Abstract
Background:
Erythropoiesis-stimulating agents (ESAs) reduce the need for red blood cell transfusions; however, they increase the risk of thromboembolic events and mortality. The impact of ESAs on quality of life (QoL) is controversial and led to different recommendations of medical societies and authorities in the USA and Europe. We aimed to critically evaluate and quantify the effects of ESAs on QoL in cancer patients.
Methods:
We included data from randomised controlled trials (RCTs) on the effects of ESAs on QoL in cancer patients. Randomised controlled trials were identified by searching electronic data bases and other sources up to January 2011. To reduce publication and outcome reporting biases, we included unreported results from clinical study reports. We conducted meta-analyses on fatigue- and anaemia-related symptoms measured with the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) and FACT-Anaemia (FACT-An) subscales (primary outcomes) or other validated instruments.
Results:
We identified 58 eligible RCTs. Clinical study reports were available for 27% (4 out of 15) of the investigator-initiated trials and 95% (41 out of 43) of the industry-initiated trials. We excluded 21 RTCs as we could not use their QoL data for meta-analyses, either because of incomplete reporting (17 RCTs) or because of premature closure of the trial (4 RCTs). We included 37 RCTs with 10 581 patients; 21 RCTs were placebo controlled. Chemotherapy was given in 27 of the 37 RCTs. The median baseline haemoglobin (Hb) level was 10.1 g dl–1; in 8 studies ESAs were stopped at Hb levels below 13 g dl–1 and in 27 above 13 g dl–1. For FACT-F, the mean difference (MD) was 2.41 (95% confidence interval (95% CI) 1.39–3.43; P<0.0001; 23 studies, n=6108) in all cancer patients and 2.81 (95% CI 1.73–3.90; P<0.0001; 19 RCTs, n=4697) in patients receiving chemotherapy, which was below the threshold (⩾3) for a clinically important difference (CID). Erythropoiesis-stimulating agents had a positive effect on anaemia-related symptoms (MD 4.09; 95% CI 2.37–5.80; P=0.001; 14 studies, n=2765) in all cancer patients and 4.50 (95% CI 2.55–6.45; P<0.0001; 11 RCTs, n=2436) in patients receiving chemotherapy, which was above the threshold (⩾4) for a CID. Of note, this effect persisted when we restricted the analysis to placebo-controlled RCTs in patients receiving chemotherapy. There was some evidence that the MDs for FACT-F were above the threshold for a CID in RCTs including cancer patients receiving chemotherapy with Hb levels below 12 g dl–1 at baseline and in RCTs stopping ESAs at Hb levels above 13 g dl–1. However, these findings for FACT-F were not confirmed when we restricted the analysis to placebo-controlled RCTs in patients receiving chemotherapy.
Conclusions:
In cancer patients, particularly those receiving chemotherapy, we found that ESAs provide a small but clinically important improvement in anaemia-related symptoms (FACT-An). For fatigue-related symptoms (FACT-F), the overall effect did not reach the threshold for a CID.
Keywords: meta-analysis, quality of life, erythropoiesis-stimulating agents
Erythropoiesis-stimulating agents (ESAs) reduce the need for red blood cell transfusions (Bohlius et al, 2006b; Ludwig et al, 2009; Tonelli et al, 2009) and may improve quality of life (QoL); however, they increase the risk of thromboembolic events and death. A large meta-analysis based on individual patient data (IPD) from 53 randomised controlled trials (RCTs) demonstrated a statistically significant, 17% higher risk of mortality during the active study phase in cancer patients who received ESAs compared with controls (Bohlius et al, 2009a, 2009b). An increased risk of mortality was also reported in each of the more recent systematic reviews and meta-analyses, which were not funded by the pharmaceutical industry (Bennett et al, 2008; Tonelli et al, 2009; Tonia et al, 2012; Grant et al, 2013) but in none of the systematic reviews and meta-analyses sponsored by the pharmaceutical industry (Aapro et al, 2008b; Glaspy et al, 2010). Several meta-analyses have shown that ESAs increase the risk of thromboembolic events in cancer patients (Bohlius et al, 2006a, 2006b; Seidenfeld et al, 2006; Aapro et al, 2008b, 2009; Bennett et al, 2008; Ludwig et al, 2009; Tonelli et al, 2009); the effects of ESAs on tumour progression remain uncertain (Aapro et al, 2012).
The impact of ESAs on QoL is controversial. Positive findings from observational studies (Glaspy et al, 1997; Demetri et al, 1998; Gabrilove et al, 2001; Quirt et al, 2001; Cella et al, 2003) and clinical trials (Littlewood et al, 2001; Fallowfield et al, 2002; Chang et al, 2005; Wilkinson et al, 2006) have not been confirmed in more recent RCTs (Smith et al, 2008; Hoskin et al, 2009; Engert et al, 2010; Fujisaka et al, 2011; Nitz et al, 2011). Previous meta-analyses have demonstrated that ESAs effectively reduce fatigue-related symptoms in cancer patients (Minton et al, 2008, 2010; Tonelli et al, 2009). However, these meta-analyses were restricted to the published literature and may be compromised by publication and outcome reporting biases (Egger and Smith, 1998; Dwan et al, 2011; Redmond et al, 2013). Publication bias refers to the fact that studies with positive results are more likely to be published compared with studies with negative results (Egger and Smith, 1998). Outcome reporting bias refers to the selective reporting of outcomes in a published study, where mainly the most statistically significant results or the ones meeting the authors' assumptions are reported (Dwan et al, 2011; Redmond et al, 2013). Meta-analyses including only published results may be prone to bias and overestimate treatment effects.
We aimed to critically evaluate and quantify the effects of ESAs on QoL in cancer. We systematically reviewed and meta-analysed RCTs that compared ESAs with controls in cancer patients. Our objectives were to examine the effects of ESAs on patient-rated fatigue- and anaemia-related symptoms and to identify groups of patients who may benefit most from treatment with ESAs. To reduce potential publication and outcome reporting biases, we included unpublished and unreported data.
Materials and methods
Study selection and data extraction
We included RCTs that compared epoetin or darbepoetin with placebo or best standard of care and assessed fatigue- and anaemia-related symptoms in cancer patients receiving or not receiving anticancer treatment. We excluded trials with high-dose myeloablative chemotherapy regimens followed by stem cell transplantation, trials in patients with myelodysplastic syndromes and acute leukaemia, and trials using ESAs for short-term pre-operative treatment. We included studies that prospectively evaluated QoL using a validated or generally accepted instrument and a planned sample size of >50 participants per study arm or 100 participants in total. Trials using different types of iron supplementation were included and evaluated in stratified analyses.
We updated literature searches from our previous meta-analyses on ESAs (Bohlius et al, 2006a, 2006b, 2009a, 2009b) in Medline, Embase, Cochrane Central Register of Controlled Trials and databases of conference proceedings for the years 2008 to January 2011 (for details, see Supplementary Webappendix Table 1). We screened the reference lists of relevant meta-analyses and clinical trials registries (http://clinicaltrials.gov/; http://www.isrctn.org/). Four reviewers (AM, JB, NR and TT) worked in pairs and independently determined study eligibility. Data on study characteristics, study quality and outcomes were extracted by one reviewer (TT) and checked for accuracy by another (JB). Our primary sources of data extraction were the published study documents. We complemented these data with information from study protocols and reports, which we had obtained from ESA manufacturers (Amgen, Thousand Oaks, CA, USA; Johnson & Johnson, New Brunswick, NJ, USA; Hoffmann-La Roche, Basel, Switzerland) and clinical study groups for a previous IPD meta-analysis (Bohlius et al, 2009a, 2009b). For that meta-analysis, we had identified published and unpublished trials through electronic searches of published abstracts and articles, screening of clinical trials registries and Oncologic Drugs Advisory Committee hearing documents, and contacting ESA manufacturers and experts in the field. We had obtained clinical study reports as requested for 98% (48 out of 49) of the trials initiated by the ESAs manufacturers and 36% (5 out of 14) of the trials run by clinical study groups, for details see Bohlius et al (2009a, 2009b). In addition, we searched for QoL results in clinical trials registries (http://clinicaltrials.gov/; http://www.isrctn.org/).
Outcomes
Our primary outcomes were fatigue- and anaemia-related symptoms measured with the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) subscale and the FACT-Anaemia (FACT-An) subscale. The FACT-F includes 13 fatigue-related questions (range of scale 0–52). The FACT-An (range of scale 0–80) includes the 13 fatigue-related items plus 7 anaemia-related questions, for example, dizziness, headaches, pain in chest and trouble walking. These instruments are widely used in ESA trials, are highly responsive to change, and have good convergent and discriminant validity (Cella, 1997, 2007; Yellen et al, 1997; Cella et al, 2002b). Secondary outcomes included changes in the cancer-specific FACT-G total score (range 0–108) and the subscales on physical, functional and social/family well-being (range 0–28) and emotional well-being (range 0–24). For sensitivity analyses, we included the fatigue- and anaemia-related subscales from studies that used instruments other than FACT-F and FACT-An, that is, EORTC QLQ-C30 (Aaronson et al, 1993), SF-36 (Ware and Sherbourne, 1992), FACT-An subscale non-fatigue items (Cella, 1997), FACT-An full scale (Cella, 1997) and visual analogue scales (VAS) assessing energy, daily activities and overall health or QoL. For each instrument, we predefined the specific domain that best corresponded to fatigue- and anaemia-related symptoms, physical, functional, social/family, emotional well-being and overall QoL as measured by FACT-F, FACT-An, FACT-G and its subscales. We defined a clinically important difference (CID) as a mean difference (MD) of ⩾3 for FACT-F (Cella et al, 2002b) and ⩾4 for FACT-An (D Cella, personal communication, March 2010). For standardised effect sizes, an effect size of 0.20–0.50 s.d. units was considered small but clinically important, whereas effect sizes of 0.50–0.80 and >0.80 were considered to be moderate and large differences, respectively (Sloan and Dueck, 2004; Sloan et al, 2006).
Statistical methods
Results from individual studies were expressed either as differences in mean changes from baseline to study end or as effect sizes. Effect sizes were calculated as the differences in mean values at the end of treatment divided by the pooled s.d. (Cohen's d) (Cohen, 1988). If the required data were not reported, we used approximations (Reichenbach et al, 2007) to calculate differences or s.d. Data were analysed according to the intention-to-treat approach, using the last observation carried forward if data were missing. In sensitivity analyses, we analysed the data measured closest to week 12, a time point frequently considered in ESA trials. We used random-effects meta-analyses to combine trials and quantified heterogeneity with the I2 statistic (Higgins et al, 2003).
In stratified analyses, we aimed to identify patient characteristics, treatment strategies and aspects of study design associated with the effect of ESAs on QoL, see Supplementary Webappendix Table 2. Tests of interactions and trends were obtained from univariate random-effects meta-regression models (Thompson and Sharp, 1999). Analyses were conducted in the entire data set, including all RCTs, only in chemotherapy trials and only in placebo-controlled RCTs in patients receiving chemotherapy. We investigated the association between trial size and treatment effects in funnel plots and regression tests (Sterne and Egger, 2001). To adjust for potential publication bias, we used the trim and fill method (sensitivity analysis) (Duval 2005). Results are presented as MDs or standardised MDs (SMDs) with 95% confidence intervals (95% CIs). We estimated treatment response as the proportion of patients achieving a CID (threshold 3 for FACT-F and 4 for FACT-An subscales). To estimate this treatment response, we used hypothetical control group risks and the SMD and the corresponding 95% CI (Furukawa and Leucht, 2011). We derived numbers needed to treat (NNT) to cause one additional treatment response on FACT-F or FACT-An in patients receiving ESA compared with control from the inverse of the absolute difference between experimental and hypothetical control group risks. Study end points, eligibility criteria, search methods and main analyses were defined in a protocol. All analyses were performed using Stata 10.0 (StataCorp, College Station, TX, USA).
Results
Number of eligible, included and excluded studies
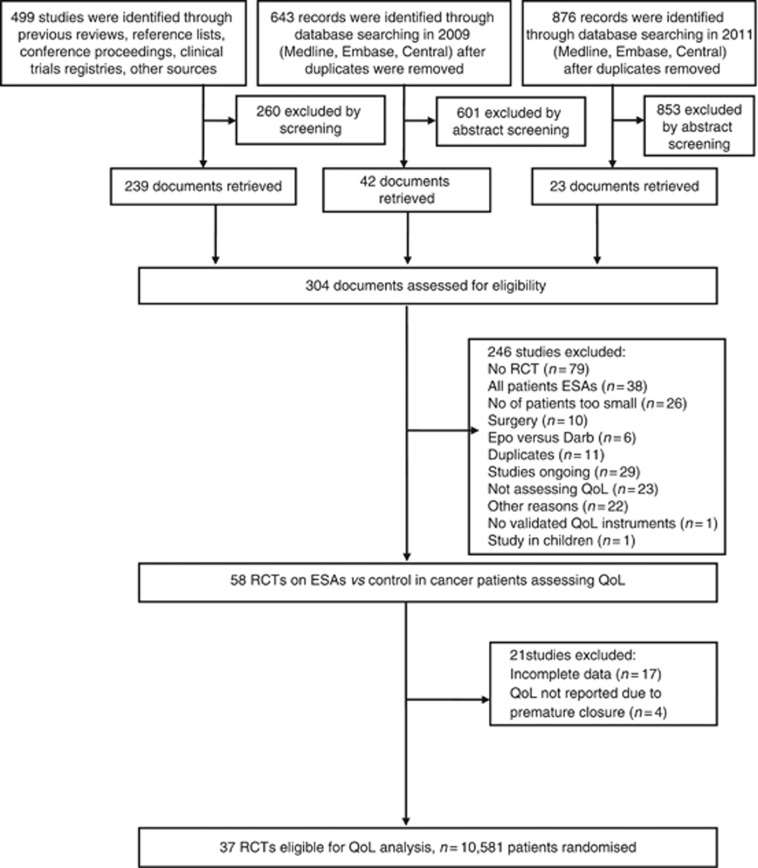
We identified 58 eligible RCTs. Clinical study reports were available for 27% (4 out of 15) of the trials run by clinical study groups and 95% (41 out of 43) of the trials initiated by the ESAs manufacturers. Of the 58 eligible RCTs, we excluded 21 RTCs for the following reasons: QoL data were not reported because of premature closure of the trials (Machtay et al, 2007; Thomas et al, 2008; AGO-OVAR 2.7; CR002305); or data reporting was too incomplete to allow any analysis (Rose et al, 1994; Dammacco et al, 2001; Quirt et al, 2001; Thomas et al, 2002; INT-1; INT-3; Leyland-Jones et al, 2005; Goss et al, 2005; Aapro et al, 2008a; Suzuki et al, 2008; EPO-GER-20; Gupta et al, 2009; Ray-Coquard et al, 2009; Yoshizaki et al, 2010; Untch et al, 2011; CDR0000069148; Moebus et al, 2013) (Figure 1). Finally, we included 37 studies with 10 581 patients randomised (Abels, 1993; Case et al, 1993; Henry and Abels, 1994; Thatcher et al, 1999; Littlewood et al, 2001; Huddart et al, 2002; Kotasek et al, 2002, 2003; Osterborg et al, 2002; Vansteenkiste et al, 2002; Boogaerts et al, 2003; Hedenus et al, 2003; Iconomou et al, 2003; Milroy et al, 2003; P-174; Chang et al, 2005; Debus et al, 2005; Mystakidou et al, 2005; O'Shaughnessy et al, 2005; Savonije et al, 2005; Witzig et al, 2005; Wilkinson et al, 2006; Charu et al, 2007; Wright et al, 2007; Gordon et al, 2008; Krzakowski, 2008; Pirker et al, 2008; Smith et al, 2008; Strauss et al, 2008; Christodoulou et al, 2009; Hernandez et al, 2009; Hoskin et al, 2009; OBE/EPO-INT-03; Tsuboi et al, 2009; Winquist et al, 2009; Engert et al, 2010; Pronzato et al, 2010).
Figure 1.
PRISMA flow diagram showing the identification of eligible trials (Moher et al, 2009).
Characteristics of included studies
Characteristics of included studies are shown in Table 1 and Supplementary Webappendix Tables 3–5. Quality of life was the primary end point in 11 (30%) studies, a secondary end point in 25 trials, and was not mentioned as a study end point in one study. Most studies (n=23) used the FACT-F subscale and/or (n=14) the FACT-An subscale. Among the studies not reporting FACT-F or FACT-An, three studies reported the total score of the full FACT-An scale (47 items), one study used EORTC QLQ-C30, one SF-36 and five studies used VAS. Twenty-one (57%) studies were placebo controlled, 11 (30%) reported sample size calculations for a QoL end point, 9 (24%) defined a QoL hypothesis, 4 (11%) reported definitions for a clinically important change and 4 (11%) reported percentages of patients completing QoL questionnaires (submission rates). Chemotherapy was given in 27 of the 37 studies included (73%). Radiotherapy or radiochemotherapy was given in two studies and no anticancer treatment was given in six (16%). In one study, <70% of the included patients received chemotherapy (P-174) for another study, the underlying anticancer therapy was unclear (Winquist et al, 2009). These two studies were categorised as ‘other/unclear'. Short-acting ESAs (epoetin α, β or δ) were given in 28 (76%) studies and darbepoetin in 9 studies. About half (20 studies, 54%) of the studies included patients with solid tumours; five included patients with haematological malignancies. Of the 37 studies included, 14 studies had a mean/median haemoglobin (Hb) at baseline below 10 g dl–1; the lowest average Hb at baseline was 8.8 g dl–1 (P-174). Seventeen studies included patients with Hb baseline levels between 10 and 12 g dl–1 and six studies included patients with Hb baseline levels above 12 g dl–1. The highest average Hb at baseline was 13.6 g dl–1 (Thatcher et al, 1999; Hoskin et al, 2009). The median baseline Hb level across all trials was 10.1 g dl–1. Most studies gave ESAs for 9–16 weeks (18 studies, 49%) or until the end of chemotherapy (13 studies, 35%). None of the included studies recommended stopping treatment at a Hb level of 12 g dl–1 or below. Eight studies (22%) stopped ESAs at Hb levels ⩽13 g dl–1 and 27 (73%) at Hb levels >13 g dl–1. Two studies did not report the Hb target. Iron supplementations were given according to a patient's transferrin saturation or ferritin levels (29 studies) or according to a fixed schedule (7 studies). All but three studies were funded by the pharmaceutical industry.
Table 1. Characteristics of included randomised controlled trials.
| Characteristic | N of studies (%) |
|---|---|
| Total number of studies |
37 (100) |
| Number of patients randomised (median (range)) |
259 (45–1379) |
| Year of publication (median (range)) |
2005 (1993–2010) |
|
Baseline Hb | |
| ⩽10 g dl–1 | 14 (37.84) |
| 10–12 g dl–1 | 17 (45.95) |
| >12 g dl–1 |
6 (16.22) |
|
Tumour type | |
| Solid | 20 (54.05) |
| Haematological | 5 (13.51) |
| Solid and haematological |
12 (32.43) |
|
Drug | |
| Epoetin α | 23 (62.16) |
| Epoetin β | 4 (10.81) |
| Epoetin δ | 1 (2.70) |
| Darbepoetin |
9 (24.32) |
|
Anticancer treatment | |
| Chemotherapy | 27 (72.97) |
| Radiotherapy | 2 (5.41) |
| No anticancer therapy | 6 (16.22) |
| Other/unclear |
2 (5.41) |
|
Duration of ESA treatment | |
| <9 Weeks | 2 (5.41) |
| 9–16 Weeks | 18 (48.65) |
| ⩾17 Weeks | 4 (10.81) |
| Until end of chemotherapy |
13 (35.14) |
|
Planned weekly ESA dose | |
| <40 000 U epo α/δ or 30,000 U epo β or 100 μg darbepo | 9 (24.32) |
| =40 000 U epo α/δ or 30,000 U epo β or 100 μg darbepo | 9 (24.32) |
| >40 000 U epo α/δ or 30,000 U epo β or 100 μg darbepo | 13 (35.14) |
| Other (e.g., weight based or Hb based) |
6 (16.22) |
|
Frequency of ESA administration | |
| TIW | 19 (51.35) |
| QW | 11 (29.73) |
| ⩽ Q2W | 6 (16.22) |
| Other |
1 (2.70) |
|
Target Hb | |
| ⩽13 g dl–1 | 8 (21.62) |
| >13–15 g dl–1 | 27 (72.97) |
| Not reported |
2 (5.41) |
|
Placebo controlled | |
| Yes | 21 (56.76) |
| No |
16 (43.24) |
|
Study completed? | |
| Terminated/halted | 7 (18.92) |
| Completed |
30 (81.08) |
|
Year last patient randomised | |
| <1995 | 5 (13.51) |
| 1995–1999 | 4 (10.81) |
| 2000–2004 | 18 (48.65) |
| 2005–2009 | 7 (18.92) |
| Not reported |
3 (8.11) |
|
Disease stage | |
| >70% Advanced disease | 16 (43.24) |
| >70% Early disease | 3 (8.11) |
| Other | 7 (18.92) |
| Not reported |
11 (29.73) |
|
QoL primary end point | |
| Yes | 11 (29.73) |
| No |
26 (70.27) |
|
Study industry funded | |
| Yes | 34 (91.89) |
| No | 3 (8.11) |
Abbreviations: ESA=erythropoiesis-stimulating agents; Hb=haemoglobin; QoL=quality of life; QW=once per week; ⩽Q2W=every second week or less frequent; TIW=three times per week.
Main results for the effects of ESAs on QoL
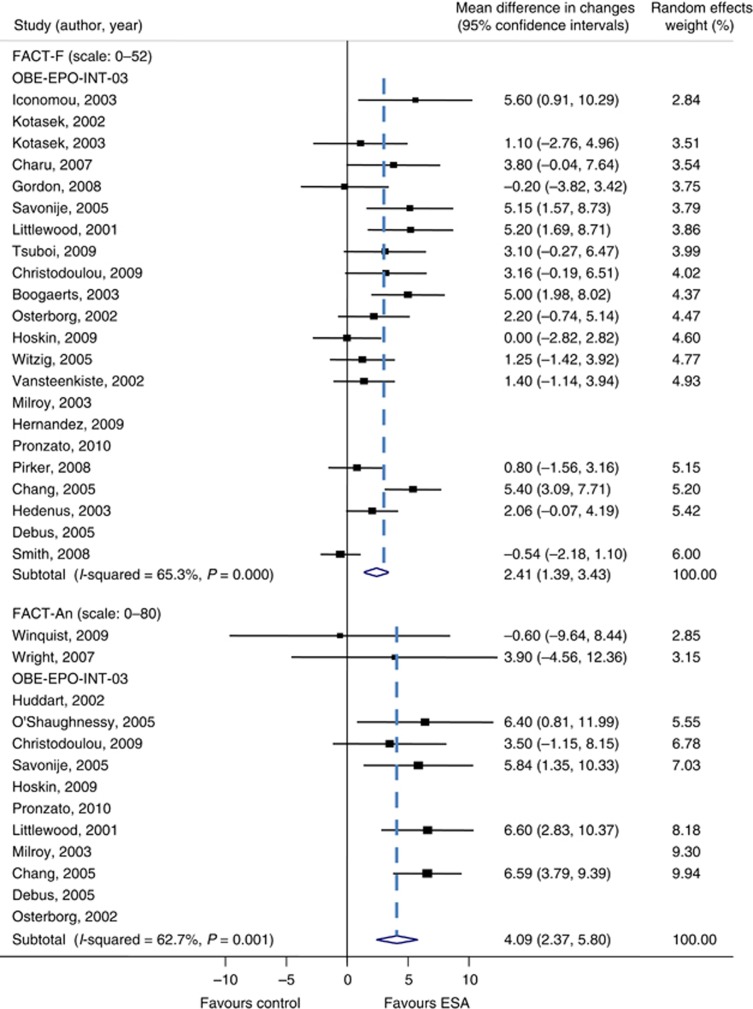
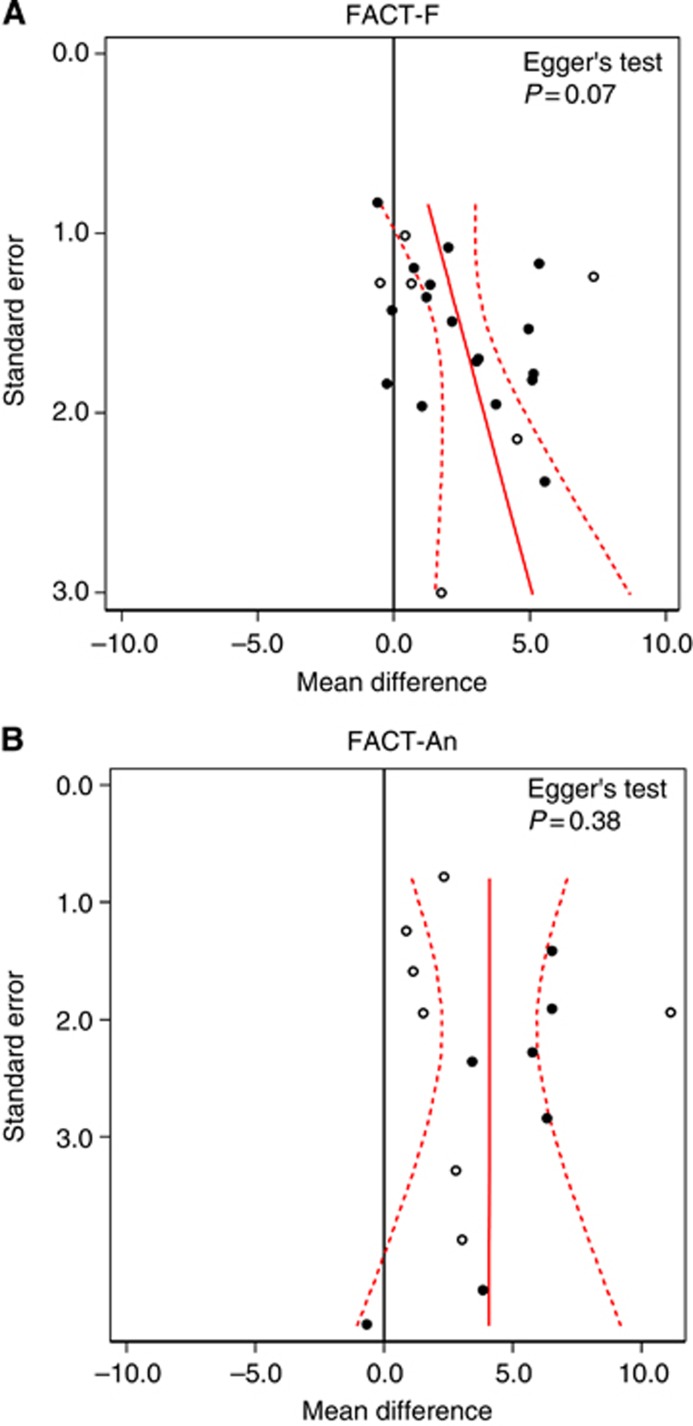
The analysis of FACT-F included results from 23 studies; 17 stemming from the published literature and 6 from clinical study reports. Of the 7624 patients initially randomised, 6108 (80%) were included in the analysis. Fatigue-related symptoms improved in patients receiving ESAs compared with controls, with a MD in FACT-F of 2.41 (95% CI 1.39–3.43, P<0.001; Figure 2), which is below the threshold (⩾3) of a CID. The SMD was 0.22 (95% CI 0.13–0.32, P<0.001). There was moderate heterogeneity among trials (I2=65%) and some evidence for funnel plot asymmetry (Egger's test P=0.07; Figure 3A). The CID for FACT-F (>3) was not reached in any of the analysis subsets, that is, all trials (see above), chemotherapy trials only (MD 2.81, 95% CI 1.73–3.90, 19 studies, n=4697) and placebo-controlled chemotherapy trials (MD 1.78, 95% CI 0.82–2.73, 10 studies, n=2714). The analysis of FACT-An included results from 14 studies; 7 were stemming from the published literature and 7 from clinical study reports. Of 3519 patients randomised, 2765 (79%) were included in the analysis. Anaemia-related symptoms improved with a MD in FACT-An of 4.09 (95% CI 2.37–5.80, P=0.001; Figure 2), which is above the threshold (⩾4) of a CID. The SMD was 0.30 (95% CI 0.17–0.42, P=0.003). There was moderate heterogeneity among trials (I2=63%) and no evidence for funnel plot asymmetry (Egger's test P=0.38; Figure 3B). Of note, the CID for FACT-An (>4) was reached throughout all analysis subsets, that is, all trials (see above), chemotherapy trials only (MD 4.50, 95% CI 2.55–6.45, 11 studies, n=2436) and placebo-controlled chemotherapy trials (MD 4.55, 95% CI 1.29–7.80, 3 studies, n=721). Results for FACT-F and FACT-An were similar when based on the data observed closest to week 12 (see Supplementary Webappendix Table 6). When using trim and fill methods, the overall result for FACT-F was reduced from MD 2.41 (95% CI 1.39 to 3.43) to MD 0.96 (95% CI −0.23 to 2.14); while the overall result for FACT-An remained unchanged (MD 4.09; 95% CI 2.37–5.80). There was little evidence for clinically important improvements in overall QoL, physical, functional, social or emotional well-being measured with FACT-G and its subscales, with MDs ranging from 0.25 (95% CI −0.09 to 0.59) for emotional well-being to 1.45 (95% CI 0.02 to 2.88) for FACT-G overall (Supplementary Webappendix Figure 1). Sensitivity analyses that included studies using other instruments produced similar results for fatigue- or anaemia-related symptoms, overall QoL and subscales (Supplementary Webappendix Table 6).
Figure 2.
Forest plots of the effect of ESAs on QoL end points assessed by scales and subscales of the FACT questionnaire. Each solid square represents the SMD between groups for individual trials, and the size of the square represents the weight of the individual study in the meta-analysis. Horizontal lines indicate 95% CIs. The dashed vertical lines indicate the thresholds for clinical important differences for FACT-F (⩾3) and FACT-An (⩾4). The width of the diamond shows the 95% CI for the pooled SMD. Trials are sorted by weight. Confidential data are masked.
Figure 3.
Funnel plots for FACT-F (A) and FACT-An (B). Closed circles=results from published literature, open circles=results from clinical study reports.
Stratified analyses for FACT-F and FACT-An
We conducted stratified analyses to identify groups of patients and treatment strategies in which ESAs had more effect on fatigue- and anaemia-related symptoms. Concerning fatigue-related symptoms, patients receiving chemotherapy showed more pronounced effects than patients receiving radiotherapy or no therapy (test for interaction P=0.079); however, in patients receiving chemotherapy the threshold for a CID was not met, see Table 2. Within the group of chemotherapy studies, trials including patients with Hb baseline below 12 g dl–1 achieved differences above the CID threshold in contrast to studies with Hb baseline above 12 g dl–1, however, the difference between these groups of trials was not statistically significant (P for interaction 0.11). Chemotherapy trials stopping ESAs at Hb levels >13 g dl–1 achieved differences above the CID threshold in contrast to studies stopping ESAs at Hb levels ⩽13 g dl–1; however, differences between these groups of studies were of borderline statistical significance (P for interaction 0.053). When we restricted the analysis to placebo-controlled chemotherapy trials, the MDs for FACT-F in trials including patients with Hb <12 g dl–1 at baseline and trials stopping ESAs at Hb levels >13 g dl–1 were below the CID threshold. The beneficial effect of ESAs on fatigue increased with the number of injections per week (test for trend P=0.032 in all trials and P=0.044 in chemotherapy trials). When we further restricted this analysis to placebo-controlled trials, the test for trend was not statistically significant (test for trend P=0.134). We observed that open-label studies showed MDs for FACT-F, which were above the CID and larger than the results stemming from placebo-controlled trials (test for interaction P=0.054 in all trials and P=0.083 in chemotherapy trials). There was some evidence that trials with QoL as primary end point achieved better results than trials with QoL as secondary end point (test for interaction P=0.027 in all trials and P=0.091 in chemotherapy trials), however, this was not confirmed when we further restricted the analysis to placebo-controlled trials. Of note, the CID was not met in trials in which a majority of patients had advanced disease (>70% of patients with metastatic/advanced disease). Results stemming from full publications and clinical study reports were similar. Studies with and without industry funding reported similar effect estimates. The corresponding results for anaemia-related symptoms (FACT-An) were similar to the FACT-F results, but were based on fewer trials; and tests for interaction failed to reach conventional levels of statistical significance, see Table 3. Additional stratified analyses for FACT-F and FACT-An are shown in Supplementary Webappendix Tables 7 and 8.
Table 2. Stratified analyses for FACT-F in (i) all included RCTs, (ii) RCTs in patients receiving chemotherapy and (iii) placebo-controlled RCTs in patients receiving chemotherapy.
| |
All RCTs |
Chemotherapy RCTs |
Placebo-controlled chemotherapy RCTs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FACT-F | Studies/ESA/control | MD (95% CI) | P-valuea | Studies/ESA/control | MD (95% CI) | P-valuea | Studies/ESA/control | MD (95% CI) | P-valuea |
| Overall |
23/3389/2719 |
2.41 (1.39 to 3.43) |
|
19/2566/2131 |
2.81 (1.73 to 3.90) |
|
10/1543/1171 |
1.78 (0.82 to 2.73) |
|
| Anticancer treatment |
|
|
0.218 |
|
|
NA |
|
|
NA |
| Chemotherapy | 19/2566/2131 | 2.81 (1.73 to 3.90) | 19/2566/2131 | 2.81 (1.73 to 3.90) | 10/1543/1171 | 1.78 (0.82 to 2.73) | |||
| Radiotherapy | 1/127/134 | 0.00 (−2.82 to 2.82) | — | — | — | — | |||
| None |
3/696/454 |
0.62 (−1.83 to 3.07) |
|
— |
— |
|
— |
— |
|
| Anticancer treatment (condensed) |
|
|
0.079 |
|
|
NA |
|
|
NA |
| Chemotherapy | 19/2566/2131 | 2.81 (1.73 to 3.90) | 19/2566/2131 | 2.81 (1.73 to 3.90) | 10/1543/1171 | 1.78 (0.82 to 2.73) | |||
| Radiotherapy, none |
4/823/588 |
0.28 (−1.34 to 1.90) |
|
— |
— |
|
— |
— |
|
| Baseline Hb |
|
|
0.424 |
|
|
0.362 |
|
|
0.153 |
| >12 g dl–1 | 3/474/478 | 0.43 (−0.94 to 1.80) | 2/347/344 | 0.56 (−1.01 to 2.13) | — | — | |||
| 10–12 g dl–1 | 12/1535/1172 | 3.07 (1.47 to 4.67) | 10/1182/1080 | 3.31 (1.52 to 5.09) | 4/596/573 | 0.97 (−0.34 to 2.28) | |||
| ⩽10 g dl–1 |
8/1380/1069 |
2.32 (0.74 to 3.90) |
|
7/1037/707 |
2.82 (1.57 to 4.08) |
|
6/947/598 |
2.41 (1.19 to 3.62) |
|
| Baseline Hb (condensed) |
|
|
0.098 |
|
|
0.11 |
|
|
NA |
| >12 g dl–1 | 3/474/478 | 0.43 (−0.94 to 1.80) | 2/347/344 | 0.56 (−1.01 to 2.13) | |||||
| ⩽12 g dl–1 |
20/2915/2241 |
2.78 (1.64 to 3.92) |
|
17/2219/1787 |
3.15 (2.00 to 4.29) |
|
10/1543/1171 |
1.78 (0.82 to 2.73) |
|
| Disease stage |
|
|
0.025b |
|
|
0.005b |
|
|
0.225b |
| >70% not metastatic/advanced | 1/168/170 | 5.40 (3.09 to 7.71) | 1/168/170 | 5.40 (3.09 to 7.71) | — | — | |||
| >70% metastatic/advanced | 11/1977/1650 | 1.15 (0.21 to 2.08) | 10/1634/1288 | 1.41 (0.49 to 2.32) | 7/1131/879 | 1.40 (0.37 to 2.42) | |||
| Other | 6/699/476 | 3.12 (0.31 to 5.93) | 3/219/250 | 5.59 (2.93 to 8.25) | — | — | |||
| Unknownc |
5/545/423 |
3.21 (1.64 to 4.77) |
|
5/545/423 |
3.21 (1.64 to 4.77) |
|
3/412/292 |
2.95 (0.68 to 5.23) |
|
| Frequency |
|
|
0.032b |
|
|
0.044b |
|
|
0.134b |
| ⩽Q2W | 6/1170/685 | 0.85 (−0.79 to 2.49) | 3/474/231 | 1.37 (−1.49 to 4.23) | 3/474/231 | 1.37 (−1.49 to 4.23) | |||
| QW | 7/849/848 | 2.20 (0.81 to 3.59) | 7/849/848 | 2.20 (0.81 to 3.59) | 4/491/481 | 1.85 (0.56 to 3.14) | |||
| TIW | 9/1125/947 | 3.72 (1.91 to 5.54) | 8/998/813 | 4.22 (2.47 to 5.97) | 2/333/220 | 3.54 (0.62 to 6.46) | |||
| Otherc |
1/245/239 |
0.80 (−1.56 to 3.16) |
|
1/245/239 |
0.80 (−1.56 to 3.16) |
|
1/245/239 |
0.80 (−1.56 to 3.16) |
|
| Target Hb |
|
|
0.008 |
|
|
0.053 |
|
|
0.105 |
| >13–15 g dl–1 | 17/2486/1903 | 3.00 (1.91 to 4.09) | 15/2156/1727 | 3.17 (2.02 to 4.31) | 9/1380/1019 | 2.06 (1.11 to 3.01) | |||
| ⩽13 g dl–1 | 5/846/761 | −0.13 (−1.20 to 0.93) | 3 /353/349 | 0.22 (−1.29 to 1.74) | 1/163/152 | −0.45 (−2.97 to 2.07) | |||
| Not reportedc |
1/57/55 |
5.60 (0.91 to 10.29) |
|
1/57/55 |
5.60 (0.91 to 10.29) |
|
— |
— |
|
| Placebo control |
|
|
0.054 |
|
|
0.083 |
|
|
NA |
| Yes | 12/2036/1583 | 1.36 (0.39 to 2.34) | 10/1543/1171 | 1.78 (0.82 to 2.73) | 10/1543/1171 | 1.78 (0.82 to 2.73) | |||
| No |
11/1353/1136 |
3.46 (1.77 to 5.16) |
|
9/1023/960 |
3.85 (1.96 to 5.74) |
|
— |
— |
|
| QoL primary end point |
|
|
0.027 |
|
|
0.091 |
|
|
0.724 |
| Yes | 8/850/878 | 3.87 (1.98 to 5.76) | 8/850/878 | 3.87 (1.98 to 5.76) | 1/151/148 | 1.25 (−1.42 to 3.92) | |||
| No |
15/2539/1841 |
1.53 (0.58 to 2.48) |
|
11/1716/1253 |
1.93 (0.88 to 2.98) |
|
9/1392/1023 |
1.87 (0.80 to 2.95) |
|
| Source of data |
|
|
0.907 |
|
|
0.537 |
|
|
0.446 |
| Full publication | 17/2628/2053 | 2.41 (1.35 to 3.47) | 13/1805/1465 | 2.98 (1.97 to 4.00) | 8/1258/990 | 1.92 (0.94 to 2.90) | |||
| Clinical study report |
6/761/666 |
2.36 (−0.37 to 5.10) |
|
6/761/666 |
2.36 (−0.37 to 5.10) |
|
2/285/181 |
1.78 (−3.14 to 6.69) |
|
| Study industry funded |
|
|
0.362 |
|
|
0.476 |
|
|
NA |
| Yes | 21/3256/2588 | 2.29 (1.22 to 3.35) | 17/2433/2000 | 2.70 (1.54 to 3.85) | 10/1543/1171 | 1.78 (0.82 to 2.73) | |||
| No | 2/133/131 | 3.99 (1.26 to 6.71) | 2/133/131 | 3.99 (1.26 to 6.71) | — | — | |||
Abbreviations: CI=confidence interval; ESA=erythropoiesis-stimulating agents; FACT-F=Functional Assessment of Cancer Therapy-Fatigue subscale; Hb=haemoglobin; MD=mean difference; NA=not applicable; QoL=quality of life; RCT=randomised controlled trial.
Frequency: ⩽Q2W=every second week or less frequent, QW=once per week, TIW=three times per week, other=frequency changing during the study.
Planned weekly ESA dose: high=>40 000 U epoetin α/δ or 30 000 U epoetin β or 100 μg darbepoetin, middle=40 000 U epoetin α/δ or 30 000 U epoetin β or 100 μg darbepoetin, low=<40 000 U epoetin α/δ or 30 000 U epoetin β or 100 μg darbepoetin, other=weight based or Hb based.
P-value: refers to test for interaction unless otherwise specified.
Test for trend.
Not used for interaction/trend test.
Table 3. Stratified analyses for FACT-An in (i) all included RCTs, (ii) RCTs in patients receiving chemotherapy and (iii) placebo-controlled RCTs in patients receiving chemotherapy.
| |
All RCTs |
Chemotherapy RCTs |
Placebo-controlled chemotherapy RCTs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FACT-An | Studies/ESA/control | MD (95% CI) | P-valuea | Studies/ESA/control | MD (95% CI) | P-valuea | Studies/ESA/control | MD (95% CI) | P-valuea |
| Overall |
14/1466/1299 |
4.09 (2.37 to 5.80) |
|
11/1310/1126 |
4.50 (2.55 to 6.45) |
|
3/413/308 |
4.55 (1.29 to 7.80) |
|
| Anticancer treatment |
|
|
0.709 |
|
|
NA |
|
|
NA |
| Chemotherapy | 11/1310/1126 | 4.50 (2.55 to 6.45) | 11/1310/1126 | 4.50 (2.55 to 6.45) | 3/413/308 | 4.55 (1.29 to 7.80) | |||
| Radiotherapy | 1/126/133 | 1.60 (−2.24 to 5.44) | — | — | — | — | |||
| None | 1/14/20 | 3.90 (−4.56 to 12.36) | — | — | — | — | |||
| Unclearb |
1/16/20 |
−0.60 (−9.64 to 8.44) |
|
— |
— |
|
— |
— |
|
| Anticancer treatment (condensed) |
|
|
0.458 |
|
|
NA |
|
|
NA |
| Chemotherapy | 11/1310/1126 | 4.50 (2.55 to 6.45) | 11/1310/1126 | 4.50 (2.55 to 6.45) | 3/413/308 | 4.55 (1.29 to 7.80) | |||
| Radiotherapy, none | 2/140/153 | 1.99 (−1.50 to 5.49) | — | — | — | — | |||
| Unclearb |
1/16/20 |
−0.60 (−9.64 to 8.44) |
|
— |
— |
|
— |
— |
|
| Baseline Hb |
|
|
0.389 |
|
|
0.567 |
|
|
0.695 |
| >12 g dl–1 | 4/511/514 | 1.64 (−0.09 to 3.36) | 3/385/381 | 1.91 (−0.58 to 4.39) | 1/40/42 | 6.40 (0.81 to 11.99) | |||
| 10–12 g dl–1 | 7/540/477 | 5.89 (3.31 to 8.48) | 5/510/437 | 6.57 (3.83 to 9.31) | — | — | |||
| <10 g dl–1 |
3/415/308 |
3.76 (0.87 to 6.64) |
|
3/415/308 |
3.76 (0.87 to 6.64) |
|
2/373/266 |
4.14 (0.08 to 8.19) |
|
| Baseline Hb (condensed) |
|
|
0.087 |
|
|
0.137 |
|
|
0.695 |
| >12 g dl–1 | 4/511/514 | 1.64 (−0.09 to 3.36) | 3/385/381 | 1.91 (−0.58 to 4.39) | 1/40/42 | 6.40 (0.81 to 11.99) | |||
| ⩽12 g dl–1 |
10/955/785 |
5.09 (2.89 to 7.29) |
|
8/925/745 |
5.44 (3.04 to 7.83) |
|
2/373/266 |
4.14 (0.08 to 8.19) |
|
| Disease stage |
|
|
0.06c |
|
|
0.064c |
|
|
0.277c |
| >70% not metastatic/advanced | 2/208/212 | 6.55 (4.05 to 9.06) | 2/208/212 | 6.55 (4.05 to 9.06) | 1/40/42 | 6.40 (0.81 to 11.99) | |||
| >70% metastatic/advanced | 7/741/661 | 2.14 (1.01 to 3.28) | 5/711/621 | 2.15 (0.98 to 3.32) | 1/173/176 | 2.40 (0.84 to 3.96) | |||
| Other | 3/254/273 | 5.50 (−1.43 to 12.43) | 2/128/140 | 7.85 (0.03 to 15.67) | — | — | |||
| Unknownb |
2/263/153 |
5.36 (2.39 to 8.34) |
|
2/263/153 |
5.36 (2.39 to 8.34) |
|
1/200/90 |
6.60 (2.83 to 10.37) |
|
| Frequency |
|
|
0.992c |
|
|
0.801c |
|
|
0.64c |
| QW | 5/409/424 | 4.11 (0.96 to 7.25) | 4/395/404 | 4.16 (0.55 to 7.76) | 1/40/42 | 6.40 (0.81 to 11.99) | |||
| TIW |
9/1057/875 |
4.09 (1.84 to 6.34) |
|
7/915/722 |
4.73 (2.10 to 7.36) |
|
2/373/266 |
4.14 (0.08 to 8.19) |
|
| Target Hb |
|
|
0.25 |
|
|
0.201 |
|
|
NA |
| >13–15 g dl–1 | 12/1279/1107 | 4.52 (2.63 to 6.41) | 9/1123/934 | 5.10 (2.92 to 7.29) | 3/413/308 | 4.55 (1.29 to 7.80) | |||
| ⩽13 g dl–1 |
2/187/192 |
1.13 (−1.22 to 3.47) |
|
2/187/192 |
1.13 (−1.22 to 3.47) |
|
— |
— |
|
| Placebo control |
|
|
0.985 |
|
|
0.912 |
|
|
NA |
| Yes | 5/443/348 | 3.91 (1.49 to 6.32) | 3/413/308 | 4.55 (1.29 to 7.80) | 3/413/308 | 4.55 (1.29 to 7.80) | |||
| No |
9/1023/951 |
4.12 (1.67 to 6.56) |
|
8/897/818 |
4.46 (1.74 to 7.17) |
|
— |
— |
|
| QoL primary end point |
|
|
0.471 |
|
|
0.489 |
|
|
NA |
| Yes | 7/568/591 | 4.69 (1.48 to 7.91) | 5/538/551 | 5.29 (1.58 to 9.00) | — | — | |||
| No |
7/898/708 |
3.21 (1.53 to 4.90) |
|
6/772/575 |
3.55 (1.60 to 5.50) |
|
3/413/308 |
4.55 (1.29 to 7.80) |
|
| Source of data |
|
|
0.229 |
|
|
0.24 |
|
|
0.259 |
| Full publication | 7/652/469 | 5.71 (4.02 to 7.39) | 5/622/429 | 6.02 (4.27 to 7.77) | 2/240/132 | 6.54 (3.41 to 9.66) | |||
| Clinical study report |
7/814/830 |
3.18 (0.72 to 5.64) |
|
6/688/697 |
3.48 (0.63 to 6.33) |
|
1/173/176 |
2.40 (0.84 to 3.96) |
|
| Study industry funded |
|
|
0.864 |
|
|
0.777 |
|
|
NA |
| Yes | 13/1403/1236 | 4.13 (2.30 to 5.97) | 10/1247/1063 | 4.61 (2.50 to 6.72) | 3/413/308 | 4.55 (1.29 to 7.80) | |||
| No | 1/63/63 | 3.50 (−1.15 to 8.15) | 1/63/63 | 3.50 (−1.15 to 8.15) | — | — | |||
Abbreviations: CI=confidence interval; ESA=erythropoiesis-stimulating agents; FACT-An=Functional Assessment of Cancer Therapy-Anaemia subscale; Hb=haemoglobin; MD=mean difference; NA=not applicable; QoL=quality of life; RCT=randomised controlled trial.
Frequency: ⩽Q2W=every second week or less frequent, QW=once per week, TIW=three times per week, other=frequency changing during the study.
Planned weekly ESA dose: high ⩾40 000 U epoetin α/δ or 30 000 U epoetin β or 100 μg darbepoetin, middle=40 000 U epoetin α/δ or 30 000 U epoetin β or 100 μg darbepoetin, low=<40 000 U epoetin α/δ or 30 000 U epoetin β or 100 μg darbepoetin, other=weight based or Hb based.
P-value: refers to test for interaction unless otherwise specified.
Not used for interaction/trend test.
Test for trend.
Percentage of patients achieving a CID and NNT
We estimated the percentage of patients achieving a CID and corresponding NNTs based on hypothetical control groups. With a hypothetical response rate of 20% in the control group, the response rate in patients receiving ESAs is 27% (95% CI 24%–30%) for FACT-F and 29% (95% CI 25%–34%) for FACT-An with corresponding NNTs of 14 (95% CI 10–26) and 10 (95% CI 7–19). With a hypothetical response rate of 40% in the control group, the response rate in patients receiving ESAs is 49% (95% CI 45–52) for FACT-F and 52% (95% CI 47%–57%) for FACT-An with corresponding NNTs of 11 (95% CI 8–19) and 8 (95% CI 5–14).
Discussion
We found that ESAs provide a small but clinically important improvement in anaemia-related symptoms (FACT-An), which was confirmed when the analysis was restricted to placebo-controlled RCTs in patients receiving chemotherapy. For fatigue-related symptoms (FACT-F), the overall effect did not reach the threshold for a CID. For FACT-F, there was some evidence that treatment effects were above the threshold for a CID in RCTs in patients receiving chemotherapy with Hb levels below 12 g dl–1 at baseline and in RCTs stopping ESAs at Hb levels above 13 g dl–1. However, these findings for FACT-F were not confirmed when we restricted the analysis to placebo-controlled RCTs in patients receiving chemotherapy.
To reduce publication and outcome reporting biases, we included unpublished and unreported results from clinical study reports. This allowed us to include more studies and patients than previous meta-analyses (Tonelli et al, 2009; Minton et al, 2010; Tonia et al, 2012; Grant et al, 2013), and to explore the effects of ESAs in different populations. Clinical study reports are not peer reviewed and the quality of these reports and the validity of the reported study data is uncertain. However, when we restricted our analyses to data from full publications our overall conclusions did not change. Similarly, our conclusions did not change when we conducted trim and fill analyses to adjust for potential publication bias. Our study does not confirm a CID for FACT-F, which had been reported in two previous literature-based meta-analyses (Minton et al, 2008, 2010; Tonelli et al, 2009). Our findings for FACT-An are more conservative compared with a recent meta-analysis based on the published literature (Tonia et al, 2012). To account for the current licensed indication and to reduce the influence of placebo effects (a potential bias in self-reported measures such as fatigue- and anaemia-related symptoms), we conducted additional analyses restricted to (1) chemotherapy RCTs regardless of blinding and (2) only placebo-controlled chemotherapy RCTs. However, there were only few placebo-controlled RCTs reporting QoL outcomes for patients receiving chemotherapy, which limited our ability to conduct stratified analyses in this setting. For example, both in the overall analyses and in those restricted to chemotherapy studies, FACT-F results were more favourable in studies that chose QoL as primary end point, compared with those that chose QoL as secondary end point. Only one study evaluating FACT-F as primary end point in patients receiving chemotherapy was placebo controlled, and so we cannot gauge the extent to which the effect observed for primary vs secondary end point was confounded by lack of blinding. The design of the included studies did not permit us to estimate the relative benefit of ESAs in Hb responders vs non-responders. This would have required RCTs that identified responders in a run in period and then randomised these responders to either stop or continue ESAs. Finally, decreased QoL in cancer patients is affected by factors other than anaemia. Correction of a single factor, as did the studies included in our meta-analyses, may not have adequately reflected the complex pathophysiological and psychological dimensions of patient-reported QoL.
Several limitations of our study underscore the need for open access to all clinical trials results including study protocols, amendments, reports and IPD as currently discussed at the European Medicines Agency (Eichler et al, 2012). First, the quality of reporting QoL data was low. Both in the published articles and the clinical study reports key information such as percentage of patients completing QoL questionnaires was missing or not clearly reported for the majority of studies. Critical review of clinical study documents by the academic community may help to improve the quality of reporting in these reports, which will only be possible with open access to these documents. Second, we identified another 16 trials (Kotasek et al, 2002, 2003; Thomas et al, 2002; Vansteenkiste et al, 2002; Boogaerts et al, 2003; Hedenus et al, 2003; Goss et al, 2005; Mystakidou et al, 2005; Witzig et al, 2005; Wilkinson et al, 2006; Aapro et al, 2008a; Gordon et al, 2008; Krzakowski, 2008; Pirker et al, 2008; Strauss et al, 2008; EPO-GER-20, 2009a) measuring FACT-An that did not or only incompletely report their FACT-An results and could therefore not be included in our analyses. Access to IPD may have permitted to include these studies in our analysis and it is possible that including these studies would change the results of our analyses. We unsuccessfully tried to retrieve the IPD and hence evaluated unpublished aggregated QoL data found in clinical study reports. However, for results, which were not reported in these documents, we made no additional attempts to obtain these results from the investigators. We also assessed whether QoL results had been published in clinical trials registries, which was not the case. Finally, our analyses are based on aggregated data and therefore analyses of variables at patient level, such as Hb at baseline and stage of disease, are prone to ecological bias (Berlin et al, 2002). This limitation could be overcome with a meta-analysis based on IPD, but this was not available for the current analyses.
When judging the efficacy of ESAs on fatigue- and anaemia-related symptoms, it is important to differentiate clinical from statistical significance. The concept of CIDs has been developed to address this problem (Cella et al, 2002a). However, defining CIDs is not straightforward. Depending on the clinical context and the methods selected, the threshold for CID could be set at different levels. For our primary analyses, we used the definition of Cella et al (2002b), which was developed to combine anchor- and distribution-based methods in populations similar to those we studied. Notably, the CIDs defined for FACT-F and FACT-An refer to changes from baseline to end of treatment. In our analyses, we used this yardstick to measure the differences in mean changes between groups from baseline to treatment, according to current practice in QoL studies (Tonelli et al, 2009; Minton et al, 2010).
Harmful effects of ESAs should be balanced against potential benefits. Previous meta-analyses have consistently shown that ESAs increase the risk of thromboembolic events in cancer patients by approximately factor 1.6 (Bohlius et al, 2006a, 2006b; Seidenfeld et al, 2006; Aapro et al, 2008b, 2009; Bennett et al, 2008; Ludwig et al, 2009; Tonelli et al, 2009). Literature-based and IPD meta-analyses showed increased mortality (Bohlius et al, 2009a, 2009b) or shortened overall survival in patients receiving ESAs (Bennett et al, 2008; Tonelli et al, 2009). Whether ESAs are safe for patients undergoing chemotherapy is a matter of debate. Our meta-analyses, and those of others based on IPD, have shown that ESAs increased short-term mortality in patients receiving chemotherapy by approximately 10% (Bohlius et al, 2009a, 2009b; Ludwig et al, 2009), not reaching conventional levels of statistical significance. Statistically, the estimated mortality increase in chemotherapy trials can be explained by the same underlying effect as that in non-chemotherapy trials (Bohlius et al, 2009a, 2009b). Clinically, the increase in mortality associated with ESAs may be less pronounced, or even absent, in patients receiving chemotherapy than in those undergoing other anticancer treatments. Two recent studies in cancer patients receiving chemotherapy did not find evidence for survival differences in patients receiving ESAs compared with controls (Engert et al, 2010; Moebus et al, 2013). In these studies, cancer patients were receiving chemotherapy with a curative intent and ESAs were stopped at Hb levels of 12 g dl–1 (Engert et al, 2010) and 14 g dl–1 (Moebus et al, 2013). Nevertheless, current evidence does not allow to conclude that ESAs are safe in patients receiving chemotherapy. Basic science studies have evaluated the presence of erythropoietin (EPO) receptors and its functionality in tumour cells (Arcasoy et al, 2005; Szenajch et al, 2010; Kumar et al, 2012). Interestingly, researchers without funding from ESA manufacturers were more likely to identify EPO receptors on cancer cells, EPO-induced signalling events or EPO-induced harmful changes of cellular function; or to conclude that ESAs had potentially harmful effects on cancer cells as compared with investigators receiving funding or being employed by ESA manufacturers (Bennett et al, 2010). Similarly, of the seven meta-analyses on the effects of ESAs in cancer patients conducted since 2008 none of the meta-analyses with funding from ESA manufacturers identified an increased mortality risk (Aapro et al, 2008b; Glaspy et al, 2010). In contrast, each of the meta-analyses conducted by researchers not receiving funding from ESA manufacturers found an increased risk either for on study mortality or overall survival (Bennett et al, 2008; Bohlius et al, 2009a, 2009b; Tonelli et al, 2009; Tonia et al, 2012; Grant et al, 2013). This observation highlights the importance of conflicts of interest both in the clinical and the basic sciences. In the case of ESAs and mortality in cancer patients, this led to misleading results and conclusions in meta-analyses funded by the pharmaceutical industry. Of note, in our analyses we found no evidence that results from industry-funded studies differed from those not funded by the industry. However, this may be due to a lack of power in a setting were >90% of studies were funded by the industry.
These observations on the harmful effects of ESAs in cancer patients led to different recommendations of medical societies and authorities in the USA and Europe (Information for Health Professions, 2007; Aapro and Link, 2008; Rizzo et al, 2010; Schrijvers et al, 2010). The FDA and the American Societies of Clinical Oncology (ASCO) and Hematology (ASH) recommend the use of ESAs only in anaemic cancer patients receiving chemotherapy (Rizzo et al, 2010) with palliative treatment intent (Information for Health Professions, 2007) up to Hb level 12 g dl–1 (Information for Health Professions, 2007) with the goal of avoiding red blood cell transfusions (Information for Health Professions, 2007; Rizzo et al, 2010). The FDA and ASCO/ASH explicitly do not recommend the use of ESAs to improve QoL because they consider the evidence inconclusive (Information for Health Professions, 2007; Rizzo et al, 2010). Similarly, in 2007, the FDA removed the claim for ESA-related QoL improvements in patients with chronic kidney disease from the product labels because of a lack of evidence from well-conducted trials. In contrast, the European Organization for Research and Treatment of Cancer (Aapro and Link, 2008) and the European Society of Medical Oncology (Schrijvers et al, 2010) recommend the use of ESAs to improve QoL in cancer patients.
Our overall analyses showed a small yet clinically important improvement for FACT-An, which was confirmed when the analysis was restricted to placebo-controlled RCTs in patients receiving chemotherapy. Of 100 patients treated, approximately 10 to 13 patients will have a clinically important improvement of anaemia-related symptoms, which can be attributed to ESA treatment. However, in patients treated with a curative approach it is unlikely that the observed benefits will outweigh the negative effects of ESAs on short-term mortality and thromboembolic events. Studies in cancer patients receiving chemotherapy with a palliative intent and receiving ESAs in accordance to current guideline recommendations (i.e., starting ESAs at Hb <10 g dl–1 and stopping at 12 g dl–1) and reporting QoL outcomes were not available. In this setting, the impact of ESAs on QoL remains unclear.
Conclusion
We found that ESAs provide a small but clinically important improvement in anaemia-related symptoms (FACT-An). For fatigue-related symptoms (FACT-F), the overall effect did not reach the threshold for a CID.
Acknowledgments
Annette Mettler (AM) and Nadège Robert (NR) screened references and assessed studies for eligibility. Martin Adam developed the EpiData format for data extraction. We thank Kali Tal for her editorial work. This study was funded by OncoSuisse, grant number OCS-02232-04-2008.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Aapro M, Jelkmann W, Constantinescu SN, Leyland-Jones B. Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer. Br J Cancer. 2012;106 (7):1249–1258. doi: 10.1038/bjc.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aapro M, Leonard RC, Barnadas A, Marangolo M, Untch M, Malamos N, Mayordomo J, Reichert D, Pedrini JL, Ukarma L, Scherhag A, Burger HU. Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol. 2008;26 (4):592–598. doi: 10.1200/JCO.2007.11.5378. [DOI] [PubMed] [Google Scholar]
- Aapro M, Osterwalder B, Scherhag A, Burger HU. Epoetin-beta treatment in patients with cancer chemotherapy-induced anaemia: the impact of initial haemoglobin and target haemoglobin levels on survival, tumour progression and thromboembolic events. Br J Cancer. 2009;101 (12):1961–1971. doi: 10.1038/sj.bjc.6605255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aapro M, Scherhag A, Burger HU. Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patients. Br J Cancer. 2008;99 (1):14–22. doi: 10.1038/sj.bjc.6604408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist. 2008;13 (Suppl 3):33–36. doi: 10.1634/theoncologist.13-S3-33. [DOI] [PubMed] [Google Scholar]
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85 (5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Abels R. Erythropoietin for anaemia in cancer patients. Eur J Cancer. 1993;29A (Suppl 2):S2–S8. doi: 10.1016/s0959-8049(05)80281-3. [DOI] [PubMed] [Google Scholar]
- AGO-OVAR 2.7 Reinduction Chemotherapy Containing Carboplatin and Paclitaxel With or Without Epoetin Alpha in Recurrent Platinum Sensitive Ovarian Cancer, Cancer of the Fallopian Tube or Peritoneum. Study terminated and unpublished (ClinicalTrials.gov Identifier: NCT00189371).
- Arcasoy MO, Amin K, Chou SC, Haroon ZA, Varia M, Raleigh JA. Erythropoietin and erythropoietin receptor expression in head and neck cancer: relationship to tumor hypoxia. Clin Cancer Res. 2005;11 (1):20–27. [PubMed] [Google Scholar]
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kuzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299 (8):914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Lai SY, Henke M, Barnato SE, Armitage JO, Sartor O. Association between pharmaceutical support and basic science research on erythropoiesis-stimulating agents. Arch Intern Med. 2010;170 (16):1490–1498. doi: 10.1001/archinternmed.2010.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI. Individual patient- versus group-level data meta-regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med. 2002;21 (3):371–387. doi: 10.1002/sim.1023. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373 (9674):1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke MJ, Weingart O, Kluge S, Piper M, Napoli M, Rades D, Steensma D, Djulbegovic B, Fey MF, Ray-Coquard I, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Erythropoietin or Darbepoetin for patients with cancer—meta-analysis based on individual patient data. Cochrane Database Syst Rev. 2009. p. CD007303. [DOI] [PMC free article] [PubMed]
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, Trelle S, Weingart O, Bayliss S, Brunskill S, Djulbegovic B, Benett CL, Langensiepen S, Hyde C, Engert E. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2006. p. CD003407. [DOI] [PubMed]
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, Trelle S, Weingart O, Bayliss S, Djulbegovic B, Bennett CL, Langensiepen S, Hyde C, Engert A. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98 (10):708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- Boogaerts M, Coiffier B, Kainz C. Impact of epoetin beta on quality of life in patients with malignant disease. Br J Cancer. 2003;88 (7):988–995. doi: 10.1038/sj.bjc.6600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case DC, Jr, Bukowski RM, Carey RW, Fishkin EH, Henry DH, Jacobson RJ, Jones SE, Keller AM, Kugler JW, Nichols CR. Recombinant human erythropoietin therapy for anemic cancer patients on combination chemotherapy. J Natl Cancer Inst. 1993;85 (10):801–806. doi: 10.1093/jnci/85.10.801. [DOI] [PubMed] [Google Scholar]
- CDR0000069148 Chemotherapy and Radiation Therapy With or Without Epoetin Alfa in Treating Patients With Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer, completed, unpublished study (ClinicalTrials.gov Identifier: NCT00028938).
- Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34 (3 Suppl 2):13–19. [PubMed] [Google Scholar]
- Cella D. The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) Scale: Summary of development and validation. FACIT.org: Elmhurst, IL; 2007. [Google Scholar]
- Cella D, Bullinger M, Scott C, Barofsky I. Group vs individual approaches to understanding the clinical significance of differences or changes in funnel plot. Mayo Clin Proc. 2002;77 (4):384–392. doi: 10.4065/77.4.384. [DOI] [PubMed] [Google Scholar]
- Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24 (6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- Cella D, Zagari MJ, Vandoros C, Gagnon DD, Hurtz HJ, Nortier JW. Epoetin alfa treatment results in clinically significant improvements in quality of life in anemic cancer patients when referenced to the general population. J Clin Oncol. 2003;21 (2):366–373. doi: 10.1200/JCO.2003.02.136. [DOI] [PubMed] [Google Scholar]
- Chang J, Couture F, Young S, McWatters KL, Lau CY. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. J Clin Oncol. 2005;23 (12):2597–2605. doi: 10.1200/JCO.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Charu V, Belani CP, Gill AN, Bhatt M, Tomita D, Rossi G, Ben-Jacob A. Efficacy and safety of every-2-week darbepoetin alfa in patients with anemia of cancer: a controlled, randomized, open-label phase II trial. Oncologist. 2007;12 (6):727–737. doi: 10.1634/theoncologist.12-6-727. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, Dafni U, Aravantinos G, Koutras A, Samantas E, Karina M, Janinis J, Papakostas P, Skarlos D, Kalofonos HP, Fountzilas G. Effects of epoetin-alpha on quality of life of cancer patients with solid tumors receiving chemotherapy. Anticancer Res. 2009;29 (2):693–702. [PubMed] [Google Scholar]
- Cohen J.1988Statistical Power Analysis for the Behavioral Sciences2nd edn.Lawrence Erlbaum: Hillsdale, NJ [Google Scholar]
- CR002305 A Phase III Clinical Trial of PROCRIT (Epoetin Alfa) Versus Placebo in Women Undergoing Adjuvant Chemotherapy for Stage I, II or III Breast Cancer. Study terminated and unpublished (ClinicalTrials.gov Identifier: NCT00246597).
- Dammacco F, Castoldi G, Rodjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol. 2001;113 (1):172–179. doi: 10.1046/j.1365-2141.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- Debus J, Hindermann S, Morr H, Metzger J, Sebastian M, Angermund R, Drings P. Epoetin alfa (EPO) and survival in patients with non-resectable NSCLC-Interim results. Lung Cancer. 2005;49 (Suppl 3):S57. [Google Scholar]
- Demetri GD, Kris M, Wade J, Degos L, Cella D. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol. 1998;16 (10):3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- Duval S. Publication Bias in Meta-Analysis – Prevention, Assessment and Adjustments. Wiley: Chichester; 2005. The trim and fill method; pp. 127–144. [Google Scholar]
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database Syst Rev. 2011. p. MR000031. [DOI] [PMC free article] [PubMed]
- Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316 (7124):61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler HG, Abadie E, Breckenridge A, Leufkens H, Rasi G. Open clinical trial data for all? A view from regulators. PLoS Med. 2012;9 (4):e1001202. doi: 10.1371/journal.pmed.1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert A, Josting A, Haverkamp H, Villalobos M, Lohri A, Sokler M, Zijlstra J, Sturm I, Topp MS, Rank A, Zenz T, Vogelhuber M, Nogova L, Borchmann P, Fuchs M, Flechtner HH, Diehl V. Epoetin alfa in patients with advanced-stage Hodgkin's lymphoma: results of the randomized placebo-controlled GHSG HD15EPO trial. J Clin Oncol. 2010;28 (13):2239–2245. doi: 10.1200/JCO.2009.25.1835. [DOI] [PubMed] [Google Scholar]
- Prospective, randomized, controlled, open phase-IV Study on the treatment of small cell lung cancer (SCLC) in the extensive disease (ED) stage per VALGB Classification with doxorubicin, cyclophosphamide, etoposide (ACE Regimen), cited in. EPO-GER-20. Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 373 (9674):1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- Fallowfield L, Gagnon D, Zagari M, Cella D, Bresnahan B, Littlewood TJ, McNulty P, Gorzegno G, Freund M, Epoetin Alfa Study Group Multivariate regression analyses of data from a randomised, double-blind, placebo-controlled study confirm quality of life benefit of epoetin alfa in patients receiving non-platinum chemotherapy. Br J Cancer. 2002;87 (12):1341–1353. doi: 10.1038/sj.bjc.6600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka Y, Sugiyama T, Saito H, Nagase S, Kudoh S, Endo M, Sakai H, Ohashi Y, Saijo N. Randomised, phase III trial of epoetin-beta to treat chemotherapy-induced anaemia according to the EU regulation. Br J Cancer. 2011;105 (9):1267–1272. doi: 10.1038/bjc.2011.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Leucht S. How to obtain NNT from Cohen's d: comparison of two methods. PLoS One. 2011;6 (4):e19070. doi: 10.1371/journal.pone.0019070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19 (11):2875–2882. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- Glaspy J, Bukowski R, Steinberg D, Taylor C, Tchekmedyian S, Vadhan-Raj S. Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. Procrit Study Group. J Clin Oncol. 1997;15 (3):1218–1234. doi: 10.1200/JCO.1997.15.3.1218. [DOI] [PubMed] [Google Scholar]
- Glaspy J, Crawford J, Vansteenkiste J, Henry D, Rao S, Bowers P, Berlin JA, Tomita D, Bridges K, Ludwig H. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102 (2):301–315. doi: 10.1038/sj.bjc.6605498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Nichols G, Ben-Jacob A, Tomita D, Lillie T, Miller C. Treating anemia of cancer with every-4-week darbepoetin alfa: final efficacy and safety results from a phase II, randomized, double-blind, placebo-controlled study. Oncologist. 2008;13 (6):715–724. doi: 10.1634/theoncologist.2007-0241. [DOI] [PubMed] [Google Scholar]
- Goss G, Feld R, Bezjak A, Perry G, Melosky B, Smith C, Snee M, Plante R, Lau C. Impact of maintaining Hb with epoetin alfa on time to progression (TTP), overall survival (OS), quality of life (QOL) and transfusion reduction in limited disease SCLC patients. Lung Cancer. 2005;49 (S53):O–154. [Google Scholar]
- Grant MD, Piper M, Bohlius J, Tonia T, Robert N, Vats V, Bonnell C, Ziegler KM, Aronson N.2013Epoetin and Darbepoetin for Managing Anemia in Patients Undergoing Cancer Treatment: Comparative Effectiveness Update Agency for Healthcare Research and Quality (US): Rockville, MD; 2013 April Report No.: 13-EHC077-EF. AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- Gupta S, Singh PK, Bisth SS, Bhatt ML, Pant M, Gupta R, Singh S, Negi MP. Role of recombinant human erythropoietin in patients of advanced cervical cancer treated ‘by chemoradiotherapy'. Cancer Biol Ther. 2009;8 (1):13–17. doi: 10.4161/cbt.8.1.7089. [DOI] [PubMed] [Google Scholar]
- Hedenus M, Adriansson M, San MJ, Kramer MH, Schipperus MR, Juvonen E, Taylor K, Belch A, Altes A, Martinelli G, Watson D, Matcham J, Rossi G, Littlewood TJ. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122 (3):394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- Henry DH, Abels RI. Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double-blind and open-label follow-up studies. Semin Oncol. 1994;21 (2 Suppl 3):21–28. [PubMed] [Google Scholar]
- Hernandez E, Ganly P, Charu V, Dibenedetto J, Tomita D, Lillie T, Taylor K. Randomized, double-blind, placebo-controlled trial of every-3-week darbepoetin alfa 300 micrograms for treatment of chemotherapy-induced anemia. Curr Med Res Opin. 2009;25 (9):2109–2120. doi: 10.1185/03007990903084164. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327 (7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin PJ, Robinson M, Slevin N, Morgan D, Harrington K, Gaffney C. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol. 2009;27 (34):5751–5756. doi: 10.1200/JCO.2009.22.3693. [DOI] [PubMed] [Google Scholar]
- Huddart RA, Welch RS, Chan S, Perren T, Atkinson R.2002A prospective randomised comparative-group evaluation of epoetin alfa for the treatment of anaemia in UK cancer patients receiving platinum-based chemotherapy. [Miscellaneous] Ann Oncol Abstract Book of the 27th ESMO Congress; Nice, France18–22 October.
- Iconomou G, Koutras A, Rigopoulos A, Vagenakis AG, Kalofonos HP. Effect of recombinant human erythropoietin on quality of life in cancer patients receiving chemotherapy: results of a randomized, controlled trial. J Pain Symptom Manage. 2003;25 (6):512–518. doi: 10.1016/s0885-3924(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Information for Health Professions. Erythropoiesis-Stimulating Agents (ESA): [Aranesp (darbepoetin), Epogen (epoetin alfa), and Procrit (epoetin alfa)] 2007 ) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126481.htm 2007, last accessed: 8 November 2012.
- INT-1 in: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen, Inc. and Procrit (epoetin alfa) Ortho Biotech, LP, for the treatment of anemia associated with cancer chemotherapy Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee.
- INT-3 in: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen, Inc. and Procrit (epoetin alfa) Ortho Biotech, LP, for the treatment of anemia associated with cancer chemotherapy Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee.
- Kotasek D, Albertsson M, Mackey J, Darbepoetin Alfa 980291 Study Group Randomized, double-blind, placebocontrolled, dose-finding study of darbepoetin alfa administered once every 3 (Q3W) or 4 (Q4W) weeks in patients with solid tumors. Proc Am Soc Clinical Oncol. 2002;21:356a. [Google Scholar]
- Kotasek D, Steger G, Faught W, Underhill C, Poulsen E, Colowick AB, Rossi G, Mackey J. Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy; results of a double-blind, placebo-controlled, randomised study. Eur J Cancer. 2003;39 (14):2026–2034. doi: 10.1016/s0959-8049(03)00456-8. [DOI] [PubMed] [Google Scholar]
- Krzakowski M. Epoetin delta: efficacy in the treatment of anaemia in cancer patients receiving chemotherapy. Clin Oncol (R Coll Radiol ) 2008;20 (9):705–713. doi: 10.1016/j.clon.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Kumar SM, Zhang G, Bastian BC, Arcasoy MO, Karande P, Pushparajan A, Acs G, Xu X. Erythropoietin receptor contributes to melanoma cell survival in vivo. Oncogene. 2012;31 (13):1649–1660. doi: 10.1038/onc.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19 (11):2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, Biakhov M, Valuckas K, Voznyi E, Liu X, Vercammen E. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23 (25):5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Crawford J, Osterborg A, Vansteenkiste J, Henry DH, Fleishman A, Bridges K, Glaspy JA. Pooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J Clin Oncol. 2009;27 (17):2838–2847. doi: 10.1200/JCO.2008.19.1130. [DOI] [PubMed] [Google Scholar]
- Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, Cmelak AJ, Schulsinger A, Fu KK. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03) Int J Radiat Oncol Biol Phys. 2007;69 (4):1008–1017. doi: 10.1016/j.ijrobp.2007.04.063. [DOI] [PubMed] [Google Scholar]
- Milroy R, Scagliotti G, van den Berg PM, Galanis NE, Gomez RG, Greil R, Krzakowski M. Early intervention with epoetin alfa maintains hemoglobin (Hb) in advanced non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2003;41 (Suppl 2):574. [Google Scholar]
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 20. 2008;100 (16):1155–1166. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010;7:CD006704. doi: 10.1002/14651858.CD006704.pub2. [DOI] [PubMed] [Google Scholar]
- Moebus V, Jackisch C, Schneeweiss A, Huober J, Lueck HJ, du Bois A, Thomssen C, Kurbacher C, Kuhn W, Nitz U, Runnebaum IB, Hinke A, Kreienberg R, Untch M. AGO Breast Study Group. Adding epoetin alfa to intense dose-dense adjuvant chemotherapy for breast cancer: randomized clinical trial. J Natl Cancer Inst. 2013;105 (14):1018–1026. doi: 10.1093/jnci/djt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 (6):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystakidou K, Kalaidopoulou O, Katsouda E, Parpa E, Kouskouni E, Chondros C, Tsiatas ML, Vlahos L. Evaluation of epoetin supplemented with oral iron in patients with solid malignancies and chronic anemia not receiving anticancer treatment. Anticancer Res. 2005;25 (5):3495–3500. [PubMed] [Google Scholar]
- Nitz U, Gluz O, Oberhoff C, Reimer T, Schumacher C, Hackmann J, Warm M, Uleer C, Runde V, Kuemmel S, Zuna I, Harbeck N. Adjuvant chemotherapy with or without Darbepoetin alpha in node-positive breast cancer: survival and Quality of Life analysis from the prospective randomized WSG ARA Plua Trial. Cancer Res. 2011;71 (Suppl 24):PD07–06. [Google Scholar]
- O'Shaughnessy JA, Vukelja SJ, Holmes FA, Savin M, Jones M, Royall D, George M, Von HD. Feasibility of quantifying the effects of epoetin alfa therapy on cognitive function in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Clin Breast Cancer. 2005;5 (6):439–446. doi: 10.3816/cbc.2005.n.002. [DOI] [PubMed] [Google Scholar]
- A randomised comparison of the effect of maintaining haemoglobin levels with weekly epoetin alfa or with conventional anaemia management in subjects with Multiple Myeloma undergoing chemotherapy (EMMY); Protocol No: OBE/EPO-INT-03. Cited in. OBE/EPO-INT-03. Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 373 (9674):1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- Osterborg A, Brandberg Y, Molostova V, Iosava G, Abdulkadyrov K, Hedenus M, Messinger D. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin Beta, in hematologic malignancies. J Clin Oncol. 2002;20 (10):2486–2494. doi: 10.1200/JCO.2002.08.131. [DOI] [PubMed] [Google Scholar]
- P-174. in: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen, Inc. and Procrit (epoetin alfa) Ortho Biotech, LP, for the treatment of anemia associated with cancer chemotherapy Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee.
- Pirker R, Ramlau RA, Schuette W, Zatloukal P, Ferreira I, Lillie T, Vansteenkiste JF. Safety and efficacy of darbepoetin alpha in previously untreated extensive-stage small-cell lung cancer treated with platinum plus etoposide. J Clin Oncol. 2008;26 (14):2342–2349. doi: 10.1200/JCO.2007.15.0748. [DOI] [PubMed] [Google Scholar]
- Pronzato P, Cortesi E, van der Rijt CC, Bols A, Moreno-Nogueira JA, de Oliveira CF, Barrett-Lee P, Ostler PJ, Rosso R. Epoetin alfa improves anemia and anemia-related, patient-reported outcomes in patients with breast cancer receiving myelotoxic chemotherapy: results of a European, multicenter, randomized, controlled trial. Oncologist. 2010;15 (9):935–943. doi: 10.1634/theoncologist.2009-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirt I, Robeson C, Lau CY, Kovacs M, Burdette-Radoux S, Dolan S, Tang SC, McKenzie M, Couture F. Epoetin alfa therapy increases hemoglobin levels and improves quality of life in patients with cancer-related anemia who are not receiving chemotherapy and patients with anemia who are receiving chemotherapy. J Clin Oncol. 2001;19 (21):4126–4134. doi: 10.1200/JCO.2001.19.21.4126. [DOI] [PubMed] [Google Scholar]
- Ray-Coquard I, Dussart S, Goillot C, Mayeur D, Debourdeau P, Ghesquieres H, Bachelot T, Le CA, Anglaret B, Agostini C, Guastalla JP, Lancry L, Biron P, Desseigne F, Blay JY. A risk model for severe anemia to select cancer patients for primary prophylaxis with epoetin alpha: a prospective randomized controlled trial of the ELYPSE study group. Ann Oncol. 2009;20 (6):1105–1112. doi: 10.1093/annonc/mdn750. [DOI] [PubMed] [Google Scholar]
- Redmond S, von Elm E, Blümle A, Gengler M, Gsponer T, Egger M. Cohort study of trials submitted to ethics committee identified discrepant reporting of outcomes in publications. J Clin Epidemiol. 2013;66 (12):1367–1375. doi: 10.1016/j.jclinepi.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Reichenbach S, Sterchi R, Scherer M, Trelle S, Burgi E, Burgi U, Dieppe PA, Juni P. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med. 2007;146 (8):580–590. doi: 10.7326/0003-4819-146-8-200704170-00009. [DOI] [PubMed] [Google Scholar]
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, Bennett CL, Bohlius J, Evanchuk D, Goode MJ, Jakubowski AA, Regan DH, Somerfield MR. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28 (33):4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- Rose E, Rai K, Revicki D, Brown R, Reblando J, and the EPO in Anemia of CLL Study Group 1994Clinical and health status assessments in anemic chronic lymphocytic leukemia (CLL) patients treated with epoetin alfa (EPO) Blood 84(10 Suppl 1)526a8025281 [Google Scholar]
- Savonije JH, van Groeningen CJ, van BA, Honkoop AH, van Felius CL, Wormhoudt LW, Giaccone G. Effects of early intervention with epoetin alfa on transfusion requirement, hemoglobin level and survival during platinum-based chemotherapy: Results of a multicenter randomised controlled trial. Eur J Cancer. 2005;41 (11):1560–1569. doi: 10.1016/j.ejca.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, De SH, Roila F. Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol. 2010;21 (Suppl 5):v244–v247. doi: 10.1093/annonc/mdq202. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J, Piper M, Bohlius J, Weingart O, Trelle S, Engert A, Skoetz N, Schwarzer G, Wilson J, Brunskill S, Hyde C, Bonnell C, Ziegler KM, Aronson N.2006. Comparative Effectiveness of Epoetin and Darbepoetin for Managing Anemia in Patients Undergoing Cancer Treatment [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); AHRQ Comparative Effectiveness Reviews. [PubMed]
- Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14 (1):73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- Sloan JA, Frost MH, Berzon R, Dueck A, Guyatt G, Moinpour C, Sprangers M, Ferrans C, Cella D. The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer. 2006;14 (10):988–998. doi: 10.1007/s00520-006-0085-y. [DOI] [PubMed] [Google Scholar]
- Smith RE, Jr, Aapro MS, Ludwig H, Pinter T, Smakal M, Ciuleanu TE, Chen L, Lillie T, Glaspy JA. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26 (7):1040–1050. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54 (10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Strauss HG, Haensgen G, Dunst J, Hayward CR, Burger HU, Scherhag A, Koelbl H. Effects of anemia correction with epoetin beta in patients receiving radiochemotherapy for advanced cervical cancer. Int J Gynecol Cancer. 2008;18 (3):515–524. doi: 10.1111/j.1525-1438.2007.01032.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Tokuda Y, Okamoto R, Nakagawa K, Ando K, Iwata H, Tobinai K, Tanigawara Y, Hotta T, Saijo N. Randomized, placebo-controlled phase II study of darbepoetin alfa (DA) administered every three weeks (Q3W) in patients with chemotherapy induced anemia (CIA) Ann Oncol. 2008;19 (Suppl 8):viii277. [Google Scholar]
- Szenajch J, Wcislo G, Jeong JY, Szczylik C, Feldman L. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells From clinic to bench - a critical review. Biochim Biophys Acta. 2010;1806 (1):82–95. doi: 10.1016/j.bbcan.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Thatcher N, De Campos ES, Bell DR, Steward WP, Varghese G, Morant R, Vansteenkiste JF, Rosso R, Ewers SB, Sundal E, Schatzmann E, Stocker H. Epoetin alpha prevents anaemia and reduces transfusion requirements in patients undergoing primarily platinum-based chemotherapy for small cell lung cancer. Br J Cancer. 1999;80 (3–4):396–402. doi: 10.1038/sj.bjc.6690369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, Abulafia O, Lucci JA, III, Begg AC. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dl with erythropoietin vs above 10.0 g/dl without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108 (2):317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, McAdam KF, Thomas RJ, Joffe JK, Sugden EM, Awwad ST, Tan LT, Murray PA, Bailey N, Branson AN.2002Early intervention with epoetin alfa for treatment of anaemia and improvement of quality of life in cancer patients undergoing myelotoxic chemotherapy Ann Oncol 13(Suppl 5)17712401687 [Google Scholar]
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18 (20):2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Hemmelgarn B, Reiman T, Manns B, Reaume MN, Lloyd A, Wiebe N, Klarenbach S. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ. 2009;180 (11):E62–E71. doi: 10.1503/cmaj.090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, Hyde C, Engert A, Bohlius J. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2012;12:CD003407. doi: 10.1002/14651858.CD003407.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi M, Ezaki K, Tobinai K, Ohashi Y, Saijo N. Weekly administration of epoetin beta for chemotherapy-induced anemia in cancer patients: results of a multicenter, Phase III, randomized, double-blind, placebo-controlled study. Jpn J Clin Oncol. 2009;39 (3):163–168. doi: 10.1093/jjco/hyn151. [DOI] [PubMed] [Google Scholar]
- Untch M, von Minckwitz G, Konecny GE, Conrad U, Fett W, Kurzeder C, Lück HJ, Stickeler E, Urbaczyk H, Liedtke B, Beckmann MW, Salat C, Harbeck N, Müller V, Schmidt M, Hasmüller S, Lenhard M, Nekljudova V, Lebeau A, Loibl S, Fasching PA, Arbeitsgemeinschaft Gynäkologische Onkologie PREPARE investigators PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer—outcome on prognosis. Ann Oncol. 2011;22 (9):1999–2006. doi: 10.1093/annonc/mdq713. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, Siena S, Gateley J, Tomita D, Colowick AB, Musil J. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94 (16):1211–1220. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30 (6):473–483. [PubMed] [Google Scholar]
- Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P. Epoetin alfa in platinum-treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. Br J Cancer. 2006;94 (7):947–954. doi: 10.1038/sj.bjc.6603004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist E, Julian JA, Moore MJ, Nabid A, Sathya J, Wood L, Venner P, Levine M. Randomized, double-blind, placebo-controlled trial of epoetin alfa in men with castration-resistant prostate cancer and anemia. J Clin Oncol. 2009;27 (4):644–646. doi: 10.1200/JCO.2008.20.4966. [DOI] [PubMed] [Google Scholar]
- Witzig TE, Silberstein PT, Loprinzi CL, Sloan JA, Novotny PJ, Mailliard JA, Rowland KM, Alberts SR, Krook JE, Levitt R, Morton RF. Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol. 2005;23 (12):2606–2617. doi: 10.1200/JCO.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L, Gagnon B, Okawara GS, Levine MN. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25 (9):1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13 (2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- Yoshizaki A, Kumagai S, Sugiyama T, Goto I, Saito H, Ariyoshi Y, Saijo N, Ohashi Y. A phase III, randomized double-blind placebo controlled study of epoetin beta in luing and gynecological cancer receiving platinum-based chemotherapy: Japan Erythropoietin study group. Ann Oncol. 2010;21 (Suppl8):viii385. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.