Abstract
Background:
Recent identification of a specific role of HSF1 in cancer progression has led to new relevance of HSF1 as both a prognostic and a predictive marker. The role of HSF1 in endometrial cancer has so far been unexplored.
Methods:
A total of 823 lesions from endometrial carcinoma precursors, primary tumours and metastases were prospectively collected and explored for HSF1 protein expression in relation to established markers for aggressive disease and survival. Transcriptional alterations related to HSF1 protein level were investigated by microarray analysis for 224 freshly frozen samples in parallel.
Results:
High expression of HSF1 protein in endometrial carcinoma is significantly associated with aggressive disease and poor survival (all P-values ⩽0.02), also among ERα-positive patients presumed to have good prognosis. The HSF1-related gene signatures increase during disease progression and were also found to have prognostic value. Gene expression analyses identified HSP90 inhibition as a potential novel therapeutic approach for cases with high protein expression of HSF1.
Conclusions:
We demonstrate for the first time in endometrial cancer that high expression of HSF1 and measures for transcriptional activation of HSF1 associate with poor outcome and disease progression. The HSP90 inhibitors are suggested as new targeted therapeutics for patients with high HSF1 levels in tumour in particular.
Keywords: endometrial cancer, prognostic marker, HSF1
Endometrial carcinoma is the fourth most common neoplasm among women in Europe, and the incidence rate is rising. The overall prognosis is good, with a 5-year survival of ∼80% in developed countries (Amant et al, 2005). Still, 15%–20% of patients suffer recurrence. Better markers are needed to earlier detect and individualise treatment. Several biomarkers have shown associations with clinical characteristics and prognosis, such as oestrogen receptor (ER) and progesterone receptor (PR) (Salvesen et al, 2012; Wik et al, 2013). However, in contrast to breast cancer (Green et al, 2013), none are currently implemented in the routine clinical practice as prognostic or predictive markers in endometrial carcinoma treatment.
Heat shock factor 1 (HSF1) is a key transcription factor in the regulation of cellular protein homeostasis. In response to various physiological stresses, HSF1 drives the transcription of heat shock proteins (HSPs), a response that is conserved from yeast to humans (Wiederrecht et al, 1988; Rabindran et al, 1991; Xiao et al, 1999). The HSF1 binds to the 5′ promoter regions of all HSP genes and triggers massive transcription of genes such as HSP90 and HSP70. The HSPs act as chaperones to facilitate normal protein folding and protect the proteome from misfolding and aggregation that could cause lethal damage. The HSF1 also protects cells from a variety of potentially lethal stressors by modulating the expression of a variety of nonchaperone proteins (Page et al, 2006; Guertin and Lis, 2010). These survival-linked roles of HSF1 might, however, be used to overcome defence mechanisms in normal cells and thereby contribute to oncogenesis. The role of HSF1 to promote tumour cell survival was demonstrated in Hsf1 knockout mice (Dai et al, 2007; Jin et al, 2011), having reduced susceptibility to tumour formation. Interestingly, the transcriptional programme regulated by HSF1 in highly malignant tumours is distinct from the heat shock programme induced by thermal stress, but are commonly activated in a range of human malignancies (lung, breast and colon cancers). HSF1 has also been identified as a potent metastasis-promoting gene in malignant melanomas (Scott et al, 2011).
Reports have suggested a potential for HSPs as prognostic biomarkers in cancers (Khalil et al, 2011); however, whether HSF1 level adds clinically relevant information for determining prognosis or response to therapy is not established. High HSF1 level is an independent predictor of poor outcome in breast cancer, also for the ER-positive patient subgroup (Santagata et al, 2011) and in hepatocellular carcinoma (HCC) (Fang et al, 2012), and the level of HSF1 has been reported to be high in a number of other cancers, without linking this to prognosis (Mendillo et al, 2012). The role of HSF1 in endometrial carcinoma is largely unexplored. We here report for the first time a significant association between high HSF1 level and endometrial cancer progression based on the analyses of more than 800 lesions from endometrial carcinoma precursors, primary tumours and metastases. In addition, based on results from comprehensive molecular profiling, HSP90 inhibitors are suggested as potential new targeting therapeutics for cases with high HSF1 levels in particular.
Materials and methods
Ethics statement
All parts of the study have been approved according to Norwegian legislation. The study was approved by the Norwegian Data Inspectorate, Norwegian Social Sciences Data Services and the Regional Committee for Medical Research Ethics, REC West (NSD15501; REK 052.01). All participants gave written informed consent.
Patient series
A total of 823 lesions from endometrial carcinoma precursors, primary tumours and metastases were prospectively collected and explored for HSF1 protein expression in formalin-fixed paraffin-embedded (FFPE) tissue in relation to established markers for aggressive disease and survival. Transcriptional alterations related to HSF1 protein level were investigated by mRNA microarray analysis for 224 freshly frozen samples collected in parallel. The patients were all diagnosed with endometrial carcinoma at Haukeland University Hospital, Bergen, Norway, during the period 2001–2012. Tumour tissue was prospectively collected and patients were staged according to FIGO 2009 criteria. Age at diagnosis, histologic subtype, histologic grade, treatment and follow-up were obtained from the clinical records and histopathology reports generated routinely in a tumour board setting. For FFPE tissues, tissue microarrays (TMAs) were constructed as previously described (Stefansson et al, 2004). Briefly, using a custom-made precision instrument (Beecher Instruments, Silver Spring, MD, USA) three cylinders (for complex atypical hyperplasias (CAHs) and primary tumours) or one cylinder (for metastatic lesions) of 0.6 mm were retrieved from the areas with high tumour purity and mounted in a paraffin block. The TMAs from 28 CAH samples and 176 metastatic lesions from a total of 84 corresponding primary tumours were included in the study to investigate the differential expression of HSF1 during disease progression.
Immunohistochemistry
Tissue microarray sections (5 μm) were dewaxed with xylene and rehydrated in ethanol before microwave antigen retrieval in target retrieval solution, pH 6. Following peroxidase block, the TMAs were incubated for 30 min at room temperature with rabbit monoclonal antibody to HSF1 (1 : 100; no. 4356, Cell Signaling, Danvers, MA, USA) followed by 30 min of incubation with secondary HRP-conjugated anti-rabbit antibody and DAB-chromogen (EnVision detection system, Dako, Glostrup, Denmark). Sections were counterstained in haematoxylin before dehydration and mounting. The immunostained sections were reviewed by light microscopy and scored visually by a semiquantitative and subjective method. Evaluation of staining was performed blinded for the clinical characteristics and outcome. A staining index was calculated as a product of staining intensity (0–3) and area of positive tumour cells (1⩽10%, 2=10%–50% and 3⩾50%). In subsequent statistical analyses, indexes were grouped in tertiles, considering the size of the subgroups and the number of events in each category. Tertile division was selected according to similarity in survival inside each tertile. Index 0–4 was considered low, index 6 intermediate and index 9 was considered high. The κ-value was calculated to be 0.72 for HSF1 in three groups.
Gene expression analyses
Gene expression alterations in relation to HSF1 expression were investigated in fresh tissues from 8 CAHs, 174 primary endometrial cancers and 42 metastatic endometrial cancer lesions, the latter from 26 individual patients. RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and hybridised to Agilent Whole Human Genome Microarrays 44k (cat. no. G4112F) according to the manufacturer's instructions, and scanned using the Agilent Microarray Scanner Bundle (Agilent, Santa Clara, CA, USA). Normalisation of raw data and expression analyses were performed using the J-Express software (Molmine, Bergen, Norway). Expression data were normalised using median over entire array. Mean spot signal was used as intensity measure.
Differentially expressed genes in tumours with high levels of HSF1 were identified using the feature subset selection (FSS) method (P<0.05, fold >1.5) when high HSF1 was defined as immunohistochemistry (IHC) staining index 9 (upper tertile), and SAM (significance analyses of microarray) when defining high HSF1 as upper tertile of mRNA values (P<0.001, FDR <0.001). Connectivity map (http://www.broadinstitute.org/cmap/) queries were performed using the identified gene lists. The presented connectivity map results represent the top-ranked compounds negatively correlated to the HSF1-defined gene lists (Lamb et al, 2006).
HSF1 gene signature analyses
The recently published HSF1-CaSig gene signature (456 genes) and the HSF1-CaSig3 gene signature (207 genes) were explored in our data set (Mendillo et al, 2012). Only genes with overlapping gene symbols between the previously published list and the Agilent gene symbol list were included in the analyses (Supplementary Table 1). For the HSF1-CaSig gene signature scores, the average value of the expression of 411 genes positively correlated with HSF1 activation were calculated for each patient. The HSF1-CaSig3 signature, the average value of defined negative genes (39), was subtracted from the average value of positive genes (149). For survival analysis, high signature scores were defined as top 25%, whereas low signature score was defined as below 75% limit.
Statistical analysis
Statistical analyses were done using IBM SPSS Statistics software version 21 (IBM, Armonk, NY, USA). Probability of <0.05 was considered statistically significant. Groups for categorical variables were compared using Pearson's χ2-test or Fisher's exact test, when appropriate. Mann–Whitney U-test was used to test correlations for continuous variables. Univariate survival analyses of time to death due to endometrial carcinoma (disease-specific survival) were performed using the Kaplan–Meier (product-limit) method. The date of primary surgery is defined as entry date. Patients who died from other causes were censored at the date of death. Differences in survival between groups were estimated by the log-rank (Mantel–Cox) test. Variables were visually examined by a log-minus-log plot to check the assumptions about proportionality over time, and tested for potential interactions before inclusion in the multivariate proportional hazards regression models (Cox analyses). Unadjusted and adjusted hazard ratios were calculated as measures of effect. Significance of change in protein expression from primary tumours to corresponding metastatic lesions was evaluated using Fisher's exact and Wilcoxon's signed-rank tests. All P-values were two sided.
Results
Nuclear HSF1 is associated with aggressive endometrial cancer and poor prognosis
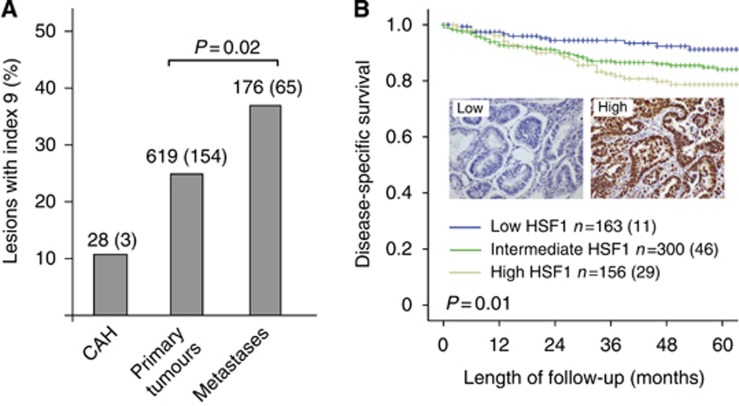
We evaluated 823 endometrial carcinoma samples by IHC for expression of HSF1. For all cases with positive staining, HSF1 staining was nuclear. The HSF1 mRNA level and proportion of lesions with high protein score (index 9) were assessed in CAHs, primary endometrial carcinomas and metastatic lesions. A significant increase in HSF1 protein level (Figure 1A) and mRNA (Supplementary Figure 1A) was detected from the primary tumours to metastases. When exploring the protein expression levels according to tertile limits for the data set, the low expression group (index 0–4) had best survival and the high expression group (index 9) demonstrated to have the worst prognosis, as illustrated in Figure 1B. The significant prognostic impact of HSF1 level was also reflected when exploring mRNA level with worst outcome for patients with expression levels above the upper tertile (P=0.03, Supplementary Figure 1B). High expression of HSF1 was significantly associated with high age, nonendometrioid histological type, high grade and aneuploidy (all P-values <0.02; Table 1). No association with PR status was seen; however, both middle and high HSF1 cases were more likely to be ERα positive (P<0.001). In a multivariate Cox analysis adjusting for age, FIGO stage, histological type and grade, HSF1 showed a tendency, although not statistically significant, as an independent marker of prognosis (P=0.08) when the whole population of endometrial cancer patients was investigated.
Figure 1.
Heat shock factor 1 (HSF1) is associated with aggressive disease in endometrial cancer.A significant increase in HSF1 protein (A) is demonstrated from primary to metastatic lesions (P=0.02). High HSF1 also associates with decreased endometrial carcinoma survival as illustrated by Kaplan–Meier survival curves for high, intermediate and low expression levels for HSF1 (B) with representative cases for low immunohistochemical protein expression of HSF1 compared with high expression in insets.
Table 1. Heat shock factor 1 (HSF1) protein expression in primary tumours in relation to clinicopathological variables in endometrial carcinoma patients.
| Variable | 0–4, n (%) | 6, n (%) | 9, n (%) | P-valuea |
|---|---|---|---|---|
| Age (years) |
|
|
|
0.02 |
| <66 | 93 (55) | 176 (57) | 71 (44) | |
| ⩾66 |
75 (45) |
133 (43) |
92 (56) |
|
| FIGO-09 stage |
|
|
|
0.75 |
| l–ll | 144 (86) | 258 (84) | 135 (83) | |
| lll–lV |
24 (14) |
49 (16) |
28 (17) |
|
| Histologic type |
|
|
|
<0.001 |
| Endometrioid | 147 (88) | 268 (87) | 114 (70) | |
| Nonendometrioid |
21 (12) |
41 (13) |
49 (30) |
|
| Histologic grade |
|
|
|
0.001 |
| Grade 1/2 | 119 (72) | 220 (72) | 88 (55) | |
| Grade 3 |
47 (28) |
87 (28) |
72 (45) |
|
| Ploidy |
|
|
|
<0.001 |
| Diploid | 80 (84) | 179 (84) | 68 (61) | |
| Aneuploid |
15 (16) |
35 (16) |
43 (39) |
|
| ERα |
|
|
|
0.001 |
| Positive | 104 (63) | 242 (79) | 125 (77) | |
| Negative |
60 (37) |
63 (21) |
38 (23) |
|
| PR |
|
|
|
0.44 |
| Positive | 124 (74) | 240 (78) | 119 (73) | |
| Negative | 43 (26) | 67 (22) | 43 (27) |
Abbreviations: ER=oestrogen receptor; FIGO=International Federation of Gynecology and Obstetrics; PR=progesterone receptor.
High level of HSF1 is associated with several of the established clinicopathological markers for aggressive disease.
Two-sided Pearson's χ2-test. Missing data for ploidy in 220 cases, PR in 4, ER in 8 and grade in 7 cases.
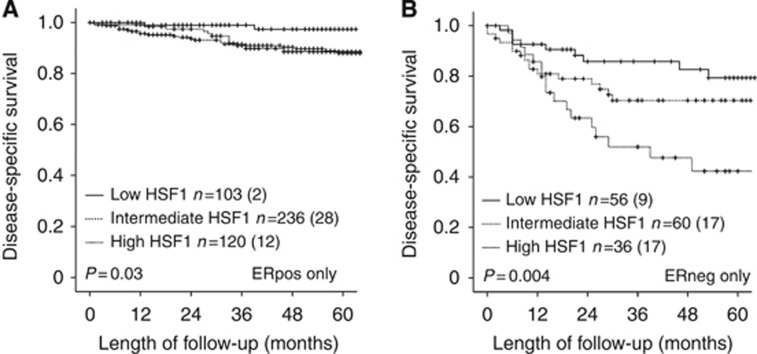
High HSF1 is associated with poor outcome in ER-positive tumours
To evaluate the observation that high HSF1 associates with intact expression of ERα, we performed subgroup analyses of patients according to receptor status. Among the 471 ERα-positive patients, high HSF1 (index 9) was significantly associated with nonendometrioid type, high grade and aneuploidy, a pattern also seen for the ERα-negative group (Table 2). In survival analysis, high HSF1 expression was significantly associated with poor survival for both ERα-positive (Figure 2A) and ERα-negative patients (Figure 2B). In a multivariate Cox analysis adjusting for age, FIGO stage, histological type and grade, we found HSF1 to be an independent prognostic marker only in the group of ERα-positive patients (hazard ratio (HR): 6.2; 95% confidence interval (CI) 1.5–26.4; P=0.014).
Table 2. Heat shock factor 1 (HSF1) protein expression in primary tumours related to clinicopathological variables stratified for ERα status.
|
ERα-positive patients |
ERα-negative patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Low, n (%) | Middle, n (%) | High, n (%) | P-valuea | Low, n (%) | Middle, n (%) | High, n (%) | P-valuea |
| Age (years) |
|
|
|
0.4 |
|
|
|
0.001 |
| <66 | 55 (53) | 139 (57) | 63 (50) | 35 (58) | 34 (54) | 8 (21) | ||
| ⩾66 |
49 (47) |
103 (43) |
62 (50) |
|
25 (42) |
29 (46) |
30 (79) |
|
| FIGO-09 stage |
|
|
|
0.9 |
|
|
|
0.03 |
| l–ll | 93 (89) | 215 (89) | 113 (90) | 49 (82) | 41 (65) | 22 (58) | ||
| lll–lV |
11 (11) |
27 (11) |
12 (10) |
|
11 (18) |
22 (35) |
16 (42) |
|
| Histologic type |
|
|
|
0.001 |
|
|
|
<0.001 |
| Endometrioid | 101 (97) | 225 (93) | 105 (84) | 43 (72) | 39 (62) | 9 (24) | ||
| Nonendometrioid |
3 (3) |
17 (7) |
20 (16) |
|
17 (28) |
24 (38) |
29 (76) |
|
| Histologic grade |
|
|
|
0.004 |
|
|
|
<0.001 |
| Grade 1/2 | 86 (84) | 197 (82) | 83 (68) | 31 (53) | 19 (31) | 5 (13) | ||
| Grade 3 |
17 (16) |
44 (18) |
39 (32) |
|
28 (47) |
43 (69) |
33 (87) |
|
| Metastatic nodes |
|
|
|
0.97 |
|
|
|
0.16 |
| Negative | 83 (92) | 183 (93) | 96 (92) | 43 (86) | 36 (75) | 22 (69) | ||
| Positive |
7 (8) |
14 (7) |
8 (8) |
|
7 (14) |
12 (25) |
10 (31) |
|
| Ploidy |
|
|
|
<0.001 |
|
|
|
0.01 |
| Diploid | 57 (89) | 145 (88) | 57 (70) | 21 (72) | 31 (67) | 11 (38) | ||
| Aneuploid | 7 (11) | 20 (12) | 25 (30) | 8 (28) | 15 (33) | 18 (62) | ||
Abbreviations: ER=oestrogen receptor; FIGO=International Federation of Gynecology and Obstetrics.
Low=index 0–4, middle=index 6 and high=index 9.
Two-sided Pearson's χ2-test; significant P-values (<0.05) are in bold.
Figure 2.
Heat shock factor 1 (HSF1) level predicts outcome in ERα-positive and -negative subgroups. The HSF1 level predicts outcome in ERα-positive and -negative subgroups, as illustrated for ERα-positive patients in (A) and ERα-negative patients in (B). Kaplan–Meier plots give number of cases in each group followed by the number of endometrial carcinoma deaths in parentheses.
HSF1-related gene signatures are associated with poor survival and increase during disease progression
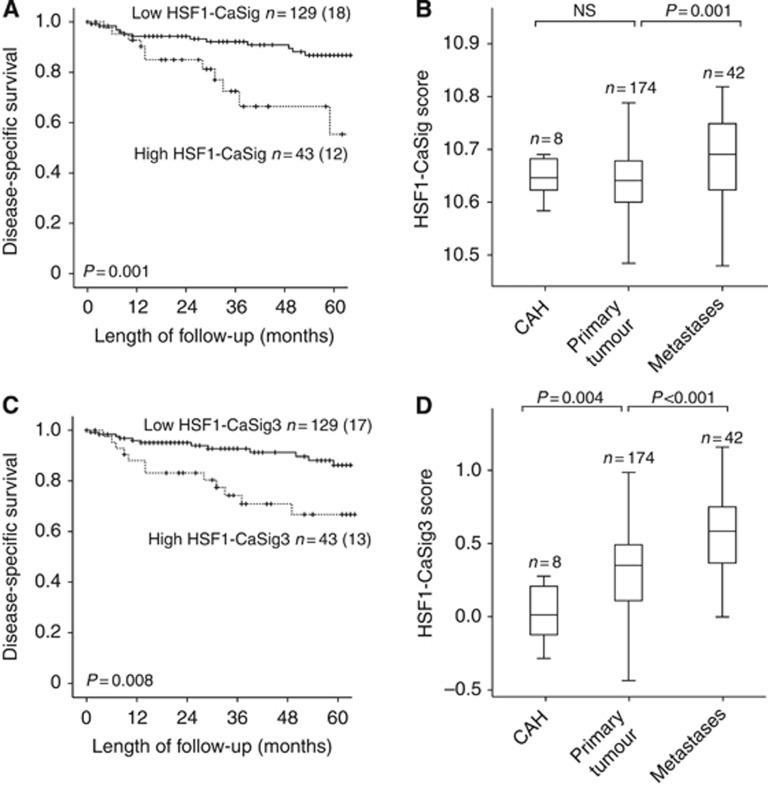
Recently, the transcriptional program regulated by HSF1 during malignant development was described to be distinct from heat shock and specific to highly malignant cells. This HSF1 cancer programme was found to be highly active in aggressive breast, lung and colon cancers and with prognostic as well as therapeutic implications (Mendillo et al, 2012). We therefore investigated whether these cancer-associated HSF1 gene signatures detected in other cancer types could also be used to detect phenotypic differences in endometrial cancer. Interestingly, both the HSF1-CaSig and the HSF1-CaSig3 scores were found to be highly significantly associated with disease-specific survival in our cohort of endometrial carcinoma patients (P=0.001, and P=0.008 respectively, Figure 3A and C). Adjusting for age, FIGO stage, histological type and grade, we found the HSF1-CaSig signature to independently predict poor survival (HR: 2.3; 95% CI 1.0–5.3; P=0.04), with a similar tendency, although not statistically significant, for the HSF1-CaSig3 (P=0.06). When exploring the signatures during disease progression, the HSF1-CaSig score (Figure 3B) increased significantly from primary tumours to metastatic lesions (P=0.001), whereas the HSF1-CaSig3 score (Figure 3D) showed significant increase from CAHs to primary tumours (P=0.004) with a further increase to the metastatic lesions (P<0.001).
Figure 3.
Heat shock factor 1 (HSF1)-related cancer programme is linked to poor prognosis in endometrial cancer. High level of the HSF1-related signatures defined in breast cancer validate to associate with poor disease-specific survival (A and C) and disease progression from CAHs through primary to metastatic endometrial carcinoma lesions for both gene signature HSF1-CaSig and HSF1-CaSig3 (B and D). Number of cases is given for each category followed by number of endometrial carcinoma deaths in parentheses for the survival curves. Numbers above the box plots represent cases investigated in each category.
HSP90 inhibitors and protein synthesis inhibitors are suggested as therapy for patients with high HSF1
To further explore the potential for HSF1 as predictive marker for response to therapy in endometrial cancer, we searched the Connectivity Map database of genome-wide transcriptional expression data from cultured human cells treated with bioactive small molecules (http://www.broadinstitute.org/cmap/). As an HSF1-transcriptional signature is difficult to implement as a predictive marker in a routine clinical setting, we defined patients as HSF1 high and HSF1 low using IHC or HSF1 mRNA levels. We generated query signatures for both cases with high HSF1 protein expression alone and cases with a combined high HSF1 protein and mRNA levels. Interestingly, we identified protein synthesis inhibitors as top-ranked anti-correlated with high protein HSF1, followed by one HSP90 inhibitor (top panel; Table 3). For patients with both high HSF1 protein and mRNA levels, the HSP90 inhibitors Geldanamycin and Tanespimycin were top-ranked anti-correlated with the gene signature for high HSF1 (lower panel; Table 3).
Table 3. Identified compounds negatively correlated to gene signatures describing primary endometrial carcinoma tumours with high HSF1 protein expression (top panel) and high HSF1 protein and mRNA levels (lower panel).
| Rank | Name of compound | Description | Na | P-valueb |
|---|---|---|---|---|
|
Negatively correlated with high HSF1 protein expression in primary tumours | ||||
| 1 | Anisomycin | Protein synthesis inhibitor | 4 | <0.0001 |
| 2 | Lycorine | Protein synthesis inhibitor | 5 | 0.0001 |
| 3 |
Monorden (Radicicol) |
HSP90 inhibitor |
22 |
0.0002 |
|
Negatively correlated with high HSF1 mRNA and high HSF1 protein expression in primary tumours | ||||
| 1 | Geldanamycin | HSP90 inhibitor | 15 | <0.0001 |
| 2 | Tanespimycin | HSP90 inhibitor | 62 | <0.0001 |
| 3 | Trichostatin A | HDAC inhibitor | 182 | <0.0001 |
| 4 | Mycophenolic acid | Immunosupressor | 3 | 0.00004 |
| 5 | Genistein | Topoisomerase II inhibitor | 17 | 0.0001 |
| 6 | Monorden (Radicicol) | HSP90 inhibitor | 22 | 0.0001 |
Abbreviations: HDAC=histone deacetylase; HSF1=heat shock factor 1; HSP90=heat shock protein 90.
N is the number of instances in which the compounds were tested in the Connectivity map.
The expression changes from the compounds tested were scored according to the HSF1 mRNA/protein expression signatures, and the P-value for each compound represents the distribution of this score in the N instances as compared with the distribution of these scores among all compounds tested, using a permutation test (Lamb et al, 2006).
Discussion
A key challenge in tailoring cancer treatments is to establish better markers to improve identification of high-risk from low-risk patients. To resolve this, better understanding of the biological processes underlying cancer progression is needed. For endometrial cancer, few prognostic markers are available and presently the therapeutic strategy is not based on molecular subclass, despite increasing evidence for this being relevant for clinical phenotypes (Salvesen et al, 2009; Kandoth et al, 2013). The HSF1 has recently been suggested as a key regulator of carcinogenesis in breast cancer, a cancer type having many similarities with endometrial carcinomas, including the hormone dependency for subgroups in particular (Salvesen et al, 2012). Here, we demonstrate that high expression of HSF1 on protein and mRNA levels in endometrial cancers reflect phenotype, and we also find a significant increase of HSF1 from primary to metastatic lesions. The potential of HSF1 detected by IHC as a prognostic marker was recently reported in breast cancer (Santagata et al, 2011) and high levels of HSF1 protein were later found in meningioma, cervix, colon, lung, pancreatic and prostate cancers (Mendillo et al, 2012). Although increased levels of HSF1 were not investigated in relation to prognosis in these cancers, the findings taken together point to a universal role for HSF1 in solid tumours. In breast cancer, high HSF1 is also an independent marker of poor survival in the ERα-positive subgroup (Santagata et al, 2011). Here, we show that HSF1 is an independent prognostic marker within the ERα-positive patients also in endometrial cancer. This finding could strengthen the hypothesis proposed that high HSF1 might promote an ERα-negative phenotype by inhibiting oestrogen-dependent transactivation (Khaleque et al, 2008). Interestingly, high HSF1 is also associated with poorer survival in the ERα-negative population. This is in line with cell line studies where depletion of HSF1 reduces the malignant state of the cell regardless of ER status (Dai et al, 2007). More work on the complex interplay between HSF1 and ERα is needed to fully understand their relation; however, our data support that HSF1 might have a potential clinical utility for identifying patients with ERα-positive tumours, normally regarded as prognostically favourable, who could benefit from adjuvant therapy.
To further evaluate the importance of HSF1 in endometrial cancer, we investigated a recently published gene expression signature describing the malignant network controlled by HSF1. In normal homeostasis, HSF1 is known to support the survival and longevity of cells under stress, a function that might be exploited by the tumour cells to prolong survival and develop a malignant state. Increasing evidence supports a role for HSF1 in carcinogenesis, and HSF1 has also recently been shown to play a fundamental role in cancer biology and cancer cell proliferation in mouse models (Dai et al, 2007). Eliminating HSF1 in mouse models has extraordinary consequences on tumour formation and survival in response to mutations in known drivers like Ras and p53. The Hsf1 knockout mice had a longer latency period before development of tumours and showed reduction in tumour incidence and lower overall tumour burden. These results pointed to an orchestrating role for HSF1 in cancer, rather than HSF1 acting as a classical oncogene or tumour suppressor. In human cancers, a direct involvement of HSF1 in cancer progression was linked to a HSF1-regulated transcriptional program distinct from heat shock in breast cancer (Mendillo et al, 2012) and the defined HSF1-regulated transcriptional programme was found to be high in both breast and colon carcinomas, and associated with poor outcome in breast cancer. Apparently in line with this, our study of a large cohort of endometrial cancer patients supports that this HSF1-related cancer signature is significantly associated with poor prognosis. In addition, the observed increase in both HSF1 protein and mRNA levels, and the increase in HSF1-signature scores from primary to metastatic lesions from endometrial cancer patients, further supports the importance of HSF1 in tumour progression. It is interesting that the link between phenotype and HSF1-related signatures derived from breast cancer cell line studies, HSF1-CaSig and HSF1-CaSig3 (Mendillo et al, 2012), are also valid in clinical samples from endometrial cancer patients, especially with regard to prognostic impact. These signatures describe a complex transcriptional program regulating cellular processes with diverse functions and our findings suggest that HSF1 might also be a potential target for developing therapeutics for metastatic endometrial carcinomas. In a routine clinical setting, a gene signature might be less applicable when determining preferred treatment strategies, and IHC-based biomarkers are more easily applied in the routinely collected formalin-fixed tissue. When exploring for agents that could revert the gene signatures of endometrial cancer patients with high HSF1 as detected by IHC in connectivity map, high levels of HSF1 in patient samples suggest drugs targeting HSP90 and protein synthesis as particularly relevant. This identification of HSP90 inhibitors among the top-ranked potential therapeutics is reassuring, given the already well-known link between HSF1 and HSP proteins. Several clinical trials are presently testing HSP90 inhibitors in cancer patients (Kim et al, 2009). Although further development of both Geldanamycin and the analogue Tanespimycin has been terminated (Neckers and Workman, 2012), our data support that targeting HSP90 in cancer is still highly relevant (Barrott and Haystead, 2013). We also identified two protein synthesis inhibitors as top-ranked anti-correlated with gene signatures for high HSF1 protein level, that is, the antibiotic Anisomycin and the alkaloid Lycorine. This finding is interesting in light of the recent publication linking HSF1 to protein translation and promising effect of the translation inhibitor rohibitin in mice experiments (Santagata et al, 2013). More work is needed to unravel whether translational inhibitors might have a role for treatment of endometrial cancer.
We here demonstrate for the first time that nuclear staining of HSF1 and HSF1-related signatures are associated with aggressive disease and poor survival in endometrial cancer. Our study also suggests that HSF1 levels may predict response to drugs targeting HSP90 or protein synthesis, and this needs further testing in the context of clinical trials. Furthermore, the identified increase in HSF1 level and HSF1-related signatures during disease progression also underline the importance of this factor in carcinogenesis and should add momentum to the emerging focus on HSF1 as an important factor for developing new cancer therapeutics.
Acknowledgments
We thank Ellen Valen, Britt Edvardsen, Kadri Madissoo, Bendik Nordanger, Hua My Hoang and Tormund S Njølstad for technical assistance. This study was supported by Helse Vest, the University of Bergen, The Norwegian Cancer Society, The Research Council of Norway and Bergen Medisinske Forskningsstiftelse.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366 (9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Barrott JJ, Haystead TA. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013;280 (6):1381–1396. doi: 10.1111/febs.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130 (6):1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Chang R, Yang L. Heat shock factor 1 promotes invasion and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer. 2012;118 (7):1782–1794. doi: 10.1002/cncr.26482. [DOI] [PubMed] [Google Scholar]
- Green AR, Powe DG, Rakha EA, Soria D, Lemetre C, Nolan CC, Barros FF, Macmillan RD, Garibaldi JM, Ball GR, Ellis IO. Identification of key clinical phenotypes of breast cancer using a reduced panel of protein biomarkers. Br J Cancer. 2013;109 (7):1886–1894. doi: 10.1038/bjc.2013.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin MJ, Lis JT. Chromatin landscape dictates HSF binding to target DNA elements. PLoS Genet. 2010;6 (9):e1001114. doi: 10.1371/journal.pgen.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Moskophidis D, Mivechi NF. Heat shock transcription factor 1 is a key determinant of HCC development by regulating hepatic steatosis and metabolic syndrome. Cell Metab. 2011;14 (1):91–103. doi: 10.1016/j.cmet.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497 (7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleque MA, Bharti A, Gong J, Gray PJ, Sachdev V, Ciocca DR, Stati A, Fanelli M, Calderwood SK. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27 (13):1886–1893. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]
- Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets. Biochim Biophys Acta. 2011;1816 (2):89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L, Trepel JB. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9 (15):1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313 (5795):1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150 (3):549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet. Clin Cancer Res. 2012;18 (1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page TJ, Sikder D, Yang L, Pluta L, Wolfinger RD, Kodadek T, Thomas RS. Genome-wide analysis of human HSF1 signaling reveals a transcriptional program linked to cellular adaptation and survival. Mol Biosyst. 2006;2 (12):627–639. doi: 10.1039/b606129j. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Giorgi G, Clos J, Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci USA. 1991;88 (16):6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, Raeder MB, Sos ML, Engelsen IB, Trovik J, Wik E, Greulich H, Bo TH, Jonassen I, Thomas RK, Zander T, Garraway LA, Oyan AM, Sellers WR, Kalland KH, Meyerson M, Akslen LA, Beroukhim R. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA. 2009;106 (12):4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13 (8):e353–e361. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, Ince TA. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci USA. 2011;108 (45):18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagata S, Mendillo ML, Tang YC, Subramanian A, Perley CC, Roche SP, Wong B, Narayan R, Kwon H, Koeva M, Amon A, Golub TR, Porco JA, Jr, Whitesell L, Lindquist S. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science. 2013;341 (6143):1238303. doi: 10.1126/science.1238303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KL, Nogueira C, Heffernan TP, van Doorn R, Dhakal S, Hanna JA, Min C, Jaskelioff M, Xiao Y, Wu CJ, Cameron LA, Perry SR, Zeid R, Feinberg T, Kim M, Vande Woude G, Granter SR, Bosenberg M, Chu GC, DePinho RA, Rimm DL, Chin L. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell. 2011;20 (1):92–103. doi: 10.1016/j.ccr.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson IM, Salvesen HB, Akslen LA. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol. 2004;22 (7):1242–1252. doi: 10.1200/JCO.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G, Seto D, Parker CS. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988;54 (6):841–853. doi: 10.1016/s0092-8674(88)91197-x. [DOI] [PubMed] [Google Scholar]
- Wik E, Raeder MB, Krakstad C, Trovik J, Birkeland E, Hoivik EA, Mjos S, Werner HM, Mannelqvist M, Stefansson IM, Oyan AM, Kalland KH, Akslen LA, Salvesen HB. Lack of estrogen receptor-alpha is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res. 2013;19 (5):1094–1105. doi: 10.1158/1078-0432.CCR-12-3039. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18 (21):5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.