular fractionation and surface biotinylation experiments, indicated that the prodomain mutations did not alter intracellular trafficking of ADAM10 to the plasma membrane or the synapse, thus eliminating the possibility that mutant ADAM10 was unable to reach its appropriate cellular destination to cleave APP. Given that the prodomain of ADAM proteases had previously been shown to possess a chaperone function that assists proper protein folding during synthesis of the enzyme, the group next investigated whether the activity of inactive prodomain- deleted ADAM10 (ADAM10Δpro) could be rescued by coexpression with WT or mutant prodomains in trans. Indeed, coexpression of WT prodomain efficiently restored the α-secretase activity of ADAM10Δpro, whereas Q170H or R181G mutant prodomains failed to do so. From these results, the authors concluded that the ADAM10 LOAD mutations Q170H and R181G impair the intramolecular chaperone protein-folding function of the ADAM10 prodomain and thus result in a misfolded enzyme with attenuated α-secretase activity.
The current Neuron article of Tanzi and colleagues is important for several reasons. First, it presents the first definitive evidence that reduction of α-secretase activity can cause AD. This hypothesis has been suggested by past cellular and animal model studies, but it has never before been demonstrated in humans with AD. The study also supports the inverse of this hypothesis, namely that therapeutic strategies for increasing α-secretase activity via ADAM10 upregulation are predicted to be efficacious for AD. Further, the team showed that ADAM10 upregulation may prove effective as an AD therapy through two distinct mechanisms that act in parallel: (1) increased α-secretase processing that competes with β-secretase cleavage of APP, resulting in reduced Aβ generation, and (2) an increased sAPPα level that leads to elevated adult neurogenesis in the hippocampus.
As a therapeutic strategy, upregulation of ADAM10 activity may prove challenging. In general, it is more feasible to develop small-molecule protease inhibitors than activators. However, in principle it may be possible to use gene-therapy approaches to increase ADAM10 expression in neurons of the brain, perhaps in a controllable fashion, to favor the nonamyloidogenic pathway of APP processing. Small-molecule activators to upregulate the expression of the endogenous ADAM10 may also be hypothetically possible, although a clear strategy for this approach is not presently obvious, and challenges concerning the specificity and control of ADAM10 activation are likely. In addition, the results of Tanzi and colleagues suggest a potential therapeutic approach to increasing adult neurogenesis in AD by raising sAPPα levels via gene therapy or direct infusion of sAPPα into the hippocampus.
Finally, the current study strengthens the causal link between abnormal Aβ metabolism and LOAD. The hypothesis that LOAD is caused by cerebral Aβ accumulation has been controversial, in part because rare highly penetrant LOAD mutations that affect Aβ metabolism have not been found, unlike the case for EO-FAD. Now, the rare highly penetrant LOAD mutations in the ADAM10 prodomain strongly support the conclusion that there is no qualitative difference between EO-FAD and LOAD and that they share a similar disease mechanism involving early cerebral Aβ accumulation but that they quantitatively differ in onset and severity depending on the rate of Aβ accumulation. In other words, EO-FAD and LOAD are the same disease but reside in different regions along a pathogenic continuum. As such, the work of Tanzi and colleagues represents an important paradigm shift in the field and further supports the amyloid cascade hypothesis of AD.
References
- Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. FASEB J. 2001;15:1837–1839. doi: 10.1096/fj.01-0007fje. [DOI] [PubMed] [Google Scholar]
- Caillé I, Allinquant B, Dupont E, Bouillot C, Langer A, Müller U, Prochiantz A. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Vassar R, Golde T. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lübke T, Lena Illert A, von Figura K, Saftig P. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, et al. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Jorissen E, Prox J, Bernreuther C, Weber S, Schwanbeck R, Serneels L, Snellinx A, Craessaerts K, Thathiah A, Tesseur I, et al. J Neurosci. 2010;30:4833–4844. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, Norton D, Tesco G, Elliott K, Wagner SL, et al. Hum Mol Genet. 2009;18:3987–3996. doi: 10.1093/hmg/ddp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, et al. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, et al. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Choi SH, Romano DM, Gannon MA, Lesinski AN, Kim DY, Tanzi RE. Neuron. 2013;80:385–401. doi: 10.1016/j.neuron.2013.08.035. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE. Cold Spring Harb Perspect Med. 2012;2:a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]