Abstract
Collective behaviours are influenced by the behavioural composition of the group. For example, a collective behaviour may emerge from the average behaviour of the group's constituents, or be driven by a few key individuals that catalyse the behaviour of others in the group. When ant colonies collectively relocate to a new nest site, there is an inherent trade-off between the speed and accuracy of their decision of where to move due to the time it takes to gather information. Thus, variation among workers in exploratory behaviour, which allows gathering information about potential new nest sites, may impact the ability of a colony to move quickly into a suitable new nest. The invasive Argentine ant, Linepithema humile, expands its range locally through the dispersal and establishment of propagules: groups of ants and queens. We examine whether the success of these groups in rapidly finding a suitable nest site is affected by their behavioural composition. We compared nest choice speed and accuracy among groups of all-exploratory, all-nonexploratory and half-exploratory–half-nonexploratory individuals. We show that exploratory individuals improve both the speed and accuracy of collective nest choice, and that exploratory individuals have additive, not synergistic, effects on nest site selection. By integrating an examination of behaviour into the study of invasive species we shed light on the mechanisms that impact the progression of invasion.
Keywords: behavioural syndrome, collective behaviour, dispersal, group composition, individual variation, Linepithema humile, personality, range expansion, relocation
Collective behaviour emerges from individual-based local rules without central control. Traditional models of collective behaviours assume that all the system's components follow identical behavioural rules (Couzin, Krause, James, Ruxton, & Franks, 2002; Seeley & Buhrman, 1999). However, individual variation is prevalent in animal groups (Jandt et al., 2014; Sih, Bell, & Johnson, 2004; Sih, Bell, Johnson, & Ziemba, 2004) and can have a great impact on the emergence of collective behaviour. For example, variation in connectivity may affect the speed at which information is transmitted among individuals (Pinter-Wollman, Wollman, Guetz, Holmes, & Gordon, 2011) and certain individuals may act as leaders (Conradt & Roper, 2005) and influence the actions of other group members.
In social insects, individual variation in behaviour among workers has great implications for the collective behaviour of the colony. Variation in which task each individual performs (i.e. division of labour) can improve colony productivity (Beshers & Fewell, 2001; Oster & Wilson, 1978) and efficiency (Dornhaus, 2008), similar to division of labour in factories (Smith, 1776). Individual variation in social insects is not confined to which task a worker performs. Within a task, there is much behavioural variation in how well it is performed (Jaisson, Fresneau, & Lachaud, 1988). For example, some workers are highly diligent in performing their task, yet others are not (Pinter-Wollman, Hubler, Holley, Franks, & Dornhaus, 2012). Such behavioural variation within a task is often overlooked, despite its potential impact on colony collective behaviour, on which natural selection acts (Jandt et al., 2014; PinterWollman, 2012).
Variation among colonies in their collective behaviour may arise from differences in worker composition. Colonies may vary in their collective behaviour because the mean behaviour of their workers differs, because of differences in the distribution of worker performance, or because of variation in both mean and distribution of worker performance (Pinter-Wollman, 2012). Thus, colony behaviour may be the outcome of additive effects (i.e. a colony's collective behaviour reflects the simple mean behaviour of its workers). For example, bumblebee colony thermoregulatory behaviour is a result of the average response threshold of its workers (Jandt & Dornhaus, 2014). Alternatively, a few individuals may influence how others behave, leading to synergistic effects on colony behaviour. Such individuals have been termed ‘key individuals’ (Modlmeier, Keiser, Watters, Sih, & Pruitt, 2014; Robson & Traniello, 1999) and may act as catalysts (i.e. increase the activity of other workers). For example, exploratory ants (Modlmeier & Foitzik, 2011) or bold individuals (Pruitt, Grinsted, & Settepani, 2013) affect how the colony responds collectively to changes in its environment. Such variation in the distribution of behavioural types within a colony can influence both the fitness of the individuals (Pruitt & Riechert, 2011) and the longevity of the colony (Pruitt, 2012, 2013).
Social insect colonies often relocate to new nest sites (Smallwood, 1982). The process of choosing a new nest site (i.e. when to move and where to settle) has been studied extensively in both honeybees (Seeley, 2010) and rock ants (Franks, Dechaume-Moncharmont, Hanmore, & Reynolds, 2009; Franks, Mallon, Bray, Hamilton, & Mischler, 2003). Nest site selection can be performed by workers of different tasks; for example, scouts locate the new nest and transporters assist in moving the colony (Franks, Mallon, et al., 2003; Seeley & Buhrman, 1999). Further variation among workers within a task group, such as experience (Langridge, Sendova-Franks, & Franks, 2008) or diligence (Pinter-Wollman et al., 2012), affects the speed of nest relocations. When making collective decisions of selecting a new nest site, there is an inherent trade-off between the speed and accuracy of the decision due to the time it takes to gather information (Chittka, Skorupski, & Raine, 2009; Franks, Dornhaus, Fitzsimmons, & Stevens, 2003). In ants, nest quality varies based on attributes such as darkness and entrance size (Franks, Mallon, et al., 2003), so colonies that make more accurate decisions are safer after relocation. In addition, the speed of relocation can affect the survival of the moving ants that are unprotected while outside the nest.
Invasive ant species expand their range through establishing new nest sites; thus, the collective process of nest selection in these species and the effects of individual variation among workers on this process have important ecological consequences. For example, behaviours such as the propensity to explore novel environments (Liebl & Martin, 2012; Rehage & Sih, 2004; Sih, Cote, Evans, Fogarty, & Pruitt, 2012) and quickly control new resources (Davidson, 1998; Holway, 1998; Holway & Case, 2001) play an important role in invasion success (Holway & Suarez, 1999). Furthermore, behavioural variation within a group determines its collective exploratory tendency (Brown & Irving, 2014). Thus, variation within a colony in the exploration and aggression of its workers may determine how well the colony as a whole expands its range.
The invasive Argentine ant, Linepithema humile, has been introduced from its native range in Argentina throughout the world (Holway, 1995; Suarez, Holway, & Case, 2001; Vogel, Pedersen, Giraud, Krieger, & Keller, 2010) and has detrimental impacts on the ecology of its introduced range (Fisher, Suarez, & Case, 2002; Human & Gordon, 1996; Peterson, Kus, & Deutschman, 2004; Suarez, Richmond, & Case, 2000). The spread of these ants at a global spatial scale is human mediated (Suarez et al., 2001), but at a local spatial scale, of a few hundred metres per year (Heller & Gordon, 2006; Markin, 1970), Argentine ants increase their range through budding: a propagule consisting of an inseminated queen and workers leaves an established nest site on foot and establishes a new nest nearby (Hee, Holway, Suarez, & Case, 2000). Propagules can disperse during any season, with or without queens (Aron, 2001), and their size (Sagata & Lester, 2009) and the number of queens (Hee et al., 2000) predict their establishment success. However, Argentine ant workers vary in their aggressive behaviour (Van Wilgenburg, Clemencet, & Tsutsui, 2010), so colonies, and potentially propagules, are composed of a behaviourally heterogeneous work-force. Thus, it is important to understand how the behavioural composition of the workers of a propagule, and not only their numbers, influences the spread of this invasive species. In addition, when choosing a new nest site, the speed–accuracy trade-off may affect how Argentine ant propagules extend the invasion range because they may be competing with other species over nest sites and other resources.
To examine how variation in exploratory behaviour of workers affects the collective speed and accuracy of choosing a new nest site by groups of Argentine ants, we induced nest relocation using nest flooding (Scholes & Suarez, 2009). We examined the choice of groups varying in composition of exploratory and nonexploratory individuals between two alternative nest sites of different quality. We asked whether exploratory individuals increase the speed of a group's search for a new nest site or improve the accuracy of distinguishing between alternative nest sites, whether there is a trade-off between the speed and the accuracy of selecting a new nest site, and whether the effect of exploratory individuals on group behaviour is additive or synergistic.
Methods
We collected 400 Argentine ant foragers from a foraging trail at the UCSD Biology Field Station on 1 March and 17 May 2013. Ants were housed in the laboratory on a 12:12 h dark:light cycle in two fluon-lined circular boxes (diameter = 25 cm, height = 13 cm) and were provided with water and sugar-water ad libitum.
Individual Exploration Assay
To determine the exploratory behaviour of individual workers, each ant was placed at the centre of an eight-arm maze and its behaviour was observed for 5 min, as in Modlmeier and Foitzik (2011). The maze comprised eight petri dishes (height = 10 mm) connected to one central dish using tygon tubes (Fig. 1a). Each of the eight dishes contained approximately 0.2 ml of a different spice (chili, cinnamon, garam masala, garlic, ginger, oregano, pilau and sage), providing novel stimuli for the ants to explore (Fig. 1a). After an ant was placed in the central chamber, we counted the number of spices it explored during 5 min. Exploring a spice was defined as entering the tube leading to a spice dish, whether or not an ant reached the spice itself. We did not observe a bias towards exploring a particular spice (all spices were visited at a similar frequency). In a preliminary test of 118 workers, we found that ants explored up to four spices (mean = 1.3 spices, median = 1 spice; Fig. 1b). Based on the distribution of the number of spices visited in these preliminary trials (Fig. 1b), we set the median number of spices visited as a threshold and defined an exploratory worker as an ant that visited two or more spices, and an ant that visited no spices or one spice as nonexploratory.
Figure 1.
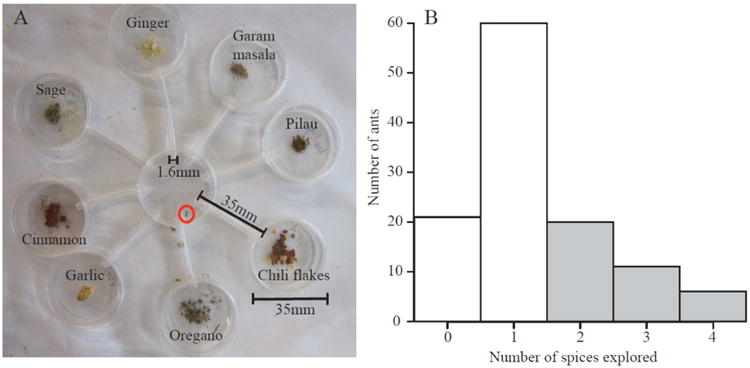
Determining exploration of individuals. (a) The eight-arm maze used to determine worker exploratory behaviour. The ant being tested is circled in red. (b) Distribution of the number of spices visited in preliminary trials. Ants that visited one spice or no spices (white bars) were defined as nonexploratory, and ants that visited two or more spices (grey bars) were defined as exploratory.
Group Nest Choice Assay
To examine the effect of group composition on their collective behaviour, we assembled three types of groups of 10 workers each. Hee et al. (2000) showed that as few as 10 workers accompanying a fertile queen were able to establish in a new nest site. Workers were scored for individual exploratory behaviour as described above and placed in one of three groups: 100% exploratory (all-exploratory), 50% exploratory and 50% nonexploratory (half-and-half) and 100% nonexploratory (all-nonexploratory). A new set of 10 workers was tested in each trial, and we replicated each type of group composition eight times, for a total of 24 group trials using 240 workers. At the end of each experiment, ants were placed in a different box from which they were previously housed in to ensure that they were not used more than once and were provided with water and sugar-water ad libitum until they died naturally. Ants were not released back to their original colony for ethical reasons to avoid aggressive rejection in case the food they were given in the laboratory changed their nestmate recognition cuticular hydrocarbons (Liang & Silverman, 2000).
To examine a group's collective choice between nest sites, we placed each group of 10 ants in a T-maze for 30 min. Three plastic containers (3.3 cm deep × 4.3 cm diameter) filled with 2 cm of dirt were connected with a bridge shaped like a T (see Fig. 2 for maze dimensions). The container at the base of the T was referred to as the ‘home nest’. One of the other two nests was identical to ‘home’ and the other was 80% covered with tin foil to produce a dark, favourable nest site (Franks, Mallon, et al., 2003; Markin, 1970; Fig. 2). The testing apparatus was set in water to prevent ants from escaping off the maze and was cleaned with ethanol between trials. Each trial began with placing 10 ants in the home nest and slowly flooding it with water to initiate nest relocation, similar to Scholes and Suarez (2009). Flooding was conducted at a rate that did not cause any ants to drown and continued until ants were no longer able to return to the home container, approximately within 30–100 s. The spatial behaviour of the ants was recorded for 30 min after flooding the home nest, as in Scholes and Suarez (2009), using an Insignia NS-DV720PBL2 video camera. Walking trajectories of the ants on the T-maze were obtained using the image analysis software AnTracks developed by Martin Stumpe (http://www.antracks.org).
Figure 2.
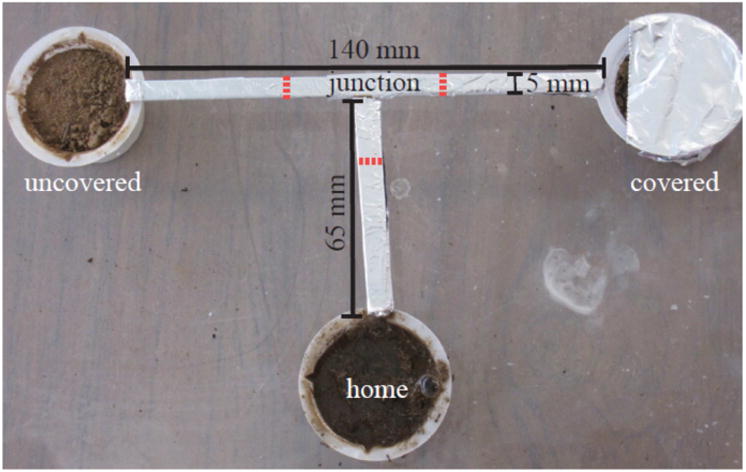
T-maze used to examine group nest choice. Dashed lines indicate the locations where we measured crossings between the junction and the various compartments.
To quantify the speed and accuracy of choosing a new nest site, we obtained the time at which each walking trajectory crossed an invisible line between the T junction and the three compartments (Fig. 2). Overall group investigative behaviour was quantified as the number of trajectory crossings from the home nest to the junction and from the junction to the two alternative nest sites, thus capturing all excursions from the home nest and all visits to the two alternative nest sites: the more excursions, the more investigative the group (similar to the measure used by Scholes & Suarez, 2009). Exploration speed was defined as the slope of the cumulative number of crossings from the home nest to the junction over time. This measure captured the rate at which ants left the home nest to investigate the two alternative nests. The shorter the latency between excursions from the nest, the steeper the slope of the cumulative number of such excursions, and thus, the faster the group's exploration. We used one-way ANOVAs with post hoc Tukey tests to compare investigative behaviour and speed among the three types of group compositions. To determine the ability of a group to accurately differentiate between the covered and uncovered nest sites, we compared the number of visits to each site by group type (i.e. number of crossings from the junction to the covered or uncovered alternative nests) using a two-tailed paired Wilcoxon signed-ranks test. More crossings to one of the two nest sites would indicate that more ants preferred that site over the other. The larger the difference in the number of visits to each of the nest sites, the greater the preference for one site over the other, and thus the greater, and more accurate the distinction between the two sites, similar to the definition of choice accuracy used by Franks, Mallon, et al. (2003) and Pratt and Sumpter (2006).
Additive versus Synergistic Effects
To determine whether the behaviour of the half-and-half groups was a result of simple additivity of the behaviour of the exploratory and nonexploratory individuals in the group or of synergistic effects of the exploratory individuals on the nonexploratory ones, we compared the behaviour of the half-and-half groups with the mean behaviour of the all-exploratory and all-nonexploratory groups. For both investigative behaviour and exploration speed, we created an estimated expected probability distribution to test the simple additivity hypothesis. We created a distribution of the 64 mean values of either investigative behaviour or exploration speed calculated for all possible combinations of the eight all-exploratory trials and the eight all-nonexploratory trials. We then calculated the probability that each of the eight half-and-half values would results from the estimated mean distribution by examining the percentile in which each of the eight half-and-half values fell within the estimated distribution. We averaged the eight obtained probabilities to estimate the P value for the test of whether the half-and-half groups significantly deviated from the mean of the behaviour of the all-exploratory and all-nonexploratory groups. A significant P value rejects the simple additivity model.
Results
Groups of all-exploratory workers investigated the T-maze significantly more than the all-nonexploratory groups but not more than the half-and-half groups. We observed more walking trajectories from the home nest to the junction and from the junction to the two alternative nest sites in the all-exploratory and the half-and-half groups than in the all-nonexploratory groups (ANOVA: F2,69=7.12, P=0.002; Fig. 3). However, when examining whether the similarity in investigative behaviour between the all-exploratory and half-and-half groups resulted from synergistic effects of the exploratory individuals on the nonexploratory ones in the half-and-half group, we did not find a difference between the behaviour of the half-and-half groups and the mean exploratory behaviour of the all-exploratory and the all-nonexploratory groups (P =0.45), suggesting an additive, not a synergistic, process.
Figure 3.
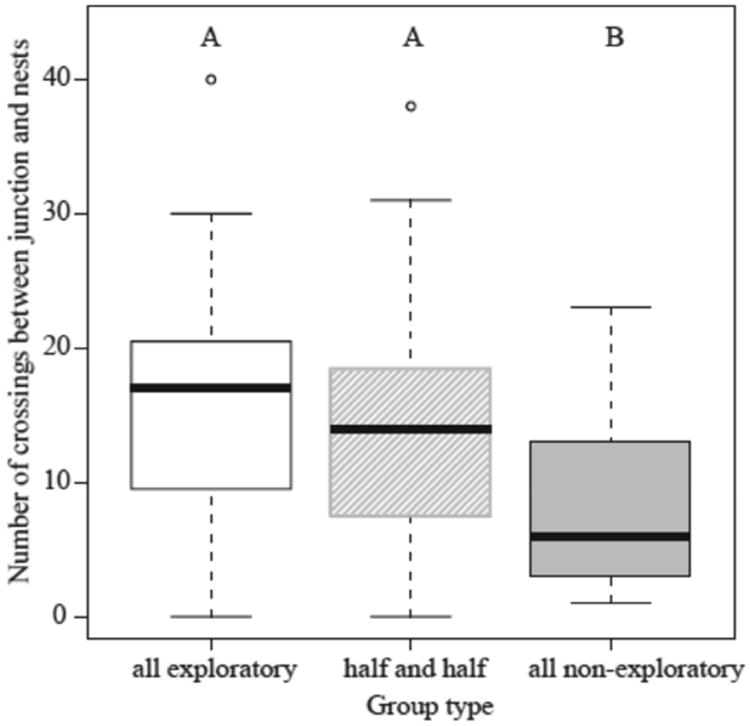
Investigative behaviour of all-exploratory, half-exploratory–half-nonexploratory and all-nonexploratory groups of ants. Different letters indicate significant differences using a post hoc Tukey test (P<0.05). Box plots: boxes indicate the lower and upper quartiles; horizontal lines within boxes indicate the median, whiskers extend to the 1.5 interquartile range from the box, and open circles indicate outliers.
Groups of all-exploratory ants were better at distinguishing between the covered and uncovered alternative nest sites than were the other two group types. When comparing the number of trajectories between the junction and the covered nest site and the junction and the uncovered nest site, the all-exploratory group visited the covered site significantly more often than the uncovered site (paired Wilcoxon signed-ranks test: V= 32, N= 8, P = 0.05), but there was no difference in number of visits of the two sites for the half-and-half (paired Wilcoxon signed-ranks test: V=22, N=8, P=0.62) or the all-nonexploratory groups (paired Wilcoxon signed-ranks test: V=21.5, N=8, P=0.67; Fig. 4).
Figure 4.
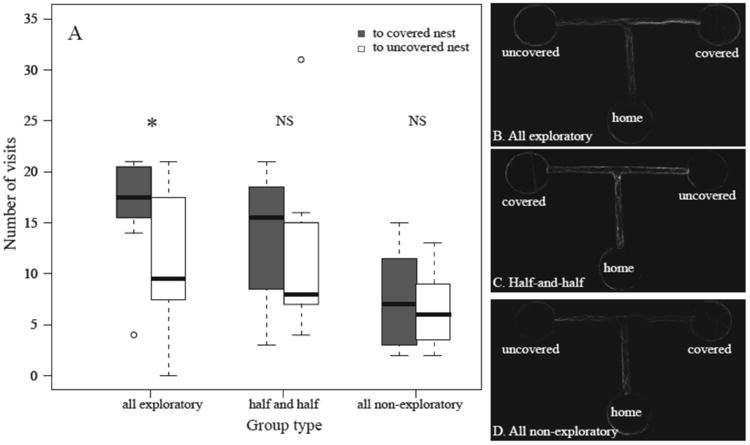
(a) Number of visits from the T junction to covered (dark boxes) or uncovered (open boxes) alternative nest sites by groups of all-exploratory, half-exploratory–half-nonexploratory and all-nonexploratory ants. Box plots as in Fig. 3. *P <0.05 (paired Wilcoxon signed-ranks test). (b–d) Representative examples of the trajectories of (b) all-exploratory, (c) half-and-half and (d) all-nonexploratory groups. Images show the density of the trajectories along the T-maze throughout the trial; brightest lines indicate the highest density of trajectories for that particular trial.
Groups of all-exploratory workers were faster at investigating the T-maze than were the all-nonexploratory groups. The slopes of the cumulative number of crossings from the home nest to the junction were significantly greater for the all-exploratory groups than for the all-nonexploratory groups, but the half-and-half groups did not significantly differ from the other two groups (ANOVA: F2,20= 4.52, P=0.02; Fig. 5). Furthermore, the investigation speed of the half-and-half groups did not differ from the mean speed of the all-exploratory and the all-nonexploratory groups (P = 0.51), suggesting an additive, not a synergistic, process.
Figure 5.
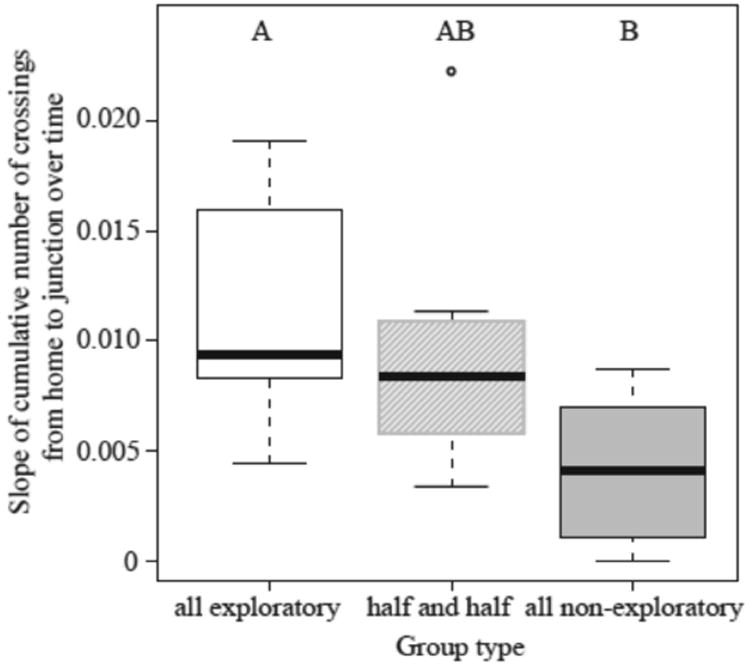
Investigation speed of all-exploratory, half-exploratory–half-nonexploratory and all-nonexploratory groups of ants. Different letters indicate significant differences using a post hoc Tukey test (P <0.05). Box plots as in Fig. 3.
Discussion
Exploratory individuals improved a group's speed and accuracy of choosing a new nest site. Groups of all-exploratory ants were faster and more investigative than groups of all-nonexploratory ants. The groups of all-exploratory ants also had a stronger preference for the covered, preferable, nest site than groups of all-nonexploratory ants or groups containing half-exploratory and half-nonexploratory ants. Thus, exploratory individuals increased both the speed and accuracy of the group's collective behaviour, but we did not find evidence for a speed–accuracy trade-off. Speed and accuracy were achieved by increasing both the number of visits to the various sites and the speed at which the visits were performed. Enhancing relocation speed while maintaining the accuracy of nest choice was also found in the emigration of Temnothorax curvispinosus colonies when faced with a crisis (Pratt & Sumpter, 2006). Furthermore, when a Temnothorax albipennis colony occupies a low-quality nest, it speeds-up the discovery of a new site by allocating more scouts to explore for new nest sites than when it occupies a high-quality nest (Doran et al., 2013). It remains to be determined whether colonies of Argentine ants allocate exploratory individuals to propagules rather than nonexploratory individuals, to increase both the speed and accuracy of propagule dispersal when expanding their invasion range.
The natural history of Argentine ants involves regular nest flooding in their native range (Wild, 2004) and seasonal nest relocations in their introduced range (Heller & Gordon, 2006; Markin, 1970). Thus, strong selective pressures of flooding and competition with other species in the Argentine ants' native range may explain their ability to find a new nest site rapidly and accurately. When directly compared to another species with similar ecology, Tapinoma sessile, Argentine ants were able to select a preferable nest site accurately under rapid nest flooding, while T. sessile were able to distinguish between preferable and mediocre sites only under slow flooding conditions (Scholes & Suarez, 2009). This difference between the two species is likely because the Argentine ant colonies sent out more workers to explore for a new nest during flooding than did T. sessile colonies (Scholes & Suarez, 2009). The increased ability of Argentine ants to select a new nest site rapidly and accurately, compared to a similar ant species, may explain the success of Argentine ants in invading and becoming established in novel habitats.
Our results suggest that exploratory ants have additive, not synergistic, effects on a group's exploration for a new nest site. We did not detect a significant difference in the investigative behaviour or speed of the all-exploratory groups and the half-exploratory–half-nonexploratory groups, as might be expected if exploratory ants had synergistic effects in the mixed group. However, the behaviour of the half-and-half groups did not significantly differ from the mean behaviour of the all-exploratory and all-nonexploratory groups, suggesting additive effects. Because we were unable to tag ants individually without causing substantial alterations to their behaviour, which precluded behavioural observations of the interactions between behavioural types, we cannot rule out the possibility that exploratory individuals in mixed groups catalysed exploratory behaviour in some nonexploratory individuals. It is possible that under different conditions, such as in larger groups or when faced with competition over nest sites, synergistic effects to expedite collective nest selection will emerge. When foragers of Argentine ants detect food, they recruit other individuals to the food source, acting as catalysts that change the foraging activity of other individuals (Aron, Pasteels, & Deneubourg, 1989; Flanegan, PinterWollman, Moses, & Gordon, 2013; Robson & Traniello, 1999), as happens in most cases of recruitment to resources. It is possible that exploratory behaviour is less plastic than foraging and therefore more difficult to induce rapidly in other individuals, suggesting that exploratory ants are perhaps highly specialized. Understanding the mechanisms underlying the variation among individuals in exploratory tendency, such as genetic or developmental factors, may help explain why certain species or colonies are better than others at invading new territory.
Maintaining variation in behavioural types within a colony is crucial for responding rapidly to environmental changes because the behaviour of each individual may not be flexible or it may take time to change (Jandt et al., 2014). Thus, group composition is likely determined by the costs and benefits associated with maintaining each behavioural type and by the relationship between the range of tasks the group must perform and the level of specialization of the workers. For example, the benefits of finding new resources, such as nest sites, by exploratory individuals need to be weighed against the costs of losing these workers to predation or other risks during their excursions (Bonte et al., 2012). So, a group with a high proportion of exploratory individuals may be able to move quickly into a new preferable nest site, but it may lose many workers to predation during the process. In addition, it may not be able to perform tasks that exploratory individuals do not engage in, and thereby limit the group's ability to perform its full range of tasks. Variation among groups in composition may lead to variation in which nest they choose, as observed here and by others (Franks, Mallon, et al., 2003). Such variation in nest choice raises an interesting hypothesis that certain nest sites are better suited for specific group compositions. Thus, as Argentine ants expand their range, it is possible that group composition might dictate the type of habitat into which they will expand.
Integrating an examination of behaviour into the study of invasive species can change our understanding of the causes and consequences of invasions (Holway & Suarez, 1999). Specifically, exploratory behaviour may prove to be an important factor explaining why some species, populations or individuals are better than others at invading new habitats (Cote, Fogarty, Brodin, Weinersmith, & Sih, 2011; Fogarty, Cote, & Sih, 2011; Sih et al., 2012). Furthermore, when studying social invasive animals, group composition may have important implications on how fast and into which habitats the population will spread. Our work shows that group composition of Argentine ant propagules, and specifically the proportion of exploratory individuals within these groups, influences both the speed and accuracy of collectively locating a new nest site. Future work should examine the exploratory behaviour of Argentine ant workers at the invasion front to determine whether colonies allocate highly exploratory individuals to where they are most needed.
Acknowledgments
We thank David Holway for his valuable advice during the initial stages of this work and Jonathan Pruitt, Stephen Pratt, Anna Dornhaus and Roy Wollman for their comments on earlier versions of the manuscript. N.P-W. was funded by a National Institutes of Health P50 grant (GM085764) to the San Diego Center for Systems Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron S. Reproductive strategy: an essential component in the success of incipient colonies of the invasive Argentine ant. Insectes Sociaux. 2001;48:25–27. [Google Scholar]
- Aron S, Pasteels JM, Deneubourg JL. Trail-laying behavior during exploratory recruitment in the Argentine ant, Iridomyrmex humilis (Mayr) Biology of Behaviour. 1989;14:207–217. [Google Scholar]
- Beshers S, Fewell JH. Models of division of labor in social insects. Annual Review of Entomology. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, et al. Costs of dispersal. Biological Reviews. 2012;87:290–312. doi: 10.1111/j.1469-185X.2011.00201.x. [DOI] [PubMed] [Google Scholar]
- Brown C, Irving E. Individual personality traits influence group exploration in a feral guppy population. Behavioral Ecology. 2014;25:95–101. [Google Scholar]
- Chittka L, Skorupski P, Raine NE. Speed–accuracy tradeoffs in animal decision making. Trends in Ecology & Evolution. 2009;24:400–407. doi: 10.1016/j.tree.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Conradt L, Roper TJ. Consensus decision making in animals. Trends in Ecology & Evolution. 2005;20:449–456. doi: 10.1016/j.tree.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A. Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1670–1678. doi: 10.1098/rspb.2010.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin ID, Krause J, James R, Ruxton GD, Franks NR. Collective memory and spatial sorting in animal groups. Journal of Theoretical Biology. 2002;218:1–11. doi: 10.1006/jtbi.2002.3065. [DOI] [PubMed] [Google Scholar]
- Davidson DW. Resource discovery versus resource domination in ants: a functional mechanism for breaking the trade-off. Ecological Entomology. 1998;23:484–490. [Google Scholar]
- Doran C, Pearce T, Connor A, Schlegel T, Franklin E, Sendova-Franks AB, et al. Economic investment by ant colonies in searches for better homes. Biology Letters. 2013;9:20130685. doi: 10.1098/rsbl.2013.0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhaus A. Specialization does not predict individual efficiency in an ant. PLoS Biology. 2008;6:2368–2375. doi: 10.1371/journal.pbio.0060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RN, Suarez AV, Case TJ. Spatial patterns in the abundance of the coastal horned lizard. Conservation Biology. 2002;16:205–215. doi: 10.1046/j.1523-1739.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- Flanegan TP, Pinter-Wollman NM, Moses ME, Gordon DM. Fast and flexible: Argentine ants recruit from nearby trails. PLoS One. 2013;8:e70888. doi: 10.1371/journal.pone.0070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty S, Cote J, Sih A. Social personality polymorphism and the spread of invasive species: a model. American Naturalist. 2011;177:273–287. doi: 10.1086/658174. [DOI] [PubMed] [Google Scholar]
- Franks NR, Dechaume-Moncharmont FX, Hanmore E, Reynolds JK. Speed versus accuracy in decision-making ants: expediting politics and policy implementation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:845–852. doi: 10.1098/rstb.2008.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NR, Dornhaus A, Fitzsimmons JP, Stevens M. Speed versus accuracy in collective decision making. Proceedings of the Royal Society B: Biological Sciences. 2003;270:2457–2463. doi: 10.1098/rspb.2003.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NR, Mallon EB, Bray HE, Hamilton MJ, Mischler TC. Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Animal Behaviour. 2003;65:215–223. [Google Scholar]
- Hee JJ, Holway DA, Suarez AV, Case TJ. Role of propagule size in the success of incipient colonies of the invasive Argentine ant. Conservation Biology. 2000;14:559–563. [Google Scholar]
- Heller NE, Gordon DM. Seasonal spatial dynamics and causes of nest movement in colonies of the invasive Argentine ant (Linepithema humile) Ecological Entomology. 2006;31:499–510. [Google Scholar]
- Holway DA. Distribution of the Argentine ant (Linepithema bumile) in northern California. Conservation Biology. 1995;9:1634–1637. [Google Scholar]
- Holway DA. Factors governing rate of invasion: a natural experiment using Argentine ants. Oecologia. 1998;115:206–212. doi: 10.1007/s004420050509. [DOI] [PubMed] [Google Scholar]
- Holway DA, Case TJ. Effects of colony-level variation on competitive ability in the invasive Argentine ant. Animal Behaviour. 2001;61:1181–1192. [Google Scholar]
- Holway DA, Suarez AV. Animal behavior: an essential component of invasion biology. Trends in Ecology & Evolution. 1999;14:328–330. doi: 10.1016/s0169-5347(99)01636-5. [DOI] [PubMed] [Google Scholar]
- Human KG, Gordon DM. Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- Jaisson P, Fresneau D, Lachaud JP. Individual traits of social behaviour in ants. In: Jeanne RL, editor. Interindividual behavioral variability in social insects. Boulder, CO: Westview Press; 1988. pp. 1–51. [Google Scholar]
- Jandt JM, Bengston S, Pinter-Wollman N, Pruitt JN, Raine NE, Dornhaus A, et al. Behavioural syndromes and social insects: personality at multiple levels. Biological Reviews. 2014;89:48–67. doi: 10.1111/brv.12042. [DOI] [PubMed] [Google Scholar]
- Jandt JM, Dornhaus A. Bumblebee response thresholds and body size: does worker diversity increase colony performance? Animal Behaviour. 2014;87:97–106. [Google Scholar]
- Langridge EA, Sendova-Franks AB, Franks NR. How experienced individuals contribute to an improvement in collective performance in ants. Behavioral Ecology and Sociobiology. 2008;62:447–456. [Google Scholar]
- Liang D, Silverman J. “You are what you eat”: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften. 2000;87:412–416. doi: 10.1007/s001140050752. [DOI] [PubMed] [Google Scholar]
- Liebl AL, Martin LB. Exploratory behaviour and stressor hyper-responsiveness facilitate range expansion of an introduced songbird. Proceedings of the Royal Society B: Biological Sciences. 2012;279:4375–4381. doi: 10.1098/rspb.2012.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin GP. Seasonal life cycle of Argentine ant, Iridomyrmex humilis (Hymenoptera: Formicidae), in southern California. Annals of the Entomological Society of America. 1970;63:1238–1242. [Google Scholar]
- Modlmeier AP, Foitzik S. Productivity increases with variation in aggression among group members in Temnothorax ants. Behavioral Ecology. 2011;22:1026–1032. [Google Scholar]
- Modlmeier AP, Keiser CN, Watters JV, Sih A, Pruitt JN. The keystone individual concept: an ecological and evolutionary overview. Animal Behaviour. 2014;89:53–62. [Google Scholar]
- Oster GF, Wilson EO. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press; 1978. [PubMed] [Google Scholar]
- Peterson BL, Kus BE, Deutschman DH. Determining nest predators of the least Bell's vireo through point counts, tracking stations, and video photography. Journal of Field Ornithology. 2004;75:89–95. [Google Scholar]
- Pinter-Wollman N. Personality in social insects: how does worker personality determine colony personality? Current Zoology. 2012;58:580–588. [Google Scholar]
- Pinter-Wollman N, Hubler J, Holley JA, Franks NR, Dornhaus A. How is activity distributed among and within tasks in Temnothorax ants? Behavioral Ecology and Sociobiology. 2012;66:1407–1420. [Google Scholar]
- Pinter-Wollman N, Wollman R, Guetz A, Holmes S, Gordon DM. The effect of individual variation on the structure and function of interaction networks in harvester ants. Journal of the Royal Society Interface. 2011;8:1562–1573. doi: 10.1098/rsif.2011.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt SC, Sumpter DJT. A tunable algorithm for collective decision-making. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15906–15910. doi: 10.1073/pnas.0604801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JN. Behavioural traits of colony founders affect the life history of their colonies. Ecology Letters. 2012;15:1026–1032. doi: 10.1111/j.1461-0248.2012.01825.x. [DOI] [PubMed] [Google Scholar]
- Pruitt JN. A real-time eco-evolutionary dead-end strategy is mediated by the traits of lineage progenitors and interactions with colony invaders. Ecology Letters. 2013;16:879–886. doi: 10.1111/ele.12123. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Grinsted L, Settepani V. Linking levels of personality: personalities of the ‘average’ and ‘most extreme’ group members predict colony-level personality. Animal Behaviour. 2013;86:391–399. [Google Scholar]
- Pruitt JN, Riechert SE. How within-group behavioural variation and task efficiency enhance fitness in a social group. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1209–1215. doi: 10.1098/rspb.2010.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehage JS, Sih A. Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biological Invasions. 2004;6:379–391. [Google Scholar]
- Robson SK, Traniello JFA. Key individuals and the organization of labor in ants. In: Detrain C, Deneubourg JL, Pasteels JM, editors. Information processing in social insects. Basel, Switzerland: Birkhäuser; 1999. pp. 239–260. [Google Scholar]
- Sagata K, Lester PJ. Behavioural plasticity associated with propagule size, resources, and the invasion success of the Argentine ant Linepithema humile. Journal of Applied Ecology. 2009;46:19–27. [Google Scholar]
- Scholes DR, Suarez AV. Speed-versus-accuracy trade-offs during nest relocation in Argentine ants (Linepithema humile) and odorous house ants (Tapinoma sessile) Insectes Sociaux. 2009;56:413–418. [Google Scholar]
- Seeley TD. Honeybee democracy. Princeton, NJ: Princeton University Press; 2010. [Google Scholar]
- Seeley TD, Buhrman SC. Group decision making in swarms of honey bees. Behavioral Ecology and Sociobiology. 1999;45:19–31. [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology & Evolution. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: an integrative overview. Quarterly Review of Biology. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Sih A, Cote J, Evans M, Fogarty S, Pruitt J. Ecological implications of behavioural syndromes. Ecology Letters. 2012;15:278–289. doi: 10.1111/j.1461-0248.2011.01731.x. [DOI] [PubMed] [Google Scholar]
- Smallwood J. Nest relocation in ants. Insectes Sociaux. 1982;29:138–147. [Google Scholar]
- Smith A. The wealth of nations. London, UK: Methuen; 1776. [Google Scholar]
- Suarez AV, Holway DA, Case TJ. Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1095–1100. doi: 10.1073/pnas.98.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez AV, Richmond JQ, Case TJ. Prey selection in horned lizards following the invasion of Argentine ants in southern California. Ecological Applications. 2000;10:711–725. [Google Scholar]
- Van Wilgenburg E, Clemencet J, Tsutsui ND. Experience influences aggressive behaviour in the Argentine ant. Biology Letters. 2010;6:152–155. doi: 10.1098/rsbl.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Pedersen JS, Giraud T, Krieger MJB, Keller L. The worldwide expansion of the Argentine ant. Diversity and Distributions. 2010;16:170–186. [Google Scholar]
- Wild AL. Taxonomy and distribution of the Argentine ant, Linepithema humile (Hymenoptera : Formicidae) Annals of the Entomological Society of America. 2004;97:1204–1215. [Google Scholar]


