Abstract
One of the major issues in clinical islet transplantation is the poor efficacy of islet isolation. During pancreas preservation and islet isolation, islets suffer from hypoxia as islets are highly sensitive to hypoxic conditions. Cold preservation has been applied to minimize hypoxia-induced cell damage during organ preservation. However, the studies related to hypoxia-induced islet cell damage during islet isolation are limited. Recently, we demonstrated that mouse islets contain high levels of high-mobility group box 1 protein (HMGB1), and during proinflammatory cytokine-induced damage, islets release HMGB1 outside the cell. The released HMGB1 is involved in the initial events of early islet loss. In the present study, we hypothesize that low temperature conditions could prevent both hypoxia induced islet cell damage and HMGB1 release from islets in a mouse model. Isolated mouse islets underwent normoxic condition (95% air and 5% CO2) at 37°C or hypoxic conditions (1% O2, 5% CO2, and 94% N2) at 37°C (hypoxia-37°C islets), 22°C (hypoxia-22°C islets), or 4°C (hypoxia-4°C islets) for 12 h. In vitro and in vivo viability and functionality tests were performed. HMGB1, IL-6, G-CSF, KC, RANTES, MCP-1, and MIP-1α levels in the medium were measured. Low temperature conditions substantially reduced hypoxia-induced necrosis (p < 0.05) and apoptosis (p < 0.05). In addition, low temperature islet culture significantly increased the insulin secretion from islets by high glucose stimulation (p < 0.05). All of the recipient mice reversed diabetes after receiving the hypoxia-4°C islets but not after receipt of hypoxia-37°C or 22°C islets. The amounts of released HMGB1, IL-6, G-CSF, KC, RANTES, MCP-1, and MIP-1α were significantly reduced in the hypoxia-4°C islets compared to those of the hypoxia-37°C islets (p < 0.05). In conclusion, low temperature conditions could prevent hypoxia-induced islet cell damage, inflammatory reactions in islets, and HMGB1 release and expression. Low temperature conditions should improve the efficacy of isolated islets.
Keywords: Islet transplantation, High-mobility group box 1 protein (HMGB1), Hypoxia, Preservation, Low temperature
INTRODUCTION
Islet transplantation is an attractive procedure for type 1 diabetes mellitus (15,16,28). Our recent survey of patients’ opinion revealed that more than 75% of type 1 diabetic patients preferred transplantation therapy in place of insulin injection therapy (6). However, there are still major issues that prevent the use of this treatment as a standard therapy, and poor efficacy of islet isolation is one of the problems (11,29). Actually, even though a normal human pancreas contains approximately 1 million islets, typically only half that number is recovered (11).
It is known that islet cells are highly sensitive to hypoxic conditions (8). Traditionally, cold temperature has been applied to reduce hypoxia-induced cell death during organ preservation (13). In addition, oxygenation of the pancreas during preservation by the two-layer method (13,14,17,18) and oxygenation of pancreatic tissue during digestion by adding oxygenated perfluorocarbon (5) were shown to improve the efficiency of isolated islets. Therefore, overcoming hypoxia is an important target to improve the efficacy of islet isolation. However, the information related to hypoxia-induced islet cell damage is limited.
Recently, we have reported that high-mobility group box 1 protein (HMGB1) was uniquely abundant in the pancreatic islets, and damaged islets released HMGB1 outside of the cells in a mouse model (19). HMGB1 was involved in the initial events of early loss of transplanted islets. Administration of anti-HMGB1 antibody into a diabetic recipient improved the results of islet transplantation in a mouse model (19). Additionally, islets damaged by proinflammatory cytokines released HMGB1 extracellularly (19). Those studies suggest that when islets are damaged by low oxygen conditions, islets might release and/or prepare to release HMGB1, and released HMGB1 might cause islet damage after islet transplantation.
From this background information, we hypothesize that hypoxic conditions could deteriorate islets and hypoxia-induced damaged islets might release HMGB1 like proinflammatory cytokine-damaged islets. In addition, low temperature conditions could prevent the damage of islets and HMGB1 release caused by hypoxic conditions.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice were purchased from Harlan Laboratories (Houston, TX) and used as diabetic recipients and islet donors. Mice weighing 23–25 g were used as recipients and those weighing 25–30 g were used as donors. Diabetes was induced in the recipients by the intravenous injection of streptozotocin (STZ) (180 mg/kg) (Sigma, St. Louis, MO). If blood glucose levels in the mice exceeded 400 mg/dl 2–3 days after the STZ injection and remained hyperglycemic at the time of islet transplantation, then these mice were used as the diabetic recipients. The experiments were approved by the Institutional Animal Care and Use Committee.
Mouse Islet Isolation
Mouse islets were isolated by the static digestion method using collagenase (Sigma-Aldrich, St. Louis, MO) and density gradient purification method (19). Islets of 150–250 μm in diameter were hand-picked using a Pasteur pipette under a dissecting microscope for the in vivo transplantation assay, because it was critical to minimize the size variation of individual islets.
Islet Culture and Exposure to Hypoxic Condition
Mouse islets were cultured in DMEM (Sigma-Aldrich) supplemented with 10% fetal bovine serum and 100 μg/ml kanamycin (Sigma-Aldrich) at 37°C in 95% air and 5% CO2 for 24 h. Then mouse islets were divided into four groups: normoxia control (control), hypoxia-37°C group, hypoxia-22°C group, and hypoxia-4°C group. In the control group, islets were washed twice with culture medium after initial culture and were cultured at 37°C in 95% air and 5% CO2 for 12 h. In the hypoxia-37°C, hypoxia-22°C, and hypoxia-4°C groups, islets were washed twice with culture medium after initial culture and placed into modular incubator chambers (Billups-Rothenberg, Inc., Del Mar, CA). The chambers were flushed with the hypoxic (1% O2, 5% CO2, and 94% N2) gas, closed to maintain the hypoxic condition, and then put into an incubator at 37°C (hypoxia-37°C), at room temperature (hypoxia-22°C) and in the refrigerator (hypoxia-4°C) for 12 h. After normoxic or hypoxic culturing, each group of islets and culture medium was used as samples.
Propidium Iodide (PI) Staining and PI+ Area Assay
Islets in each group were incubated in phosphate-buffered saline (PBS) containing 20 μg/ml Hoechst 33342 (HO342) (Sigma-Aldrich) and 10 μg/ml propidium iodide (PI) (Sigma-Aldrich) for 10 min at 37°C, as previously described (26). Then islets were washed with PBS and examined with fluorescence microscopy. PI PI+ area and islet area were measured by digital image soft, Image J (version 1.40g) (NIH, Bethesda, MD). Twenty samples were obtained by three independent experiments in each group. PI+ area and islet area (%) were expressed as mean ± SD.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Assay
The TUNEL assay was performed to detect the level of apoptosis in the islets of each group. The ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) was used according to the manufacturer’s instructions. Twenty samples were obtained by three independent experiments in each group. TUNEL+ cells and all cells were counted to calculate the ratio of TUNEL+ cells.
Caspase-3/7 Activity Assay
Caspase-3/7 activity was determined using the Apo-ONE Homogeneous Caspase-3/7 Activity Kit (Promega, Madison, WI), according to the manufacturer’s instruction. Fifteen samples were obtained by three independent experiments in each group.
Assay of ATP Content
To measure the adenosine triphosphate (ATP) content of islets in each group, islets were washed twice and sonicated in 1 ml of PBS. The amount of ATP was measured using an ATP assay system (ATP-lite, Perkin Elmer, Groningen, Netherlands) according to the manufacturer’s instructions. Five samples (100 islets in one sample) were obtained in each group. The data were normalized to total DNA and expressed as mean ± SD.
Glucose-Stimulated Insulin Secretion Test (GSIS)
Islets in each group were incubated with low (2.8 mM) and high (20.0 mM) concentrations of glucose solution in Functionality/Viability Medium CMRL1066 (Mediatech, Inc., Manassas, VA) for 1 h at 37°C. Insulin concentrations were measured by an insulin ELISA kit (ALPCO Diagnostics, Salem, NH). The insulin secretion levels were normalized to total DNA of islets. Five samples (100 islets in one sample) were obtained in each group. The data were expressed as mean ± SD.
In Vivo Assessment
Two hundred mouse islets in the each group were transplanted under the left kidney capsule of STZ-induced diabetic mice; this number was chosen based on Sakata et al.’s report that STZ-induced diabetic mice became normoglycemic after receiving 200 islets under the kidney capsule (25). Five cases of transplantation were performed in each group. The nonfasting blood glucose levels were measured using Accu-Chek Aviva (Roche Diagnostics, Indianapolis, IN) three times a week in all the recipients for 3 weeks after islet transplantation. Normoglycemia after transplantation was defined as two consecutive blood glucose levels reading <200 mg/dl. The curves of curative rate were obtained using Kaplan-Mayer’s method, and data were analyzed using log-rank test.
Immunohistochemistry
Each group of islets were preserved in 10% formalin, embedded in paraffin, and sectioned at 5 μm. Tissue sections were depaparaffinized, and heat-mediated antigen retrieval was performed. The sections were stained immunohistochemically with rabbit anti-HMGB1 antibody (Abcam, Cambridge, UK), guinea pig anti-insulin antibody (Sigma-Aldrich), and DAPI (Sigma-Aldrich) (19).
Assay of Medium HMGB1 Levels
At the end of hypoxic culture, islet culture media from each well was collected and stored at −80°C until assay. The amount of HMGB1 was measured using an HMGB1 ELISA kit II (Shino-test, Kanagawa, Japan) according to the manufacturer’s instructions (31). Five samples (100 islets in one sample) were obtained in each group. The amount of HMGB1 in media was normalized to total DNA of cultured islets and the data were expressed as mean ± SD.
Quantitative Real-Time PCR
Four groups of the islets were analyzed by the quantitative real-time PCR (qRT-PCR) studies. Total RNA was extracted from the each group of islets using a RNeasy® Mini Kit (QIAGEN Inc., Valencia, CA), and was reverse transcribed into complementary DNA using an RT2 FirstStrand Kit (QIAGEN Inc.). Template cDNA was mixed with RT2 SYBR green/ROX PCR master mix (QIAGEN Inc.), and hmgb1 primer (QIAGEN Inc.). The reverse transcription-PCR reaction was run using the Stratagene MX3000 system with the following program: 95°C for 10 min, and 40 cycles of 95°C for 15 s, and 60°C for 60 s. Quantitative analysis was performed by the ΔΔCt method by using β-actin (QIAGEN Inc.) as an internal control.
Measurement of Secreted Inflammatory Cytokines and Chemokines
Secretion of interleukin-6 (IL-6), granulocyte-colony stimulating factor (G-CSF), keratinocyte chemoattractant [KC; also known as chemokine (CXC motif) ligand 1 or CXCL1], Regulated upon Activation, Normal T-cell Expressed, and Secreted [RANTES; also known as chemokine (C-C motif) ligand 5 or CCL5], monocyte chemotactic protein-1 (MCP-1 or CCL2), and macrophage inflammatory protein-1 (MIP-1α or CCL3) in the culture medium was determined by measuring cell culture supernatant in a Luminex 200 using xMAP technology (Millipore, Billerca, MA). The bead assay was performed according to the manufacturer’s instructions with samples in duplicate. Five samples (100 islets in one sample) were obtained in each group. The each amount in media was normalized to total DNA of cultured islets and the data were expressed as mean ± SD.
Statistical Analysis
The statistical significance of PI+ area assay, the percentage of TUNEL+ cells, caspase-3/7 activity, ATP/DNA assay, insulin secretion levels, released HMGB1 levels, mRNA levels of HMGB1, and released cytokines and chemokines levels were determined by one-way ANOVA and Tukey/Kramer post hoc test. The statistical significance with respect to the rate of euglycemia in STZ-induced diabetic mice after islet transplantation was determined by Fisher’s exact test. All statistical analysis was performed using StatView 5.0 (SAS Institute Inc, Cary, NC). Differences were considered significant when values were p < 0.05.
RESULTS
Morphological Appearance of Four Groups of Islets
In the control islets, islet surface was smooth and no dark spot was seen and very few PI+ and TUNEL+ cells were seen. However, in the hypoxia-37°C and hypoxia-22°C islets, the islet surface was becoming rougher and the dark spots more noticeable, and the PI+ and TUNEL+ cells were increased compare to that of control islets. On the other hand, in the hypoxia-4°C islets, the surface was smooth with no dark spots apparent, and few cells were positive for PI and TUNEL staining (Fig. 1).
Figure 1.
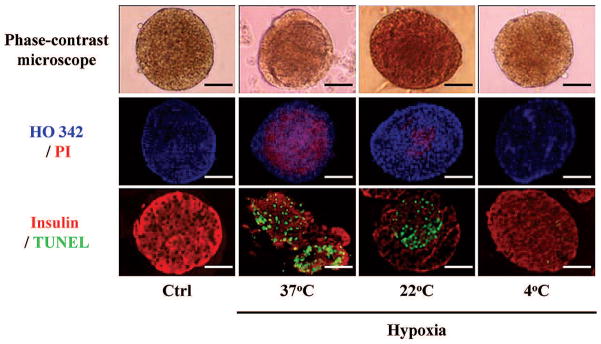
Morphological appearance of four groups of islets. Control (Ctrl), hypoxia-37°C, hypoxia-22°C and hypoxia-4°C islets were examined by phase-contrast microscopy, Hoechst33342 (blue)/propidium iodide (PI; red) staining and insulin (red)/Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL; green) staining. Scale bars: 50 μm.
Low Temperature Conditions Prevented Hypoxia-Induced Cell Necrosis and Cell Apoptosis of Islets
The four groups of islets were evaluated using PI staining, TUNEL staining, and caspase-3/7 activity assay. PI+ area/islet area in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 2.3 ± 0.7%, 35.2 ± 10.5%, 10.5 ± 5.8%, and 3.5 ± 4.1%, respectively (Fig. 2A). A significant difference was found between the control and hypoxia-37°C, control and hypoxia-22°C, hypoxia-22°C and hypoxia-37°C, and the hypoxia-4°C and hypoxia-37°C islets (*p < 0.05). There was no significant difference between control and hypoxia-4°C islets. The percentage of TUNEL+ cells in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 2.8 ± 1.4%, 23.6 ± 6.4%, 14.0 ± 14.3%, and 7.4 ± 6.5%, respectively (Fig. 2B). A significant difference was found between the control and hypoxia-37°C, control and hypoxia-22°C, and the hypoxia-4°C and hypoxia-37°C islets (*p < 0.05). There was no significant difference between control and hypoxia-4°C islets. The caspase-3/7 activity assay in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 6521.9 ± 2126.9, 13332.9 ± 7019.5, 10890.9 ± 2443.4 and 9898.7 ± 29979.1 rate fluorescence (RFU), respectively (Fig. 2C). A significant difference was found between the control and hypoxia-37°C, and control and hypoxia-22°C islets (*p < 0.05). There was no significant difference between control and hypoxia-4°C islets. These results indicate that low temperature could prevent cell necrosis and cell apoptosis of islets while islets were exposed to hypoxic conditions.
Figure 2.
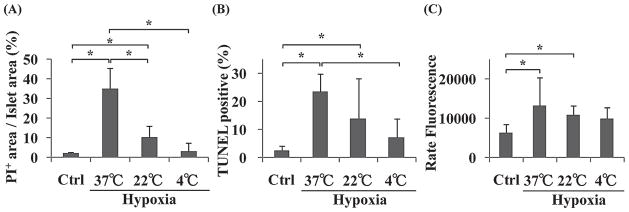
PI+ area assay, TUNEL assay, and caspase-3/7 activity assay of four groups of islets. Control (Ctrl), hypoxia-37°C, hypoxia-22°C, and hypoxia-4°C islets were examined by PI+ area assay (A), TUNEL assay (B), and caspase-3/7 activity assay (C). *p < 0.05.
ATP/DNA Assay for Four Groups of Islets
The ATP/DNA ratios in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 22.2 ± 4.0, 15.9 ± 1.5, 16.3 ± 10.3, and 24.0 ± 3.1 pmol/μg DNA, respectively (Fig. 3). There were no significant differences among four groups of islets.
Figure 3.
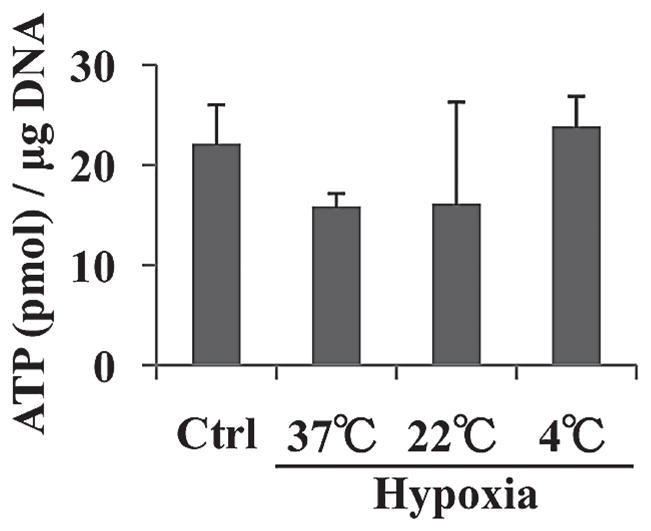
ATP/DNA levels of four groups of islets. The ATP/DNA of the control (Ctrl), hypoxia-37°C, hypoxia-22°C, and hypoxia-4°C islets were measured.
Hypoxia-4°C Islets Showed Better Insulin Release Function Compared to That of Hypoxia-37°C Islets
Insulin levels in response to low glucose (2.8 mM) in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 0.17 ± 0.05, 0.43 ± 0.06, 0.18 ± 0.12, and 0.16 ± 0.03 ng insulin/μg DNA, respectively (Fig. 4A). A significant difference was found between the control and hypoxia-37°C, hypoxia-22°C and hypoxia-37°C, and hypoxia-4°C and hypoxia-37°C islets (*p < 0.05). Insulin secretion levels in response to high-glucose stimulation (20.0 mM) in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 5.6 ± 0.8, 2.2 ± 0.3, 4.2 ± 1.9, and 6.8 ± 2.5 ng insulin/μg DNA, respectively (Fig. 4B). A significant difference was found between the control and hypoxia-37°C, and hypoxia-4°C and hypoxia-37°C islets (*p < 0.05). The stimulation index in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 36.5 ± 12.5, 5.1 ± 1.1, 27.4 ± 12.8, and 42.1 ± 14.2, respectively (Fig. 4C). A significant difference was found between the control and hypoxia-37°C, hypoxia-22°C and hypoxia-37°C, and hypoxia-4°C and hypoxia-37°C islets (*p < 0.05). These results demonstrated that low temperature conditions could prevent loss of islets’ ability to secrete insulin even though islets were under hypoxic conditions.
Figure 4.
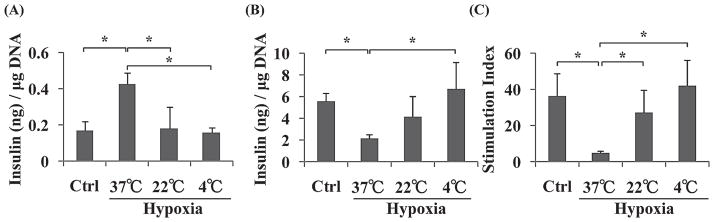
Glucose-stimulated insulin secretion (GSIS) test of the four groups of islets. Four groups of islets were stimulated by 2.8 mM glucose (A) and 20.0 mM glucose (B). The stimulation index was calculated by determining the ratio of insulin concentrations in the high-glucose solution to that in the low-glucose solution (C). *p < 0.05.
Reversal of STZ-Induced Diabetes After Receipt of Hypoxia-4°C Islets But Not Hypoxia-37°C and Hypoxia-22°C Islets
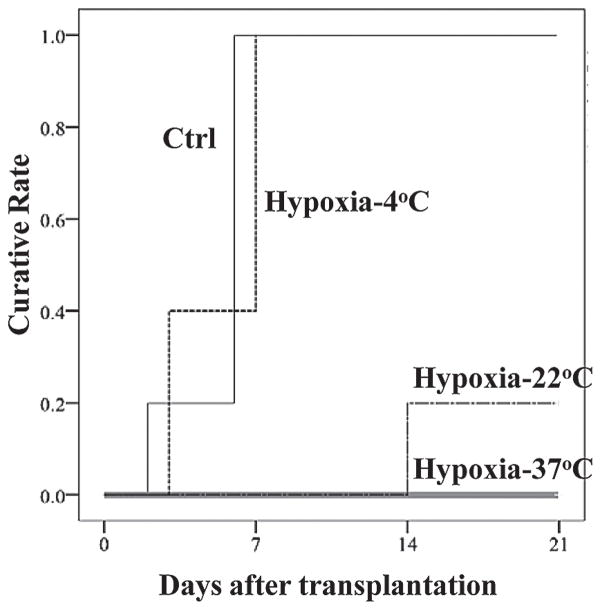
We examined the islets’ in vivo function by transplantation assay using diabetic mice for the four groups of islets. In the control group, all mice were cured after receiving 200 islets under the kidney capsule (Fig. 5). However, 5/5 of hypoxia-37°C islet group and 4/5 of hypoxia-22°C islet group were not cured after receiving same number of islets. On the other hand, in the hypoxia-4°C islet group, all of the STZ-induced diabetic mice (5/5) became normoglycemic after receiving the same number of islets (Fig. 5). There was a significant difference between control and hypoxia-37°C, control and hypoxia-22°C, hypoxia-4°C and hypoxia-37°C, and hypoxia-4°C hypoxia-22°C using log-rank test (p < 0.05). These results demonstrated that islet in vivo function was well maintained at 4°C, even when islets were exposed to hypoxic condition.
Figure 5.
Streptozotocin (STZ)-induced diabetic mice were ameliorated after receiving control and hypoxia-4°C islets but not after receiving hypoxia-37°C and hypoxia-22°C islets. The curative rates after receiving 200 of control (Ctrl), hypoxia-4°C, hypoxia-22°C, and hypoxia-37°C were 100%, 100%, 20%, and 0%, respectively (n = 5 in each group).
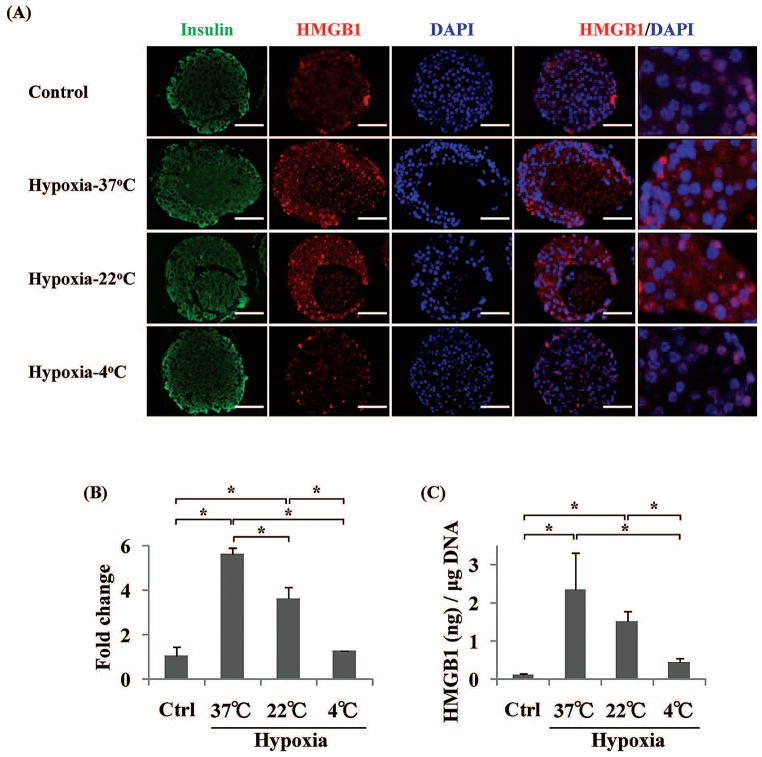
The Expression and the Amount of Released HMGB1 Was Decreased for Hypoxic-4°C Islets
In order to confirm the HMGB1 expression in four groups of islets, next we performed immunohistochemistry and qRT-PCR for HMGB1. In the control islets, HMGB1 was mainly stained in the nucleus (Fig. 6A, upper panels); however, HMGB1 was presented in not only the nucleus, but also cytoplasm in the hypoxia-37°C and hypoxia-22°C islets (Fig. 6A, middle panels). In contrast, HMGB1 was mainly stained in the nucleus of hypoxia-4°C islets (Fig. 6A, lower panel). In the HMGB1 qRT-PCR, the fold change against β-actin in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 1.1 ± 0.4, 5.6 ± 0.3, 3.7 ± 0.5, and 1.3 ± 0.0, respectively (Fig. 6B). A significant difference was found between the control and hypoxia-37°C, control and hypoxia-22°C, hypoxia-22°C and hypoxia-37°C, hypoxia-4°C and hypoxia-22°C, and hypoxia-4°C and hypoxia-37°C islets (*p < 0.05). There was no significant difference between control and hypoxia-4°C islets. Next, we measured the amount of released HMGB1 from the four groups of islets. The amount of released HMGB1 in the control, hypoxia-37°C, hypoxia-22°C, and the hypoxia-4°C islets were 0.13 ± 0.04, 2.36 ± 0.96, 1.52 ± 0.28, and 0.46 ± 0.12 ng HMGB1/μg DNA, respectively (Fig. 6C). A significant difference was found between the control and hypoxia-37°C, control and hypoxia-22°C, hypoxia-4°C and hypoxia-22°C, and hypoxia-4°C and hypoxia-37°C islets (*p < 0.05). There was no significant difference between control and hypoxia-4°C islets. From these results, we demonstrated that low temperature condition could prevent upregulation of HMGB1 expression and HMGB1 release from islets when islets were under hypoxic conditions.
Figure 6.
High-mobility group box 1 protein (HMGB1) expression and the amount of released HMGB1 from four groups of islets. Four groups of islets were stained with insulin (green), HMGB1 (red), and DAPI (blue) (A). HMGB1 mRNA levels were analyzed by qRT-PCR (B). The amount of released HMGB1 was measured by ELISA (C). Scar bars: 50 μm. *p < 0.05.
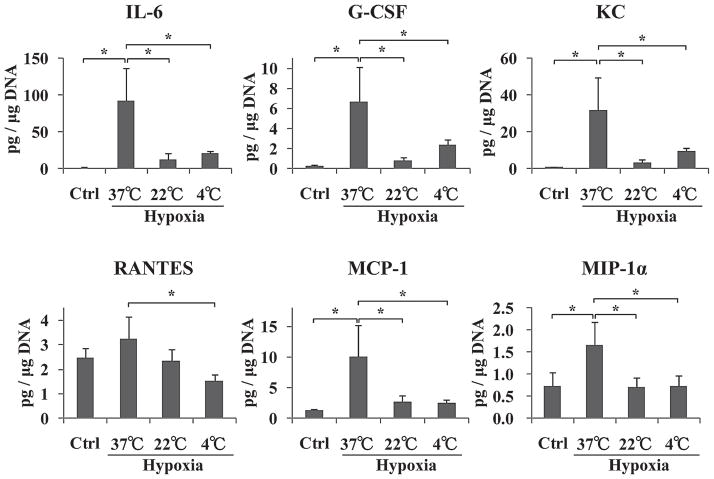
Low Temperature Conditions Could Prevent Hypoxia-Induced Inflammatory Reactions in Islets
At the end of this study, we measured inflammatory cytokine and chomokine production levels in the islet culture medium. IL-6, G-CSF, KC, MCP-1, and MIP-1α were significantly increased in hypoxia-37°C islets group when compare to those of control islets group (*p < 0.05) (Fig. 7). However, IL-6, G-CSF, KC, RANTES, MCP-1, and MIP-1α production from hypoxia-4°C islets group was significantly lower than that of hypoxia-37°C islets group (*p < 0.05) (Fig. 7) and there were no significant differences between control and hypoxia-4°C islets group. Taken collectively, these results demonstrated that low temperature conditions could prevent release of inflammatory cytokines and chemokines from hypoxia-induced damaged islets.
Figure 7.
Inflammatory cytokines and chemokines production from four groups of islets. The amounts of released interleukin (IL)-6, granulocyte-colony stimulating factor (G-CSF), keratinocyte chemoattractant [KC; also known as chemokine (CXC motif) ligand 1 or CXCL1], Regulated upon Activation, Normal T-cell Expressed, and Secreted [RANTES; also known as chemokine (C-C motif) ligand 5 or CCL5], monocyte chemotactic protein-1 (MCP-1 or CCL2), and macrophage inflammatory protein-1 (MIP-1α or CCL3) from four groups of islets. *p < 0.05.
DISCUSSION
During pancreas preservation, pancreas digestion, and islet purification, islets suffer from hypoxic conditions and it is known that islets are sensitive to hypoxic damage (8). The effects of hypoxia to whole pancreata have been well examined as a part of whole pancreas preservation research (4,13,14,24). However, the majority of the pancreas consists of exocrine tissue; therefore, pancreas preservation research provides limited information related to islets. Once islets are isolated, they can obtain oxygen from culture medium and generate ATP (2). Therefore, to investigate the effect of hypoxia on islets, an appropriate model for hypoxia is necessary. In this study, we simulated low oxygen conditions by utilizing a hypoxic chamber, which was originally established as a way to elicit hypoxia for hepatic cells (23). After the pancreas procurement, islets are no longer attached to the vascular system until revascularization takes places within 7–10 days after implantation (21). During organ procurement, islet isolation, and early posttransplantation period, islets suffer PaO2 5–10 mmHg (about 1% O2) (3,21); therefore, we selected 1% O2, 5% CO2, and 94% N2 mixture hypoxic gas for this study. We selected 12 h of hypoxic conditions to simulate the clinical transplantation process, which requires time for organ procurement, organ transportation, and islet isolation. In the period during organ procurement, organ transportation, islet isolation, islets were put at 37°C, 22°C, or 4°C conditions (11). Therefore, in the present study, we used those three temperature conditions.
In this study, we clearly demonstrated that hypoxia at 37°C deteriorated islet cells accompanied with HMGB1 release from damaged islets and maintaining low temperature conditions could prevent both hypoxia-induced islet cell damage and HMGB1 release for 12 h.
We analyzed the ability of low temperature conditions to prevent cell necrosis using PI staining and apoptosis using TUNEL staining and caspase-3/7 activity assay. Both the PI+ area and the rate of TUNEL+ cells in the hypoxia-4°C islets were significantly lower compared to those of the hypoxia-37°C islets. The caspase-3/7 activity was significantly increased in hypoxia-37°C islets when compare to that of control islets. However, there was no significant difference between control and hypoxia-4°C islets. From these results, we demonstrated that 12 h of hypoxia caused both necrosis and apoptosis in islets and that low temperature condition could prevent both of them.
The ATP/DNA levels of the four groups of islets showed no significant differences by one-way ANOVA analysis. However, when comparing between hypoxia-4°C and hypoxia-37°C islets using Student t-test, hypoxia-4°C islets were significantly higher than that of the hypoxia-37°C islets (p = 0.0007). The level of ATP/DNA of the hypoxia-4°C islets was almost the same level as normoxia control islets. Since the metabolic rate at 4°C is only 5% of normal physiological levels (9), islet cells can save ATP at the lower temperatures. This extra ATP can be utilized to repair cell damage and/or maintain cell function after returning to normothermic conditions (12).
Next, we analyzed the in vitro and in vivo islet functions. In GSIS test, the hypoxia-4°C islets showed significantly higher insulin secretion against 20.0 mM glucose solution compare to that of the hypoxia-37°C islets and almost the same level as control islets. This proved that hypothermic conditions could maintain islets’ ability to secrete insulin even when exposed to hypoxic damage. However, the hypoxia-37°C islets showed significantly higher insulin secretion against 2.8 mM glucose solution compared to those of the control, hypoxia-22°C, and hypoxia-4°C islets. When islets are stimulated with hypoglycemic solution, intact islets can detect the hypoglycemic condition and minimize the secretion of insulin but damaged islets leak insulin. Thus, high insulin secretion of hypoxia-37°C islets against hypoglycemic solution indicated that these islets were damaged and broken the mechanism to minimize insulin secretion against the hypoglycemic condition.
In order to confirm in vivo functionality of islets, we performed islet transplantation into STZ-induced diabetic recipient mice. All of diabetic mice were ameliorated of diabetes just like the glycemic control after receiving 200 of the control and hypoxia-4°C islets but were not ameliorated after receiving the same number of the hypoxia-37°C and hypoxic-22°C islets. Taken together, these results demonstrated that low temperature conditions could prevent the loss of islet function caused by a hypoxic environment.
Next we analyzed HMGB1 expression and the amount of released HMGB1 levels from each group of islets. HMGB1 protein was initially found as a DNA-binding protein. It presents in almost all eukaryotic cells to stabilize nucleosome formation and acts as a nuclear factor that enhances gene transcription (22,27,30). It was shown that HMGB1 played a crucial role in response to tissue damage, indicating that HMGB1 was a prototype of the emerging damage-associated molecular pattern molecule (10,27,30). Previously we demonstrated that HMGB1 was released from transplanted mouse islets and released HMGB1 caused the early loss of transplanted islets (19). Proinflammatory cytokine-induced damaged islet cells also released HMGB1 to the extracellular environment (19).
In this study, we demonstrated that hypoxia-37°C islets upregulate the HMGB1 mRNA; however, it was prevented by low temperature conditions. Furthermore, in the intact (control) islets, HMGB1 was mainly stained in the nucleus; however, HMGB1 was presented in not only the nucleus, but also the cytoplasm. In contrast, HMGB1 was mainly stained in nucleus of hypoxia-4°C islets. In addition, the amount of released HMGB1 was significantly decreased in hypoxia-4°C islets compared to that of hypoxia-22°C and hypoxia-37°C islets. The most recent of our experiments have indicated that the amount of released HMGB1 from hypoxia-damaged islets correlates with the length of time islets are subjected to hypoxic conditions and inversely correlated with the viability of islets in a mouse model (Itoh, et al., unpublished data). Therefore, it can be inferred that the low temperature condition can prevent hypoxic islet cell damage. These low levels of HMGB1 released from islets might reduce the inflammatory responses after islet transplantation. The clear success of our in vivo data might be results of not only high islet viability but also due to reduced levels of HMGB1 released from islets. Further investigation is necessary to evaluate the importance of islet viability versus the amount of released HMGB1 on islet graft survival after islet cell transplantation. In this study, we measured not only HMGB1, but also inflammatory cytokines and chemokines, which were previously reported to have been released by damaged islets in humans and mice, including IL-6, G-CSF, KC (CXCL1), RANTES (CCL5), MCP-1 (CCL2), and MIP-1α (CCL3) (1,7,20). The hypoxia-4°C islet group showed significantly lower levels of these cytokine and chemokine productions compare to those of the hypoxia-37°C islet group. Melzi et al. have reported that the amount of released MCP-1 level from human islets was correlated to the outcomes of clinical islet transplantation (20). In the present study, low temperature conditions could prevent MCP-1 release and correlated to the outcomes of islet transplantation in a mouse model.
Most of the HMGB1 was thought to be released passively by damaged islet cells but the precise mechanism is still unclear. In the present study, we demonstrated that HMGB1 mRNA levels were upregulated in damaged islets. Therefore, there is some possibility that hypoxia-induced damaged islets can synthesize the HMGB1. We will analyze the more precise mechanisms of the relationship between HMGB1 expression and islet damage in a future study.
Taken collectively, low temperature conditions could prevent hypoxia-induced islet cell damage, inflammatory reactions in islets, and HMGB1 release and expression. Maintaining low temperature conditions during islet isolation should improve the efficacy of islet isolation in a clinical setting.
Acknowledgments
This study was partially supported by grants from the National Institute of Diabetes and Digestive and Kidney Disease (1R21DK090513-019) (to M.F.L.) and the Juvenile Diabetes Research Foundation (#3-2011-447 to M.T.). The authors thank Ms. Anne-Marie Brun for technical support.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Barbé-Tuana FM, Klein D, Ichii H, Berman DM, Coffey L, Kenyon NS, Ricordi C, Pastori RL. CD40-CD40 ligand interaction activates proinflammatory pathways in pancreatic islets. Diabetes. 2006;55:2437–2445. doi: 10.2337/db05-1673. [DOI] [PubMed] [Google Scholar]
- 2.Brandhorst D, Brandhorst H, Hering BJ, Federlin K, Bretzel RG. Large variability of the intracellular ATP content of human islets isolated from different donors. J Mol Med. 1999;77:93–95. doi: 10.1007/s001090050310. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 4.D’Alessandro AM, Stratta RJ, Sollinger HW, Kalayoglu M, Pirsch JD, Belzer FO. Use of UW solution in pancreas transplantation. Diabetes. 1989;38(Suppl 1):7–9. doi: 10.2337/diab.38.1.s7. [DOI] [PubMed] [Google Scholar]
- 5.Goto T, Tanioka Y, Sakai T, Terai S, Kamoda Y, Li S, Tanaka T, Tsujimura T, Matsumoto I, Fujino Y, Suzuki Y, Kuroda Y. Application of the two-layer method on pancreas digestion results in improved islet yield and maintained viability of isolated islets. Transplantation. 2007;83:754–758. doi: 10.1097/01.tp.0000256338.53305.a9. [DOI] [PubMed] [Google Scholar]
- 6.Hatanaka N, Takita M, Yamaguchi T, Kami M, Matsumoto S. Interests in beta-cell replacement therapies among Japanese patients with type 1 diabetes. Diabetes Res Clin Pract. 2010;89:e5–8. doi: 10.1016/j.diabres.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Klein D, Timoneri F, Ichii H, Ricordi C, Pastori RL. CD40 activation in human pancreatic islets and ductal cells. Diabetologia. 2008;51:1853–1861. doi: 10.1007/s00125-008-1092-y. [DOI] [PubMed] [Google Scholar]
- 8.Lau L, Henriksnas J, Svensson J, Carlsson PO. Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant. 2009;14:688–693. doi: 10.1097/MOT.0b013e32833239ff. [DOI] [PubMed] [Google Scholar]
- 9.Levy MN. Oxygen consumption and blood flow in hypothermic, perfused kidney. Am J Physiol. 1959;197:1111–1114. doi: 10.1152/ajplegacy.1959.197.5.1111. [DOI] [PubMed] [Google Scholar]
- 10.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S. Islet cell transplantation for type 1 diabetes. J Diabetes. 2010;2:16–22. doi: 10.1111/j.1753-0407.2009.00048.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto S, Fujino Y, Suzuki Y, Tanioka Y, Muramatsu S, Sugimoto T, Ku Y, Yasunami Y, Kuroda Y. Evidence of protein synthesis during resuscitation of ischemically damaged canine pancreas by the two-layer method. Pancreas. 2000;20:411–414. doi: 10.1097/00006676-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto S, Kuroda Y. Perfluorocarbon for organ preservation before transplantation. Transplantation. 2002;74:1804–1809. doi: 10.1097/00007890-200212270-00030. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Kuroda Y, Hamano M, Kim Y, Suzuki Y, Saitoh Y. Direct evidence of pancreatic tissue oxygenation during preservation by the two-layer method. Transplantation. 1996;62:1667–1670. doi: 10.1097/00007890-199612150-00023. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto S, Noguchi H, Hatanaka N, Shimoda M, Kobayashi N, Jackson A, Onaca N, Naziruddin B, Levy MF. SUITO index for evaluation of efficacy of single donor islet transplantation. Cell Transplant. 2009;18:557–562. doi: 10.1177/096368970901805-611. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Noguchi H, Shimoda M, Ikemoto T, Naziruddin B, Jackson A, Tamura Y, Olson G, Fujita Y, Chujo D, Takita M, Kobayashi N, Onaca N, Levy M. Seven consecutive successful clinical islet isolations with pancreatic ductal injection. Cell Transplant. 2010;19:291–297. doi: 10.3727/096368909X481773. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Qualley SA, Goel S, Hagman DK, Sweet IR, Poitout V, Strong DM, Robertson RP, Reems JA. Effect of the two-layer (University of Wisconsin solution-perfluorochemical plus O2) method of pancreas preservation method on human islet isolation, as assessed by the Edmonton isolation protocol. Transplantation. 2002;74:1414–1419. doi: 10.1097/00007890-200211270-00013. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Rigley TH, Qualley SA, Kuroda Y, Reems JA, Stevens RB. Efficacy of the oxygen-charged static two-layer method for short-term pancreas preservation and islet isolation from nonhuman primate and human pancreata. Cell Transplant. 2002;11:769–777. [PubMed] [Google Scholar]
- 19.Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, Taniguchi M, Yasunami Y. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120:735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melzi R, Mercalli A, Sordi V, Cantarelli E, Nano R, Maffi P, Sitia G, Guidotti LG, Secchi A, Bonifacio E, Piemonti L. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant. 2010;19:1031–1046. doi: 10.3727/096368910X514639. [DOI] [PubMed] [Google Scholar]
- 21.Moritz W, Meier F, Stroka DM, Giuliani M, Kugelmeier P, Nett PC, Lehmann R, Candinas D, Gassmann M, Weber M. Apoptosis in hypoxic human pancreatic islets correlates with HIF-1alpha expression. FASEB J. 2002;16:745–747. doi: 10.1096/fj.01-0403fje. [DOI] [PubMed] [Google Scholar]
- 22.Müller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255:332–343. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki M, Haga S, Zhang HQ, Irani K, Suzuki S. Inhibition of hypoxia/reoxygenation-induced oxidative stress in HGF-stimulated antiapoptotic signaling: Role of PI3-K and Akt kinase upon rac1. Cell Death Differ. 2003;10:508–515. doi: 10.1038/sj.cdd.4401172. [DOI] [PubMed] [Google Scholar]
- 24.Ploeg RJ, Goossens D, Sollinger HW, Southard JH, Belzer FO. Efficacy of 48-hour pancreas preservation with UW solution in the dog allograft model. Transplant Proc. 1988;20:1026–1028. [PubMed] [Google Scholar]
- 25.Sakata N, Tan A, Chan N, Obenaus A, Mace J, Peverini R, Sowers L, Chinnock R, Hathout E. Efficacy comparison between intraportal and subcapsular islet transplants in a murine diabetic model. Transplant Proc. 2009;41:346–349. doi: 10.1016/j.transproceed.2008.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saldeen J. Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology. 2000;141:2003–2010. doi: 10.1210/endo.141.6.7523. [DOI] [PubMed] [Google Scholar]
- 27.Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 30.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Yakabe K, Ishii J, Imaizumi H, Maruyama I. New high mobility group box 1 assay system. Clin Chim Acta. 2006;372:173–178. doi: 10.1016/j.cca.2006.04.016. [DOI] [PubMed] [Google Scholar]