Abstract
Background:
Little is known about the epidemiology of basal cell carcinoma (BCC).
Methods:
Using the Clinical Practice Research Datalink, we calculated annual incidence rates. In a case–control analysis, we examined lifestyle factors and comorbidities.
Results:
Incidence rose significantly between 2000 and 2011. Basal cell carcinoma risk was increased in alcohol drinkers (slightly) and immunocompromised patients, but reduced in smokers and individuals with abnormal weight.
Conclusions:
Basal cell carcinoma places a growing public health burden. Lifestyle factors do not play a major role in pathogenesis, but immunosuppression is important.
Keywords: basal cell carcinoma, epidemiology, incidence, smoking, alcohol, body mass index, comorbidities, immunosuppression
Cutaneous basal cell carcinoma (BCC) represents the most common malignancy in Caucasian populations, and the incidence is rising (Lomas et al, 2012). Nevertheless, it is often omitted from official cancer statistics, as little is known about the true extent of the disease. Cancer registries, if at all, only include histologically confirmed tumours and do not take into account the substantial proportion of BCCs diagnosed clinically without histology (Flohil et al, 2012).
Basal cell carcinoma is primarily caused by heavy episodic and chronic sun exposure (Armstrong and Kricker, 2001). Predisposing factors include fair skin type, immunosuppression, and certain genetic disorders (e.g., albinism, Gorlin syndrome, xeroderma pigmentosum; Baxter et al, 2012). Data on the relationship between BCC, other diseases, and lifestyle factors are limited.
Using the Clinical Practice Research Datalink (CPRD), we aimed at estimating BCC incidence in the United Kingdom (UK) and at characterising affected patients regarding lifestyle factors and comorbidities.
Materials and methods
Data source
The CPRD is a large primary care database containing computerised longitudinal patient records for about 6% of the UK population. Available data include demographics, lifestyle factors, medical diagnoses, and prescribed drugs. Numerous studies have demonstrated the completeness and high validity of the records (Wood and Martinez, 2004; Herrett et al, 2010).
Study design
We calculated incidence rates (IRs) of BCC in adults between 2000 and 2011, stratified by age, sex, and year of diagnosis.
Using a case–control design, we compared alcohol consumption, smoking status, BMI, and selected comorbidities present before diagnosis between patients with incident BCC and a disease-free control group.
Study population
We identified all patients aged 18 years or older in the CPRD with a BCC first-time diagnosis between 2000 and 2011.
Patients with less than 3 years of history in the CPRD before diagnosis as well as those with a record of albinism, Gorlin syndrome, or xeroderma pigmentosum were excluded.
For the case–control analysis, we randomly selected a group of controls (patients with no recorded BCC) matched 1:1 to BCC cases on age, sex, general practice, calendar time, and years of history in the database. The same exclusion criteria were applied to controls as to cases.
Statistical analysis
We calculated crude IRs as the number of new BCC cases during the study period divided by the total number of person-years at risk (person-years of all adult individuals at risk in the CPRD between start of the study period and end of follow-up, i.e., the day of first BCC diagnosis, death, leaving the practice, or the end of the study period, whichever came first). We also computed directly age-standardised incidence rates (ASRs, reference: European standard population, Waterhouse et al, 1976) and standardised rate ratios (SRRs) to compare rates between sexes and over time.
For comparison of alcohol consumption (non, current, ex; units per week), smoking status (non, current, ex; cigarettes per day), BMI (<18.5, 18.5–24.9, 25–29.9, ⩾30 kg m−2), and comorbidities (Table 1) between cases and controls, we conducted conditional logistic regression analyses and presented relative risk estimates as odds ratios (ORs) with 95% confidence intervals (CIs).
Table 1. Distribution of comorbidities among basal cell carcinoma cases and their matched controls.
| BCC cases (n=57 121), n (%) | BCC-free controls (n=57 121), n (%) | OR crude (95% CI) | |
|---|---|---|---|
|
Diseases of internal organs | |||
| COPD | 2663 (4.7) | 2922 (5.1) | 0.90 (0.86–0.96) |
| Diabetes mellitus | 5009 (8.8) | 5709 (10.0) | 0.86 (0.83–0.90) |
| Hypertension | 22 235 (38.9) | 21 807 (38.2) | 1.04 (1.01–1.06) |
| Gout | 3660 (6.4) | 3483 (6.1) | 1.06 (1.01–1.11) |
| Rheumatoid arthritis | 1571 (2.8) | 1322 (2.3) | 1.20 (1.11–1.29) |
| Inflammatory bowel disease | 729 (1.3) | 589 (1.0) | 1.24 (1.11–1.39) |
| Depression | 8784 (15.4) | 8758 (15.3) | 1.00 (0.97–1.04) |
| Schizophrenia | 271 (0.5) | 381 (0.7) | 0.71 (0.61–0.83) |
| Dementia | 672 (1.2) | 945 (1.7) | 0.70 (0.63–0.77) |
| Malignancies (excl. skin cancer) | 5247 (9.2) | 4015 (7.0) | 1.35 (1.29–1.41) |
| Solid organ transplantation |
205 (0.4) |
41 (0.1) |
5.10 (3.63–7.16) |
|
Skin diseases | |||
| Atopic dermatitis | 3761 (6.6) | 3305 (5.8) | 1.16 (1.10–1.22) |
| Seborrhoeic dermatitis | 3514 (6.2) | 2686 (4.7) | 1.34 (1.27–1.41) |
| Skin mycoses | 8560 (15.0) | 7263 (12.7) | 1.22 (1.18–1.27) |
| Bacterial skin infections | 3948 (6.9) | 3388 (5.9) | 1.18 (1.13–1.24) |
| Warts | 5462 (9.6) | 3642 (6.4) | 1.58 (1.51–1.65) |
| Herpes infection | 6923 (12.1) | 6236 (10.9) | 1.13 (1.09–1.17) |
| Psoriasis | 2480 (4.3) | 2319 (4.1) | 1.07 (1.01–1.14) |
| Rosacea | 2346 (4.1) | 1671 (2.9) | 1.43 (1.34–1.53) |
| Cutaneous malignant melanoma | 810 (1.4) | 332 (0.6) | 2.46 (2.16–2.80) |
Abbreviations: BCC=basal cell carcinoma; CI=confidence interval; COPD=chronic obstructive pulmonary disease; OR=odds ratio.
We controlled for confounding by running a multivariate model incorporating all examined lifestyle factors and the number of general practitioner visits in the year before diagnosis (marker for medical attention). Comorbidities were not included, as they were only thought to descriptively characterise the study population.
Analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC, USA). Statistical significance was defined at the α-level of 0.05.
Results
UK IRs
We identified 57 123 adults with a BCC first-time diagnosis between 2000 and 2011. The overall crude IR and ASR were 201.7 (95% CI: 200.1–203.4) and 151.8 (95% CI: 150.5–153.1) per 100 000 person-years, respectively. Basal cell carcinoma incidence sharply increased with increasing age. Although men had a higher aggregate risk than women (SRR: 1.27, 95% CI: 1.25–1.29), BCC was more common among the latter in individuals younger than 55 years (Supplementary Table 1).
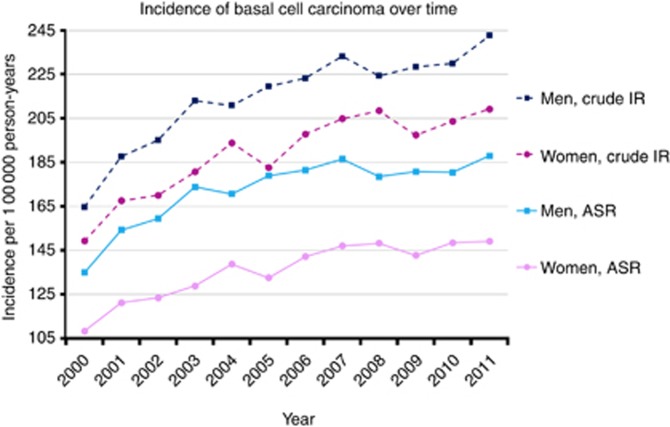
Basal cell carcinoma incidence rose over time in both sexes and in all age groups except in those under 30 years, with an overall increase of 39% between 2000 and 2011 (SRR: 1.39, 95% CI: 1.33–1.45, Figure 1).
Figure 1.
Sex-specific crude incidence rates (IRs) and age-standardised incidence rates (ASRs) of basal cell carcinoma first-time diagnoses in the United Kingdom from 2000 to 2011 (reference: European standard population, Waterhouse et al, 1976).
Lifestyle factors
The case–control analysis comprised 57 121 cases and the same number of matched controls (mean age 69.5 years (s.d.: 13.3 years), 51.3% males). Current alcohol drinkers had a slightly elevated BCC risk compared with non-drinkers. However, the risk only marginally increased with increasing number of alcohol units consumed per week. Smokers had a significantly reduced BCC risk compared with non-smokers. The lowest risk was observed in current heavy smokers (⩾40 cigarettes per day), indicating a negative dose–response relationship between smoking and BCC. Individuals with a BMI outside the normal range (BMI<18.5 or ⩾25) were less likely to develop BCC than normal-weight individuals (Table 2 and Supplementary Table 2).
Table 2. Distribution of lifestyle factors among basal cell carcinoma cases and their matched controls.
| BCC cases (n=57 121), n (%) | BCC-free controls (n=57 121), n (%) | OR crude (95% CI) | OR adjusteda (95% CI) | |
|---|---|---|---|---|
|
Alcohol status | ||||
| Non | 8592 (15.0) | 9442 (16.5) | 1.00 (ref.) | 1.00 (ref.) |
| Ex | 1069 (1.9) | 1193 (2.1) | 1.00 (0.92–1.09) | 0.96 (0.87–1.05) |
| Current | 42 640 (74.7) | 40 552 (71.0) | 1.18 (1.14–1.22) | 1.19 (1.15–1.23) |
| Unknown |
4820 (8.4) |
5934 (10.4) |
0.87 (0.82–0.91) |
1.05 (0.99–1.11) |
|
Smoking status | ||||
| Non | 28 058 (49.1) | 26 439 (46.3) | 1.00 (ref.) | 1.00 (ref.) |
| Ex | 19 607 (34.3) | 19 033 (33.3) | 0.98 (0.95–1.00) | 0.91 (0.89–0.94) |
| Current | 6999 (12.3) | 8359 (14.6) | 0.78 (0.75–0.80) | 0.77 (0.74–0.80) |
| Unknown |
2457 (4.3) |
3290 (5.8) |
0.65 (0.62–0.70) |
0.92 (0.86–0.99) |
|
BMI (kg m−2) | ||||
| 12.0–18.4 | 868 (1.5) | 910 (1.6) | 0.86 (0.79–0.95) | 0.86 (0.78–0.95) |
| 18.5–24.9 | 20 522 (35.9) | 18 591 (32.6) | 1.00 (ref.) | 1.00 (ref.) |
| 25.0–29.9 | 19 849 (34.8) | 19 246 (33.7) | 0.93 (0.91–0.96) | 0.90 (0.87–0.92) |
| 30.0–60.0 | 8907 (15.6) | 10 072 (17.6) | 0.80 (0.78–0.83) | 0.73 (0.70–0.75) |
| Unknown | 6975 (12.2) | 8302 (14.5) | 0.73 (0.70–0.76) | 0.89 (0.85–0.94) |
Abbreviations: BCC=basal cell carcinoma; BMI=body mass index; CI=confidence interval; OR=odds ratio.
Adjusted for alcohol status, smoking status, BMI, and number of general practitioner visits 1 year before BCC diagnosis.
Comorbidities
Compared with controls, BCC cases were significantly more likely to have a medical history of rheumatoid arthritis (RA), inflammatory bowel disease (IBD), extra-cutaneous malignancies, solid organ transplantation, and various skin disorders. On the other hand, they were less likely to have been diagnosed with chronic obstructive pulmonary disease (COPD), diabetes mellitus, schizophrenia, and dementia. The prevalences of the remaining examined comorbidities were equally distributed between the two groups (Table 1).
Discussion
The observed BCC incidence in the UK is considerably high, particularly in the elderly. Projecting the crude IR in the CPRD population to the total UK population aged 18 years or older, we estimate that approximately 110 000 adults developed BCC for the first time in 2011 alone. Taking into account the ageing of the UK population and the increasing IRs over the last decade, BCC places a growing burden on the National Health Service.
In accordance with three large cohort studies (Fung et al, 2002; Freedman et al, 2003; Jensen et al, 2012), we observed an elevated BCC risk in alcohol drinkers. Several mechanisms have been suggested to explain how alcohol may initiate and promote skin carcinogenesis. These comprise impairment of the immune system, poor nutritional status as well as photosensitising and direct mutagenic effects of acetaldehyde, the primary oxidative metabolite of ethanol (Poschl and Seitz, 2004; Saladi et al, 2010). Nonetheless, the detected association between alcohol intake and BCC risk was weak and there was no evidence of a clear dose–response relationship. Two US surveys reported an increased prevalence and severity of sunburns in alcohol drinkers. Thus, alcohol consumption could also be a marker for willingness to take health risks including excessive sun exposure, which then increases the risk of BCC, rather than being a causal factor for BCC itself (Warthan et al, 2003; Mukamal, 2006).
A meta-analysis of 11 case–control and 3 cohort studies (Leonardi-Bee et al, 2012) and two subsequently published individual studies (a case–control study and a study based on two cohorts) (Rollison et al, 2012; Song et al, 2012) found that smoking was not related to an increased BCC risk. Some of these studies (Freedman et al, 2003; Marehbian et al, 2007; Rees et al, 2007; Song et al, 2012) and our results even suggest a lower risk for smokers, which seems paradoxical in view of the carcinogenic effects of cigarette smoke. Aside from non-causal explanations (cigarette smoking may for example be associated with a lower socioeconomic status and fewer opportunities to go on sunny holidays), an underlying mechanism might be an attenuated cutaneous inflammatory response to ultraviolet radiation in smokers, possibly by nicotine altering prostaglandin metabolism (Mills et al, 1993).
The relationship between overweight and a decreased BCC risk has already been reported by others (Gilbody et al, 1994; van Dam et al, 1999; Gerstenblith et al, 2012; Pothiawala et al, 2012). It has been proposed that obese individuals engage less in physical activity, therefore spend less time outdoors, and wear less revealing clothing, which leads to reduced sun exposure of the skin. The same might be true for underweight people.
The analysis of comorbidities revealed significant associations between BCC and diseases related to iatrogenic or non-iatrogenic immunosuppression (RA, IBD, organ transplantation, malignancies, skin infections, seborrhoeic dermatitis). Besides specific photosensitising and oncogenic effects of certain immunosuppressive drugs, it is believed that impaired immune surveillance facilitates unrestricted growth of cancer-initiated cells (Athar et al, 2011). The increased risk of non-melanoma skin cancer in organ transplant recipients has been extensively discussed in the literature, and regular dermatological examinations are an integral part of post-transplant care (Mudigonda et al, 2013). Evidence of a heightened susceptibility in other immunocompromised populations such as RA and IBD patients has been growing only recently, but skin cancer screening should be considered likewise in these individuals (Krathen et al, 2010; Long et al, 2011).
A plausible reason for the overrepresentation of rosacea and cutaneous malignant melanoma among BCC cases is the role of sun exposure in the pathogenesis of all three diseases, even though we cannot rule out some degree of detection and misclassification bias.
Considering the strong correlation between a history of tobacco smoking and COPD, the slightly reduced BCC risk of these patients most likely underscores the protective effect of smoking discussed above.
Similar to our observations, a few other studies also found inverse associations of non-melanoma skin cancer with diabetes mellitus, schizophrenia, and dementia. Suggested explanations include again confounding by sun exposure (possibly mediated by its role in vitamin D synthesis), detection bias, and complex biological mechanisms such as the maintenance of insulin-like growth factor-1 receptor activity (important in the response of keratinocytes to ultraviolet radiation) through exogenous insulin in diabetics (Chuang et al, 2005; Goldacre et al, 2005; White et al, 2013).
In conclusion, the presented IRs highlight the growing burden of BCC in the UK. Along with sun exposure, immunosuppression is an important factor in tumour pathogenesis, whereas lifestyle factors do not appear to have a major role.
Acknowledgments
We thank Pascal Egger for his invaluable technical support and programming.
CS was associated with Spirig Pharma Ltd, Egerkingen, Switzerland. He is a consultant to Galderma SA, Lausanne, Switzerland.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63 (1-3):8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Athar M, Walsh SB, Kopelovich L, Elmets CA. Pathogenesis of nonmelanoma skin cancers in organ transplant recipients. Arch Biochem Biophys. 2011;508 (2):159–163. doi: 10.1016/j.abb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter JM, Patel AN, Varma S. Facial basal cell carcinoma. Br Med J. 2012;345:e5342. doi: 10.1136/bmj.e5342. [DOI] [PubMed] [Google Scholar]
- Chuang TY, Lewis DA, Spandau DF. Decreased incidence of nonmelanoma skin cancer in patients with type 2 diabetes mellitus using insulin: a pilot study. Br J Dermatol. 2005;153 (3):552–557. doi: 10.1111/j.1365-2133.2005.06738.x. [DOI] [PubMed] [Google Scholar]
- Flohil SC, Proby CM, Forrest AD, van Tiel S, Saksela O, Pitkanen S, Ahti T, Micallef R, de Vries E, Group E. Basal cell carcinomas without histological confirmation and their treatment: an audit in four European regions. Br J Dermatol. 2012;167 (Suppl 2):22–28. doi: 10.1111/j.1365-2133.2012.11083.x. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Sigurdson A, Doody MM, Mabuchi K, Linet MS. Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer Epidemiol Biomarkers Prev. 2003;12 (12):1540–1543. [PubMed] [Google Scholar]
- Fung TT, Hunter DJ, Spiegelman D, Colditz GA, Rimm EB, Willett WC. Intake of alcohol and alcoholic beverages and the risk of basal cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2002;11 (10 Pt 1):1119–1122. [PubMed] [Google Scholar]
- Gerstenblith MR, Rajaraman P, Khaykin E, Doody MM, Alexander BH, Linet MS, Freedman DM. Basal cell carcinoma and anthropometric factors in the U.S. radiologic technologists cohort study. Int J Cancer. 2012;131 (2):E149–E155. doi: 10.1002/ijc.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody JS, Aitken J, Green A. What causes basal cell carcinoma to be the commonest cancer. Aust J Public Health. 1994;18 (2):218–221. doi: 10.1111/j.1753-6405.1994.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V. Schizophrenia and cancer: an epidemiological study. Br J Psychiatry. 2005;187:334–338. doi: 10.1192/bjp.187.4.334. [DOI] [PubMed] [Google Scholar]
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69 (1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A, Birch-Johansen F, Olesen AB, Christensen J, Tjonneland A, Kjaer SK. Intake of alcohol may modify the risk for non-melanoma skin cancer: results of a large Danish prospective cohort study. J Invest Dermatol. 2012;132 (12):2718–2726. doi: 10.1038/jid.2012.198. [DOI] [PubMed] [Google Scholar]
- Krathen MS, Gottlieb AB, Mease PJ. Pharmacologic immunomodulation and cutaneous malignancy in rheumatoid arthritis, psoriasis, and psoriatic arthritis. J Rheumatol. 2010;37 (11):2205–2215. doi: 10.3899/jrheum.100041. [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J, Ellison T, Bath-Hextall F. Smoking and the risk of nonmelanoma skin cancer: systematic review and meta-analysis. Arch Dermatol. 2012;148 (8):939–946. doi: 10.1001/archdermatol.2012.1374. [DOI] [PubMed] [Google Scholar]
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166 (5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- Long MD, Kappelman MD, Pipkin CA. Nonmelanoma skin cancer in inflammatory bowel disease: a review. Inflamm Bowel Dis. 2011;17 (6):1423–1427. doi: 10.1002/ibd.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marehbian J, Colt JS, Baris D, Stewart P, Stukel TA, Spencer SK, Karagas MR. Occupation and keratinocyte cancer risk: a population-based case-control study. Cancer Causes Control. 2007;18 (8):895–908. doi: 10.1007/s10552-007-9034-4. [DOI] [PubMed] [Google Scholar]
- Mills CM, Hill SA, Marks R. Altered inflammatory responses in smokers. BMJ. 1993;307 (6909):911. doi: 10.1136/bmj.307.6909.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudigonda T, Levender MM, O'Neill JL, West CE, Pearce DJ, Feldman SR. Incidence, risk factors, and preventative management of skin cancers in organ transplant recipients: a review of single- and multicenter retrospective studies from 2006 to 2010. Dermatol Surg. 2013;39 (3 Pt 1):345–364. doi: 10.1111/dsu.12028. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ. Alcohol consumption and self-reported sunburn: a cross-sectional, population-based survey. J Am Acad Dermatol. 2006;55 (4):584–589. doi: 10.1016/j.jaad.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39 (3):155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- Pothiawala S, Qureshi AA, Li Y, Han J. Obesity and the incidence of skin cancer in US Caucasians. Cancer Causes Control. 2012;23 (5):717–726. doi: 10.1007/s10552-012-9941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JR, Stukel TA, Perry AE, Zens MS, Spencer SK, Karagas MR. Tea consumption and basal cell and squamous cell skin cancer: results of a case-control study. J Am Acad Dermatol. 2007;56 (5):781–785. doi: 10.1016/j.jaad.2006.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollison DE, Iannacone MR, Messina JL, Glass LF, Giuliano AR, Roetzheim RG, Cherpelis BS, Fenske NA, Jonathan KA, Sondak VK. Case-control study of smoking and non-melanoma skin cancer. Cancer Causes Control. 2012;23 (2):245–254. doi: 10.1007/s10552-011-9872-y. [DOI] [PubMed] [Google Scholar]
- Saladi RN, Nektalova T, Fox JL. Induction of skin carcinogenicity by alcohol and ultraviolet light. Clin Exp Dermatol. 2010;35 (1):7–11. doi: 10.1111/j.1365-2230.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- Song F, Qureshi AA, Gao X, Li T, Han J. Smoking and risk of skin cancer: a prospective analysis and a meta-analysis. Int J Epidemiol. 2012;41 (6):1694–1705. doi: 10.1093/ije/dys146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam RM, Huang Z, Rimm EB, Weinstock MA, Spiegelman D, Colditz GA, Willett WC, Giovannucci E. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150 (5):459–468. doi: 10.1093/oxfordjournals.aje.a010034. [DOI] [PubMed] [Google Scholar]
- Warthan MM, Sewell DS, Marlow RA, Warthan ML, Wagner RF., Jr The economic impact of acute sunburn. Arch Dermatol. 2003;139 (8):1003–1006. doi: 10.1001/archderm.139.8.1003. [DOI] [PubMed] [Google Scholar]
- Waterhouse JAH, Muir CS, Correa P, Powell J.eds (1976Cancer Incidence in Five ContinentsVol. IIIIARC: Lyon [Google Scholar]
- White RS, Lipton RB, Hall CB, Steinerman JR. Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology. 2013;80 (21):1966–1972. doi: 10.1212/WNL.0b013e3182941990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L, Martinez C. The general practice research database: role in pharmacovigilance. Drug Saf. 2004;27 (12):871–881. doi: 10.2165/00002018-200427120-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.