Abstract
Dornase alfa has been shown to reduce markers of inflammation and neutrophil-associated metalloproteinases in cystic fibrosis (CF), suggesting a potential benefit from use of this therapy early in the disease. However, observational studies indicate that dornase alfa is often reserved for “sicker” patients. A 2-year, early intervention study of dornase alfa in CF patients with early lung disease demonstrated significant improvements in lung function and risk of exacerbation compared to placebo. A more recent analysis, using the database of the large observational Epidemiologic Study of Cystic Fibrosis (ESCF), found that initiation of dornase alfa has the potential to alter the course of CF by decreasing the rate of lung function decline in children and adults. These encouraging results, possibly linked to indirect effects on inflammation, suggest a greater role for dornase alfa therapy in the early treatment of CF, where it may help preserve lung function and potentially extend survival.
Keywords: dornase alfa, cystic fibrosis, inflammation, pulmonary function
Cystic fibrosis (CF) is a hereditary, life-shortening disease in which a progressive decline in pulmonary function is the major source of mortality [1]. The lung destruction occurring in CF is caused by a vicious cycle of airway obstruction, infection, and an exaggerated inflammatory response [2,3]. Although treatments aimed at relieving obstruction and treating infection have long been the foundations of CF therapy, the recognition of inflammation as an important driver of lung destruction has focused greater attention on anti-inflammatory therapies [4].
Dornase alfa, a recombinant human deoxyribonuclease (DNase), cleaves extracellular DNA in the airway to facilitate mucus clearance in the lung [5]. It has been known for many years that the accumulation and breakdown of neutrophils in the infected lungs of CF patients leads to the release of large amounts of DNA, which increases the viscosity of the infected sputum and decreases ciliary transport [5,6]. By decreasing the size and viscosity of DNA in sputum [6], dornase alfa has the direct effect of relieving obstruction, which was the original rationale for its development. Clinically, this translates into a significant improvement in lung function for CF patients treated with dornase alfa [7–9].
In addition to its effect on obstruction, dornase alfa also significantly reduces the risk of exacerbations or infections requiring intravenous antibiotics [7,9]. This may be linked to reduced bacterial infection as a result of improved mucociliary clearance [10], and/or broader effects on host defenses against infection [11,12]. For example, a randomized 1-year trial of dornase alfa (single-blinded to the clinical microbiologists) reported a 30% prevalence of positive Staphylococcus aureus cultures in untreated patients vs 16% in the group treated with dornase alfa (P<0.0001) [10]. The study researcher attributed the reduced number of lower respiratory tract (LRT) pathogens in patients treated with dornase alfa to improved mucociliary clearance and further speculated that reduced LRT infection could be one of the mechanisms for the anti-inflammatory effects of dornase alfa. Other study results, however, have suggested that the action of dornase alfa may have a more direct impact on infection rates by releasing antimicrobial peptides bound in DNA/actin bundles in CF sputum [11], as well as degrading bacterial extracellular matrix DNA, which is an important proinflammatory component of bacterial biofilms triggering neutrophil activation [12].
The observed clinical benefits on both obstruction and infection have led the Cystic Fibrosis Foundation to strongly recommend dornase alfa for treatment in patients aged 6 years and older with moderate to severe lung disease and to recommend dornase alfa for treatment in patients aged 6 years and older with mild lung disease to improve lung function and reduce exacerbations [13].
The clinical efficacy of dornase alfa also may be related to an indirect effect on enhanced early nutritional status in young children with CF [14]. In a retrospective cohort study of 165 infants using Cystic Fibrosis Foundation registry data from 3 centers, prescription of dornase alfa prior to age 2 was associated with a 10-percentile increase in body mass index (BMI) through age 6 compared with infants not prescribed dornase alfa. This result accounted for confounding factors, such as demographics, CF center, age at diagnosis, and other clinical factors, and use of supplemental feedings and pancreatic enzymes. Nevertheless, the mechanisms of dornase alfa on nutritional status and clinical implications of this finding are not yet clear.
Despite clinical data demonstrating the efficacy of dornase alfa in treating CF patients with early lung disease, data from observational studies reflecting conventional clinical practice demonstrate that it is often reserved for “sicker” patients, ie, those with lower lung function, who are more likely to experience more frequent pulmonary exacerbations [15,16]. Recent evidence indicates that dornase alfa can slow the rate of lung function decline, and thus, may be beneficial if initiated earlier in the course of the disease [17,18]. We speculate that the ability of dornase alfa to modify the rate of lung function decline may be related to its indirect effects on inflammation and by-products of the inflammatory cascade, including neutrophil-derived proteases. This review examines the evidence for anti-inflammatory effects of dornase alfa in the CF lung and explores possible mechanistic links between this anti-inflammatory activity and clinical benefit as they relate to slowing disease progression.
Early Inflammation in CF
The defective CF gene leads to defective or deficient CF transmembrane conductance regulator (CFTR) protein and thus abnormal salt and water transport. The resulting alterations to mucus secretions and mucociliary clearance contribute to airway obstruction and set up a cycle of obstruction, infection, and inflammation [3]. The exact order of events within this cycle remains a matter of controversy, as there is evidence that inflammation may actually precede infection or, alternatively, may persist in the airway after the infection has been cleared [3,4,19] Nevertheless, it is clear that the neutrophil-driven lung inflammation that characterizes CF begins very early in the course of disease, becomes prolonged, and is disproportionate to the burden of infection. Bronchoalveolar lavage (BAL) fluid from infants with CF, for example, has been shown to contain high levels of inflammatory markers, even in those without active infection [20]; in those infected only with H influenzae, BAL fluid contains more neutrophils and interleukin-8 (IL-8) than BAL fluid from infants who have other chronic respiratory conditions and are infected with H influenzae [21,22].
Neutrophils are present in large quantities in the lungs of CF patients even in infants and in older patients who are clinically stable with early stage lung disease [20,23]. These neutrophils initially function to control infection but, in doing so, release oxidants and proteases (eg, elastase and matrix metalloproteinases 8 and 9 [MMP-8 and MMP-9]) that may destroy lung tissue when they are present at high concentrations [2,24]. Elastase is also a potent mucus secretagogue that increases airway obstruction and contributes to the generation of neutrophil chemoattractants such as interleukin-8 (IL-8), further amplifying the inflammatory response. Finally, the decomposition of neutrophils leads to the accumulation of extracellular DNA and actin in the airway, increasing the viscosity of sputum and further hindering airway clearance [2,25].
These findings, taken together, have prompted efforts to better define the role of anti-inflammatory therapies for use earlier in the course of CF lung disease [4].
Indirect Anti-inflammatory Effects of Dornase Alfa
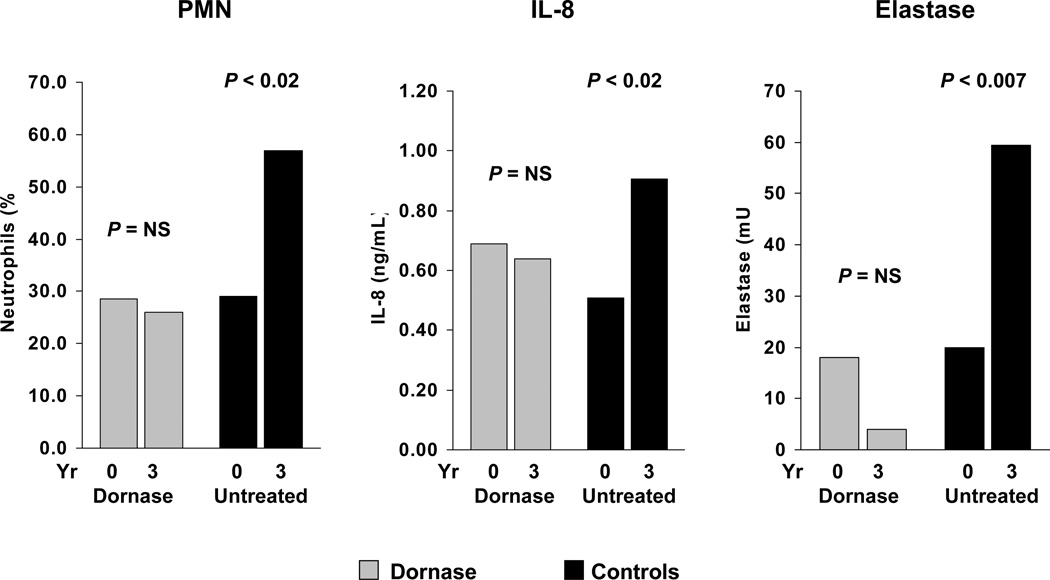
The Bronchoalveolar Lavage for the Evaluation of Anti-inflammatory Treatment (BEAT) study was a 3-year BAL study conducted to assess the anti-inflammatory activity of dornase alfa in patients with CF with early lung disease [26,27]. The study included 105 patients (mean age, 11.8 years) who had a forced expiratory volume in 1 second (FEV1) of >80% predicted and were clinically stable. After the first BAL, patients with elevated neutrophil counts (>10% for children aged ≤8 years and >5% for patients older than 8 years) were randomized to dornase alfa (n=46) or no treatment (n=39). Twenty patients were not randomized due to lower neutrophil counts. Follow-up BAL was performed after 18 and 36 months. During the 3-year study period, the percentage of neutrophils in BAL fluid significantly increased in untreated patients (P<0.02) while remaining constant in the dornase alfa group [Figure 1]. Levels of elastase and IL-8 also significantly increased from baseline in the untreated group (P<0.007 and P<0.02 for elastase and IL-8, respectively) but remained stable in patients receiving dornase alfa. Myeloperoxidase levels showed similar trends but the changes were not statistically significant.
Figure 1. Effect of dornase alfa on markers of inflammation over the 3-year BEAT study [26].
[reprint permission needed] Median neutrophils (% of total cell population), interleukin-8 levels, and elastase activity in bronchoalveolar lavage fluid (BALF) of patients randomized to treatment with dornase alfa or no dornase alfa treatment, at baseline and after 3 years.
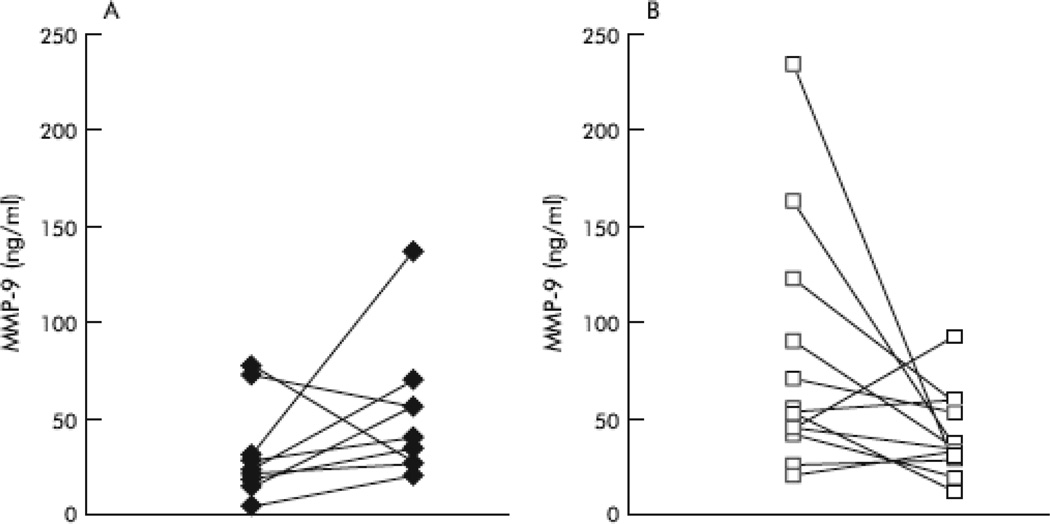
In a substudy of 23 patients participating in the BEAT study (n=13, treated with dornase alfa; n=10, not treated), levels of MMP-8 and MMP-9 were measured due to their central role in CF airway inflammation and potential role in lung tissue destruction [24]. Compared with the BAL fluid of 11 children without pulmonary disease, the BAL fluid of CF patients with early lung disease showed a 300-fold increase in MMP-8 and 116-fold increase in MMP-9 levels. As shown in Figure 2, after 18 months, patients treated with dornase alfa showed a decrease from baseline in median MMP-8 and MMP-9 levels (-23 and -20 ng/mL, respectively) while those not treated showed an increase (15 and 16 ng/mL in MMP-8 and MMP-9 levels, respectively; P=0.007 and P=0.02 for between-group differences). It can therefore be speculated that by decreasing MMP levels associated with lower airway inflammation, treatment with dornase alfa may diminish long-term structural lung damage.
Figure 2.
Changes in MMP-9 concentrations in BAL fluid for patients who were untreated (A) or treated with dornase alfa (B) over 18 months [24]. [Reprint permission needed]
Another study that assessed airway inflammation after treatment with dornase alfa [28] reported negative results that conflict with the findings from the BEAT study. However, it was a short-term study with an older patient population (mean age 16.8 years; range 6.7–27.5 years) and other methodological differences relative to the BEAT study. Briefly, 20 patients with CF were treated for 1 month with dornase alfa, and sputum samples before and after the 1-month trial were analyzed for total cell and neutrophil counts. There were no significant changes in cell counts after dornase alfa treatment in either clinical responders (n=13) or nonresponders (n=7). Mean FEV1 increased significantly, however, from 62.3% predicted at baseline to 70.8% after 1 month of treatment (P=0.02), and 13 patients had a 10% or greater increase in FEV1. The 1-month time scale (compared with 3 years for the BEAT study) and lack of an untreated control group may have prevented this study from demonstrating the anti-inflammatory effect of dornase alfa reported in the BEAT study, which found increased levels of neutrophils over time in the untreated patients vs unchanged levels in the dornase alfa group.
Effect of Dornase Alfa on Rate of Lung Function Decline
In patients with CF, disease progression is assessed by plotting their FEV1 % predicted value vs time [29]. The age at which this curve intersects 20% predicted FEV1 is a strong predictor of mortality, and a steeper slope indicates a more rapid rate of lung function decline [29–31]. Although there is a high degree of variability in the progression of CF lung disease, several risk factors are known to predict the rate of decline in lung function [32]. In particular, young patients with high lung function have a significantly elevated risk of immediate rapid decline, underscoring the need for early and aggressive treatment.
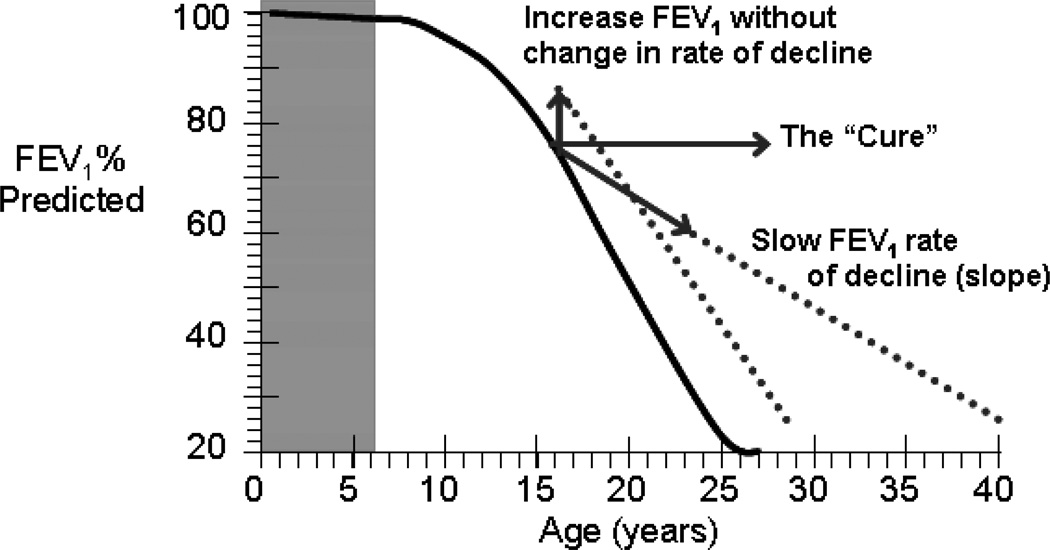
As shown in Figure 3, therapies with the best chance of ultimately improving patient survival would be those not only providing immediate improvement in lung function but also altering the underlying rate of decline in FEV1 (% predicted/year) [17]. The BEAT study of dornase alfa in patients with early lung disease of CF provided encouraging results in this direction. Although the study was not designed to assess the effect of dornase alfa on decline in lung function, it demonstrated a trend toward a slower rate of FEV1 decline over the 3-year treatment period in those receiving dornase alfa vs those not treated (−1.99% predicted/year vs - 3.26% predicted/year, respectively; P = not significant) [26].
Figure 3.
Benefit of increasing forced expiratory volume in 1 second (FEV1) vs slowing the rate of decline of FEV1 [17]. [Reprint permission required]
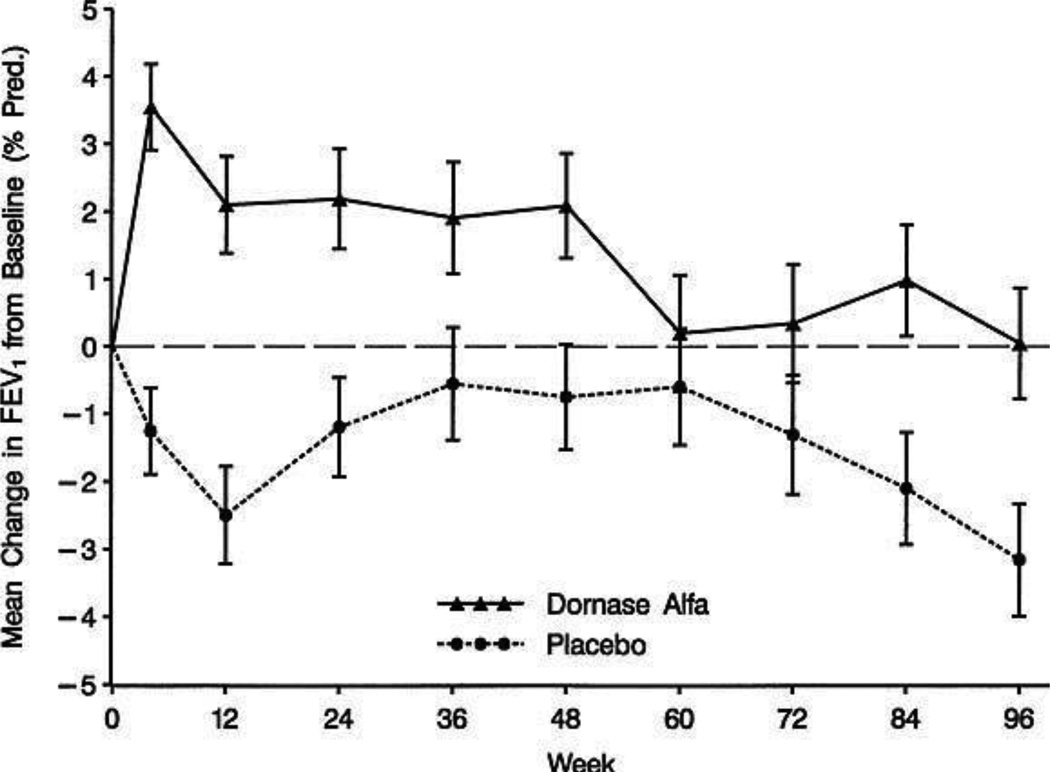
The inherent difficulty in demonstrating an effect on the rate of lung function decline in a clinical trial is that studies powered to do so require several years’ duration and/or a large number of patients, even in the presence of a large therapeutic effect [33,34]. This clinical reality is illustrated by the Pulmozyme Early Intervention Trial (PEIT)—a 2-year longitudinal study of 474 young CF patients (aged 6–10 years) with mean FEV1 of 95% predicted) [9]. This study demonstrated a treatment benefit with dornase alfa (Figure 4), with the dornase alfa-treated patients maintaining lung function over the 2-year study period but the placebo group experiencing a mean decrease in FEV1 of 3.2% predicted from baseline (P=0.006, treatment difference). Use of dornase alfa also reduced the risk of exacerbations by 34% compared with placebo (P=0.048). However, this study did not permit analysis of the rate of decline in lung function due to limitations in study size and duration. As the authors of the study pointed out, a 2-year trial comparing rates of decline in FEV1 % predicted between treatment and placebo would have required 1500 patients per arm to have 90% power to detect a 50% reduction in the rate of decline [9]. Thus, the limited number of CF patients in this age group precluded a study assessing rate of decline.
Figure 4.
Mean change in FEV1 from baseline through 96 weeks in a study of children with early-stage CF lung disease [9]. [Reprint permission required]
A more practical approach to evaluating the effect of treatments on the rate of decline in lung function is to examine the databases of large observational studies spanning several years. The Epidemiologic Study of Cystic Fibrosis (ESCF)—a prospective observational study with data from more than 24,000 patients with CF collected from 1994 through 2005—has provided a wealth of opportunities to evaluate the effects of dornase alfa in clinical practice [35]. This study was designed to collect detailed, longitudinal data regarding clinical care for CF on an encounter-by-encounter basis at a large number of treatment centers in the United States and Canada. The primary objectives were to characterize the decline of lung function in CF patients, rates of acute pulmonary exacerbations requiring antibiotic therapy, and safety and effectiveness of long-term treatment with dornase alfa [35].
In a recently published analysis of the association between dornase alfa therapy and the rate of lung function decline, 2230 patients aged 8 to 38 years (mean age 14.5, baseline FEV1 76.0 % predicted) initially treated with dornase alfa (index event) were compared with 5970 patients age 8 to 38 years (mean age 14.5, baseline FEV1 86.6% predicted) who had never used dornase alfa or had not started dornase alfa for at least 4 years after enrollment in ESCF [18]. Patients treated with dornase alfa were included in the analysis if they had been enrolled in ESCF 2 years prior to the initiation of dornase alfa (baseline period) and were recorded as receiving dornase alfa for 2 subsequent years for ≥80% of visits. An improvement in FEV1 was observed shortly after the initiation of dornase alfa (3.95% predicted for 8- to 17-year-olds and 2.64% for ≥18-year-olds, both P<0.001) but children in the comparator group showed a significant decrease in FEV1 after the index event (−0.67% predicted, P<0.001). Adults in the comparator group demonstrated a small decrease in lung function (−0.14% predicted, P=0.66) around a comparable index event that was not statistically significant. These observations confirm results from previous controlled clinical trials of dornase alfa [7,9]. More importantly, a reduction in the mean annual rate of FEV1 decline for the 2 years after the index event was observed for children (32.1%, P<0.001) and adults (28.8%, P=0.068) in the dornase alfa group, although the difference did not reach statistical significance for adult patients. By contrast, the mean annual rate of decline significantly increased (P<0.001) for children in the comparator group, and did not change (P=0.92) for adults in the comparator group.
These results suggest that early initiation of therapy with dornase alfa slows the rate of lung function decline in patients with CF and has the theoretical potential to prolong survival. These beneficial effects of early dornase therapy may relate to its indirect effects on inflammation. In that regard, it is interesting to note that the only other interventions thus far shown to slow the rate of lung function decline in CF have been anti-inflammatory therapies (high-dose ibuprofen and inhaled corticosteroids [ICS]). In the case of ibuprofen, observational data from the Cystic Fibrosis Foundation Patient Registry revealed a 29% reduction in the rate of decline of FEV1 % predicted in children 6–17 year years treated with ibuprofen (n=1365) vs those not treated (n=8960) (P<0.0001) [36]. This study of the CFF registry with a large patient population supported the results of a 4-year prospective clinical trial conducted in a smaller number of patients [37]. With regard to ICS, an analysis of observational data from ESCF examining the effect of ICS therapy in 2978 children aged 6 to 17 years also revealed a 29% reduction in the rate of decline in FEV1 % predicted after initiating ICS [38]. These results are supported by a recent European observational study of the rate of lung function decline with ICS treatment [39].
Conclusions
The pathophysiology of CF is characterized by a continuous cycle of obstruction, infection, and inflammation. Given the role of the inflammatory process as a driver of irreversible lung destruction, there is an increasing interest in exploring the ability of therapies with anti-inflammatory effects to slow disease progression when used early in the course of disease. Dornase alfa has well-documented beneficial clinical effects on the obstruction and infection components of the disease process (improvement in lung function and decrease in the rate of exacerbations requiring antibiotics), and has also shown positive effects on markers of inflammation. In addition, recent observational data from the ESCF study has suggested that early initiation of dornase alfa can not only improve lung function shortly after initiating therapy, but also can reduce the rate of lung function decline over several years. Slowing progression of lung disease should ultimately extend survival. Results from a recent single-center cohort study of adult CF patients with low lung function (FEV1 <30% predicted) in the United Kingdom suggests that the introduction of dornase alfa as a chronic maintenance therapy is associated with improved survival in patients with late-stage CF lung disease [40]. Additional data will be needed to determine whether this will translate more generally into improved patient survival and whether treatment initiated in infants and your children will stabilize lung function over time. In the meantime, these encouraging results concerning the long-term benefits of dornase alfa therapy suggest a potentially greater role for this therapy in the early treatment of CF.
Figure 5.
Annual rates of decline in FEV1 % predicted before and after index PFT in children and adults for the dornase alfa and comparator groups [18]. [Reprint permission required]
Acknowledgments
The authors would like to thank Embryon for editorial support. Support for third-party assistance for this paper was provided by Genentech, Inc., South San Francisco, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Michael Konstan has received honoraria from Genentech for serving as co-chair of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF) and for other consulting activities. Felix Ratjen has received honoraria from Genentech for consulting activities. No compensation was provided to these authors in exchange for production of this manuscript.
References
- 1.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24:137–142. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Ratjen F. What's new in CF airway inflammation: an update. Paediatr Respir Rev. 2006;7(Suppl 1):S70–S72. doi: 10.1016/j.prrv.2006.04.170. [DOI] [PubMed] [Google Scholar]
- 4.Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;28:331–346. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Pulmozyme® (dornase alfa) Inhalation Solution. South San Francisco, CA: Genentech Inc.; 2005. Ref Type: Data File. [Google Scholar]
- 6.Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A. 1990;87:9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 8.McCoy K, Hamilton S, Johnson C. Effects of 12-week administration of dornase alfa in patients with advanced cystic fibrosis lung disease. Pulmozyme Study Group. Chest. 1996;110:889–895. doi: 10.1378/chest.110.4.889. [DOI] [PubMed] [Google Scholar]
- 9.Quan JM, Tiddens HA, Sy JP, et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813–820. doi: 10.1067/mpd.2001.118570. [DOI] [PubMed] [Google Scholar]
- 10.Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr. 2006;95:1070–1074. doi: 10.1080/08035250600752466. [DOI] [PubMed] [Google Scholar]
- 11.Bucki R, Byfield FJ, Janmey PA. Release of the antimicrobial peptide LL-37 from DNA/F-actin bundles in cystic fibrosis sputum. Eur Respir J. 2007;29:624–632. doi: 10.1183/09031936.00080806. [DOI] [PubMed] [Google Scholar]
- 12.Fuxman Bass JI, Russo DM, Gabelloni ML, et al. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. J Immunol. 2010;184:6386–6395. doi: 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- 13.Flume PA, O'Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 14.Padman R, Werk LN, Ramirez-Garnica G, Ye G, Nathanson I. Association between practice patterns and body mass index percentile in infants and young children with cystic fibrosis. J Cyst Fibros. 2008;7:385–390. doi: 10.1016/j.jcf.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Konstan MW, Butler SM, Schidlow DV, Morgan WJ, Julius JR, Johnson CA. Patterns of medical practice in cystic fibrosis: part II. Use of therapies. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Pediatr Pulmonol. 1999;28:248–254. doi: 10.1002/(sici)1099-0496(199910)28:4<248::aid-ppul3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Johnson CA, Butler SM, Konstan MW, Breen TJ, Morgan WJ. Estimating effectiveness in an observational study: a case study of dornase alfa in cystic fibrosis. The Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. J Pediatr. 1999;134:734–739. doi: 10.1016/s0022-3476(99)70290-8. [DOI] [PubMed] [Google Scholar]
- 17.Konstan MW. Dornase Alfa and Progression of Lung Disease in Cystic Fibrosis. Pediatr Pulmonol. 2008;43:S24–S28. [Google Scholar]
- 18.Konstan MW, Wagener JS, Pasta DJ, et al. Clinical use of dornase alfa is associated with a slower rate of FEV(1) decline in cystic fibrosis. Pediatr Pulmonol. 2011;46:545–553. doi: 10.1002/ppul.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol. 1995;20:63–70. doi: 10.1002/ppul.1950200203. [DOI] [PubMed] [Google Scholar]
- 20.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 21.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis. 1997;175:638–647. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 22.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160:186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 23.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 24.Ratjen F, Hartog CM, Paul K, Wermelt J, Braun J. Matrix metalloproteases in BAL fluid of patients with cystic fibrosis and their modulation by treatment with dornase alpha. Thorax. 2002;57:930–934. doi: 10.1136/thorax.57.11.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchner KK, Wagener JS, Khan TZ, Copenhaver SC, Accurso FJ. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1426–1429. doi: 10.1164/ajrccm.154.5.8912759. [DOI] [PubMed] [Google Scholar]
- 26.Paul K, Rietschel E, Ballmann M, et al. Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. Am J Respir Crit Care Med. 2004;169:719–725. doi: 10.1164/rccm.200307-959OC. [DOI] [PubMed] [Google Scholar]
- 27.Ratjen F, Paul K, van KS, Breitenstein S, Rietschel E, Nikolaizik W. DNA concentrations in BAL fluid of cystic fibrosis patients with early lung disease: influence of treatment with dornase alpha. Pediatr Pulmonol. 2005;39:1–4. doi: 10.1002/ppul.20134. [DOI] [PubMed] [Google Scholar]
- 28.Henry RL, Gibson PG, Carty K, Cai Y, Francis JL. Airway inflammation after treatment with aerosolized deoxyribonuclease in cystic fibrosis. Pediatr Pulmonol. 1998;26:97–100. doi: 10.1002/(sici)1099-0496(199808)26:2<97::aid-ppul4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Konstan MW, Wagener JS, VanDevanter DR. Characterizing aggressiveness and predicting future progression of CF lung disease. J Cyst Fibros. 2009;8(Suppl 1):S15–S19. doi: 10.1016/S1569-1993(09)60006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 31.Schluchter MD, Konstan MW, Davis PB. Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med. 2002;21:1271–1287. doi: 10.1002/sim.1104. [DOI] [PubMed] [Google Scholar]
- 32.Konstan MW, Morgan WJ, Butler SM, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139. 139. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Davis PB, Byard PJ, Konstan MW. Identifying treatments that halt progression of pulmonary disease in cystic fibrosis. Pediatr Res. 1997;41:161–165. doi: 10.1203/00006450-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Konstan MW, Wagener JS, Yegin A, Millar SJ, Pasta DJ, VanDevanter DR. Design and powering of cystic fibrosis clinical trials using rate of FEV(1) decline as an efficacy endpoint. J Cyst Fibros. 2010;9:332–338. doi: 10.1016/j.jcf.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan WJ, Butler SM, Johnson CA, et al. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176:1084–1089. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332:848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 38.Ren CL, Pasta DJ, Rasouliyan L, Wagener JS, Konstan MW, Morgan WJ. Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. J Pediatr. 2008;153:746–751. doi: 10.1016/j.jpeds.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 39.De Boeck K, Vermeulen F, Wanyama S, Thomas M. Inhaled corticosteroids and lower lung function decline in young children with cystic fibrosis. Eur Respir J. 2011;37:1091–1095. doi: 10.1183/09031936.00077210. [DOI] [PubMed] [Google Scholar]
- 40.George PM, Banya W, Pareek N, et al. Improved survival at low lung function in cystic fibrosis: cohort study from 1990 to 2007. BMJ. 2011;342:d1008. doi: 10.1136/bmj.d1008. [DOI] [PMC free article] [PubMed] [Google Scholar]