Abstract
Objectives
Obesity is associated with increased risk and worse outcomes for ovarian cancer. Thus, we examined the effects of obesity on ovarian cancer progression in a genetically engineered mouse model of serous ovarian cancer.
Methods
We utilized a unique serous ovarian cancer mouse model that specifically deletes the tumor suppressor genes, Brca1 and p53, and inactivates the retinoblastoma (Rb) proteins in adult ovarian surface epithelial cells, via injection of an adenoviral vector expressing Cre (AdCre) into the ovarian bursa cavity of adult female mice (KpB mouse model). KpB mice were subjected to a 60% calories-derived from fat in a high fat diet (HFD) versus 10% calories from fat in a low fat diet (LFD) to mimic diet-induced obesity. Tumors were isolated at 6 months after AdCre injection and evaluated histologically. Untargeted metabolomic and gene expression profiling was performed to assess differences in the ovarian tumors from obese versus non-obese KpB mice.
Results
At sacrifice, mice on the HFD (obese) were twice the weight of mice on the LFD (non-obese) (51 g versus 31 g, p = 0.0003). Ovarian tumors were significantly larger in the obese versus non-obese mice (3.7 cm2 versus 1.2 cm2, p = 0.0065). Gene expression and metabolomic profiling indicated statistically significant differences between the ovarian tumors from the obese versus non-obese mice, including metabolically relevant pathways.
Keywords: Obesity, Ovarian cancer, Mouse model, Metabolomics, Genomics, Biomarkers
Introduction
Obesity has been linked to increased risk of many cancers, including breast, colon, endometrial, among others [1]. Currently, new cancer cases are in the order of 1.5 million with half a million cancer deaths per year, and nearly one in five are due to obesity [1,2]. It is postulated that hyperglycemia and hyperinsulinemia resulting from over-nutrition in obese patients provide abundant nutrients and growth factors to cancer cells, resulting in the ideal environment for tumor initiation and promotion [3]. Chronic inflammation and immunosuppression are also thought to be a link between obesity and cancer [3].
Epithelial ovarian cancer (OC) is one of the most deadly cancers with an overall 5-year survival of only 30–40%. Increasing evidence suggests that obesity is a significant risk factor for OC and associated with worse outcomes for this disease [1,4–20]. Given the overall poor prognosis of OC and the rising rate of obesity, it is imperative to investigate obesity as a potential modifiable risk factor that may reverse risk and lead to the prevention and improvement of outcomes for OC. We hypothesize that the metabolic consequences of obesity may play a contributing role in the pathogenesis of OC and may lead to biologically and phenotypically different cancers than those that arise in normal weight women, possibly necessitating distinct treatment strategies. Herein, we assessed the impact of obesity on OC development and progression in a genetically engineered mouse model of serous OC and comprehensively interrogated the obesity-induced carcinogenesis signature through genomic and metabolomic analysis.
Materials and methods
Obesity and the ;p53fl/fl;Brca1fl/fl mouse model
The ;p53 fl/fl;Brca1fl/fl (KpB) mouse model (Terry Van Dyke, PhD, NIH) is a unique serous OC mouse model, wherein the tumor suppressor genes, Brca1 and p53 are specifically and somatically deleted and the retinoblastoma (Rb) proteins are inactivated in the adult ovarian surface epithelium [21]. Inactivation of all 3 Rb proteins by T121 (a fragment of the SV40 large T antigen) is driven by the keratin 18 (K18) promoter [21]. Expression of the T121 transgene and knockout of p53 and Brca1 are conditional and only activated via injection of an adenoviral vector expressing Cre (AdCre) into the ovarian bursa cavity of adult female mice. At approximately 6 months after AdCre injection, tumors develop in the affected ovary, while the un-injected ovary remains normal.
All experimental animals were maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) and the NIH guide for the Care and Use of Laboratory Animals. Recombinant adenovirus Ad5-CMV-Cre (AdCre) was purchased from the University of Iowa Transfer Vector Core at a titer of 1011–1012 infectious particles/ml. To maximize weight gain, mice were provided a high-fat diet (HFD, obese group) (60% kcal from fat, Research Diets, New Brunswick, NJ) and control mice (non-obese group) were provided a low-fat diet (LFD) (10% kcal from fat, Research Diets, New Brunswick, NJ) ad libitum, beginning at 6 weeks of age. AdCre injection occurred at 8 weeks to induce OC 6 months later (at 8 months of age) [21]. Thirty-six hours following superovulation, the mice were anesthetized, and a single 1 cm incision was made on the dorsal surface of each mouse. The AdCre was then injected via a needle introduced into the oviduct near the infundibulum and into the ovarian bursa, and the incision was closed. All mice were sacrificed at 8 months of age.
The primary outcome comparison between non-obese and obese mice was the response of tumor growth to the obesity exposure. This was assessed via direct measurement of the tumor at the time of sacrifice. At the time of sacrifice, the ovarian tumors were harvested, wet tumor weights recorded, and tissue was snap frozen in liquid nitrogen for later harvest of mRNA for microarray analysis and metabolites for metabolomic analysis.
Body weight & composition
Prior to starting mice on diet and weekly until sacrifice, body weight was measured. Body composition, including lean mass, fat mass, free water content and total water content, of non-anesthetized mice was also measured at pre- and post-diet exposures using the EchoMRI-100 quantitative magnetic resonance whole body composition analyzer (Echo Medical Systems, Houston, TX).
Blood glucose
Random blood glucose was measured prior to start of diet and at sacrifice using a Bayer Contour Blood Glucose Monitor (Bayer HealthCare LLC, Tarrytown, NY).
mRNA isolation
Approximately 25–50 mg of frozen OC tissue in small fragments was homogenized in RLT lysis buffer. Total RNA was isolated using the RNeasy mini kit and QIAshredder kit (Qiagen Inc., Mississauga, ON) following the manufacturer’s instructions. RNA quantity and quality were analyzed by Nanodrop (Thermoscientific, Wilmington, DE).
Gene expression profiling
Microarrays were performed on ovarian tumors from non-obese and obese mice (N = 5/group) using Affymetrix GeneChip Mouse Genome 430 2.0 Arrays. These samples were processed in the Lineberger Comprehensive Cancer Center Genomics Core Facility. The image files were analyzed with GenePix Pro 4.1 and pre-processed via the UNC-Chapel Hill Microarray Database (https://genome.unc.edu) where a Lowess normalization procedure was performed to adjust for Cy3 and Cy5 channel biases [22]. In addition, probes with missing values in 3 or more samples in each of the obese and non-obese groups were removed. Two-class SAM (Significance Analysis of Microarrays, http://www-stat.stanford.edu/~tibs/SAM/) was performed to identify significantly differentially expressed genes using FDR < 0.2. EASE (Expression Analysis Systematic Explorer, http://david.niaid.nih.gov/david/ease.htm) analysis was used to interpret and identify biological themes (gene ontology categories) overrepresented in the gene list obtained from SAM results. The EASE Score was used as statistical measure of overrepresentation of a biological theme. Specifically, the EASE Score is a jackknifed one-tailed Fisher’s exact probability which is calculated by removing one gene within the given category from the list and penalizes the statistical significance of categories supported by fewer genes; thus is a more robust measure than the Fisher’s exact probability [23].
Metabolomic profiling
Gas chromatography time-of-flight mass spectrometry (GC-TOFMS, Leco Corporation, St Joseph, MI) and liquid chromatography coupled with time-of-flight mass spectrometry (LC-TOFMS, Agilent Corporation, Santa Clara, CA) were used to analyze tumors from non-obese and obese mice (N = 5/group). Metabolite extraction followed previous publication with minor revision through the UNC/Nutrition Obesity Research Center (NORC) Core facility [24]. Briefly, 50 mg samples were extracted with 0.5 ml of methanol:chloroform:water = 3:1:1 (v:v:v) with homogenization for 3 min using 1-mm inner diameter balls in a Bullet Blender (Next Advance, Averill Park, NY). Two aliquots of 150 μl of supernatant were used for GC-TOFMS and LC-TOFMS analysis, separately. After removal of the extra supernatant, the remainder was extracted with 500 μl of methanol. Two aliquots of 150 μl of supernatant were combined into the tube containing first step extraction for GC and LC-TOFMS analysis, separately. Metabolite annotation was performed by comparing the mass spectrum and retention time to an in-house library and NIST library (GC-TOMFS) or HMDB (LC-TOFMS) [25,26].
Statistical methods
Unpaired Student’s t-test was used to determine statistical difference between non-obese and obese treatment groups using STATA software (College Station, TX). A p-value <0.05 was considered significant. For metabolomics, after normalization to the internal standard and sample weight, the data set was imported into SIMCA-p software (Umeå, Sweden) for multivariate analysis. Principle component analysis (PCA) was first performed to check the outliers and the separation tendency (data not shown). A supervised orthogonal partial least squares-discriminant analysis (OPLS-DA) analysis was then performed. Differentiating metabolites were selected with the criteria of the variable importance in the projection (VIP) value >1 and p value (Student’s t test) lower than 0.05.
Results
Obesity drove significant tumor progression in KpB mice
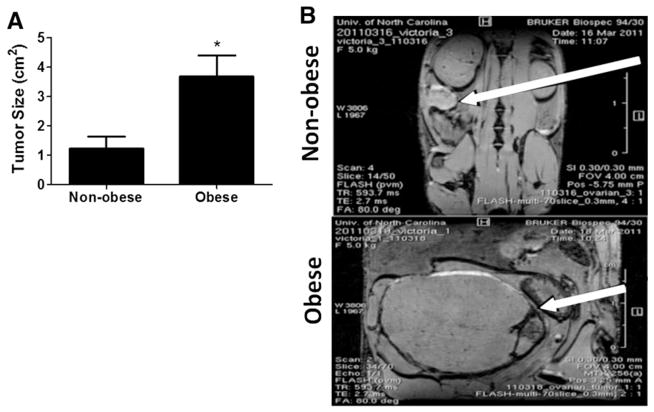
KpB mice were subjected to 60% calories-derived from fat in a high fat diet (HFD) versus 10% calories from fat in a low fat diet (LFD) to induce diet-induced obesity (N = 14/group) starting at 6 weeks of age and until sacrifice. After 8 months of exposure to the HFD or LFD, obese mice weighed significantly greater than non-obese mice (p = 0.003, Table 1). There was no effect of HFD on non-fasted blood glucose levels in KpB mice over the course of the diet (Table 1). Body composition was significantly altered in obese KpB mice compared to non-obese controls. Percent body fat was six-fold greater in obese mice (Table 1, p = 0.0001), while percent lean mass increased by 25% (p = 0.0006, Table 1). The ovarian tumors were tripled in size in the obese mice as compared to non-obese mice (mean size of 3.7 cm2 versus 1.2 cm2, Fig. 1, p = 0.0065).
Table 1.
Diet-induced metabolic characteristics in non-obese and obese KpB mice.
| Non-obese | Obese | p-Value | |
|---|---|---|---|
| Weight (g) | 31.14 ± 5.26 | 50.71 ± 16.73 | p = 0.0003 |
| Glucose (mg/dl) | 186.81 ± 26.99 | 214.38 ± 58.11 | p = 0.053 |
| % fat | 3.28 ± 1.51 | 19.58 ± 7.88 | p = 0.00001 |
| % lean | 22.89 ± 2.11 | 28.66 ± 5.24 | p = 0.0006 |
N = 14 mice per group. Mean ± SD. % fat or % lean = each mass / total body mass as measured by MRI.
Fig. 1.
Obesity increases tumor size in KpB mice. KpB mice were fed low fat or high fat diets to induce obesity for 6 months during tumorigenesis. (A) Comparison of tumor size from non-obese and obese mice (N = 14). These mice were sacrificed 6 months after ovarian tumor induction via injection of AdCre into the ovarian bursa cavity. For the calculation of tumor size, the greatest longitudinal diameter (length) and the greatest transverse diameter (width) were determined and multiplied (m2). *p = 0.0065. (B) MRI images of tumors (arrow) from non-obese (top image) and obese (bottom image) mice demonstrate representative tumors.
Obesity induces genomic differences between obese and non-obese ovarian tumors
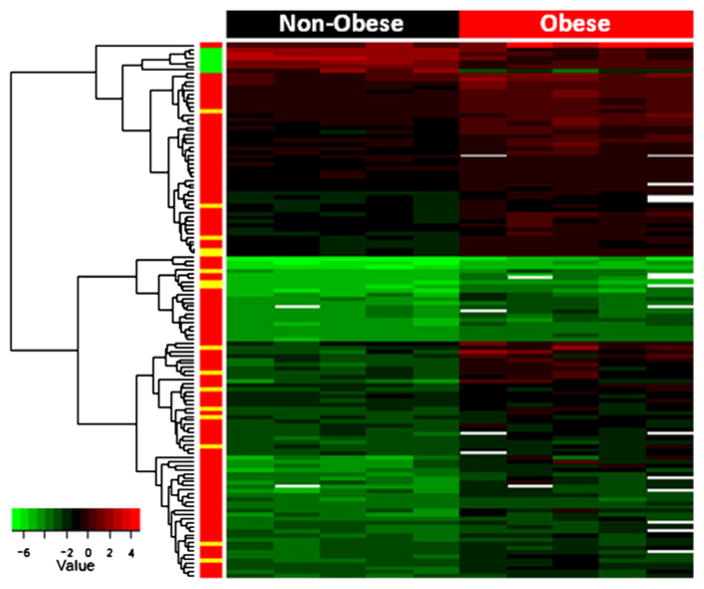
439 genes were found to be significantly up-regulated (417 genes) or down-regulated (22 genes) in the ovarian tumors from obese KpB mice versus non-obese mice (FDR < 0.2, Supplemental Table 1). Fig. 2 is a heat map of 131 genes up- and down-regulated at a FDR < 0.1. Metabolically relevant genes were significantly upregulated in the ovarian tumors from the obese versus non-obese mice, such as lipocalin (2.7 fold), fatty acid amide hydrolase (2.7 fold), fatty acid 2-hydroxylase (2.2 fold), glycerol-3-phosphate acyltransferase (1.5 fold), protein phosphatase (1.2 fold), AMP deaminase 3 (1.6 fold), and protein kinase C (1.7 fold) (Supplemental Table 1). Arginase 1 was the most upregulated gene (7.3 fold) and plays a role in the urea cycle, tissue remodeling and inflammation. Other upregulated genes identified in the ovarian tumors from the obese mice were related to cell adhesion, including neurotrimin (2.2 fold) and desmoglein 1-alpha (2.0 fold). Increased expression of histone 1 (2.3 fold), endothelin-1 (5.8 fold), ectonucleoside triphosphate diphosphohydrolase (3 fold) and serotonin transporter solute carrier family 6 member 4 (Slc6a4) (5.4 fold) were also associated with obesity in the KpB mouse model. Significantly downregulated genes with obesity included spermidine synthase and thrombospondin 4.
Fig. 2.
Genomic differences between ovarian tumors from obese versus non-obese KpB mice reveal alterations in metabolically relevant genes. Heat map representation of 131 genes found to be significantly up- or down-regulated in the ovarian tumors from the obese versus non-obese KpB mice (FDR < 0.1). Many metabolically relevant genes, such as lipocalin, fatty acid amide hydrolase, ectonucleoside triphosphate diphosphohydrolase, fatty acid 2-hydroxylase, glycerol-3-phosphate acyltransferase, protein phosphatase, protein kinase C and AMP deaminase 3, were upregulated in obese tumors.
In the ovarian tumors from the obese versus non-obese mice, EASE over-representation analysis revealed significant enrichment in “phospholipid binding” (EASE score of 0.008), “regulation of apoptosis” (EASE score of 0.014), “lipid binding” (EASE score of 0.015), “endopeptidase activity” (EASE score of 0.03) and “cell–cell signaling” (EASE score of 0.44) for those identified genes.
Metabolic differences between ovarian tumors from obese and non-obese KpB mice
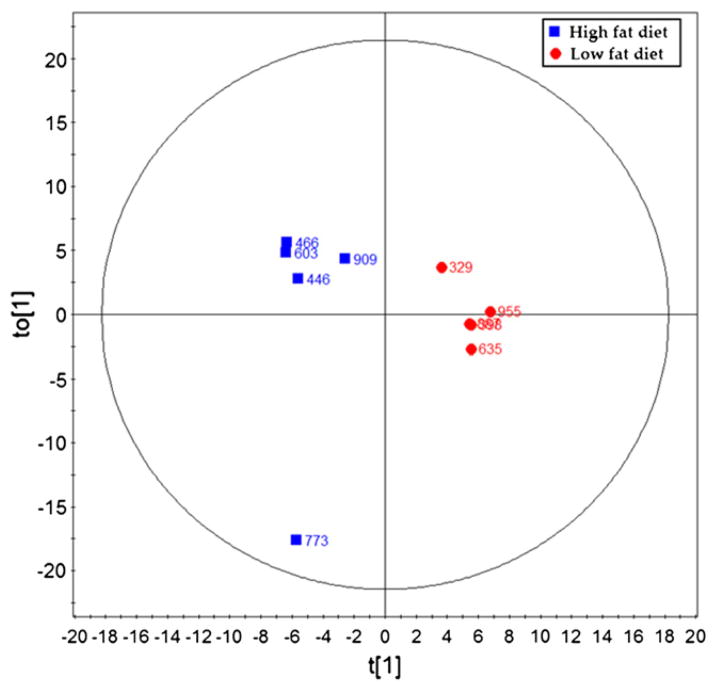
Principle component analysis defined a clear separation between obese and non-obese samples (Fig. 3, 3 components, R2X = 0.563, R2Ycum = 0.95, Q2cum = 0.411). Differentiating metabolites were selected with the criteria of the variable importance in the projection (VIP) value >1 and p value (Student’s t test) lower than 0.05. Twenty metabolites were identified using this criterion, all of which were up-regulated in the ovarian tumors of the non-obese versus obese KpB mice (Table 2).
Fig. 3.
Several metabolites define a clear separation using principal component analysis between the ovarian tumors in the non-obese group and obese group. PLS-DA scores plot of the ovarian tumors in the non-obese group (low fat diet) and obese (high fat diet) group.
Table 2.
Metabolic alterations in tumors from non-obese and obese KpB mice.
| Compound name | VIPa | pb | Fold change (non-obese/obese)c | Analysis method | Identification methodd |
|---|---|---|---|---|---|
| N-glycylproline | 2.27 | 0.0043 | 1.95 | LC-ES+ | Std |
| Oxidized glutathione | 2.25 | 0.0047 | 3.45 | LC-ES+ | Std |
| N-acetylaspartic acid | 2.22 | 0.0059 | 2.31 | LC-ES− | HMDB |
| Vanillactic acid | 2.17 | 0.0079 | 2.23 | LC-ES+ | HMDB |
| 3-Amino-2-piperidone | 2.14 | 0.0099 | 1.75 | GCTOF | NIST |
| Cytidine | 2.10 | 0.0122 | 4.52 | LC-ES+ | Std |
| Cytosine | 2.05 | 0.0158 | 4.11 | LC-ES+ | Std |
| LysoPC(16:1(9Z)) | 1.99 | 0.0205 | 1.83 | LC-ES+ | HMDB |
| 8-Hydroxy-deoxyguanosine | 1.97 | 0.0230 | 2.45 | LC-ES+ | HMDB |
| Adenosine monophosphate | 1.94 | 0.0257 | 1.61 | LC-ES- | HMDB |
| Arginine | 1.93 | 0.0268 | 1.93 | LC-ES+ | Std |
| Gluconolactone | 1.89 | 0.0311 | 2.97 | LC-ES+ | Std |
| Glutathione | 1.89 | 0.0313 | 3.10 | LC-ES+ | Std |
| Glutamate | 1.89 | 0.0318 | 1.52 | GCTOF | Std |
| Guanosine diphosphate | 1.82 | 0.0404 | 2.39 | LC-ES− | HMDB |
| Cytidine | 1.81 | 0.0424 | 4.97 | GCTOF | NIST |
| Inodxyl glucuronide | 1.80 | 0.0439 | 3.05 | LC-ES+ | HMDB |
| Phenylethanolamine | 1.80 | 0.0446 | 1.69 | GCTOF | NIST |
| Succinic acid | 1.78 | 0.0465 | 1.90 | GCTOF | Std |
| 5-Hydroxyindoleacetic acid | 1.76 | 0.0498 | 1.85 | LC-ES+ | HMDB |
Variable importance in the projection (VIP) was obtained from OPLS-DA with a threshold of 1.0.
p value was calculated from Student’s t test.
Fold change with a value larger than 1 indicates a relatively higher concentration in tumors from non-obese (low fat diet-fed) KpB mice, while a value less than 1 means a relatively lower concentration as compared to tumors from obese (high fat diet-fed) KpB mice.
The metabolites were identified by in-house library (Std), NIST library (NIST) or HMBD database (HMDB).
Metabolites involved in inflammatory signaling and protein/collagen metabolism were down-regulated in the ovarian tumors of obese mice as compared to non-obese mice, including arginine (p = 0.0268), N-glycylproline (p = 0.0043) and 3-amino-2-piperidone (p = 0.0099). Components and markers of oxidative stress were also downregulated in the tumors from obese mice: glutathione (p = 0.0313), oxidized glutathione (p = 0.0047), gluconolactone (p = 0.0311) and 8-hydroxy-deoxyguanosine (p = 0.0230). Lower levels of nucleotides (i.e. cytidine (p = 0.0122 and p = 0.0424), cytosine (p = 0.0158), guanosine diphosphate (GDP, p = 0.0404)) and adenosine monophosphate (AMP, p = 0.0257) were detected with obesity. The serotonin metabolite, 5-hydroxyindoleacetic acid (5HIAA, p = 0.0498), and the catecholamine metabolites, vanillactic acid (p = 0.0079) and phenylethanolamine (p = 0.0446), were found to be lower in the ovarian tumors of obese versus non-obese mice. Glutamate (p = .0318), N-acetylaspartic acid (p = 0.0059) and succinic acid (p = 0.0465) are involved in energy metabolism, and were decreased in the ovarian tumors of obese KpB mice. LysoPC(16:1(9Z)) (p = 0.0205), a lysophospholipid, was also lower in the ovarian tumors from obese animals.
Discussion
Recent evidence suggests that obesity may be a significant risk factor and associated with worse outcomes for OC [1,4–20]. Therefore, a metabolic approach to the diagnosis and treatment of OC may provide a novel strategy to improve outcomes for this invariably lethal disease. Hence, we induced obesity in the KpB mouse, a faithful murine model of serous OC, to ask if obesity alters tumorigenesis. KpB mice fed a HFD had significant increases in their body weight and fat mass compared to mice fed a LFD. Herein, we report that obesity promoted tumor progression in the KpB mouse model of OC with a tripling of ovarian tumor size. Obesity has been associated with more rapid tumor growth in animal models of other cancer types, such as breast, colon and lung cancer [27,28], but this is the first study to demonstrate this for OC.
Genomic and metabolomic analyses were utilized to identify obesity-induced alterations in tumors with the intention of identifying significant pathways or biomarkers to aid in explaining why obese mice developed larger, more aggressive tumors. The metabolically relevant genes, lipocalin, ectonucleoside triphosphate diphosphohydrolase and fatty acid amide hydrolase, were upregulated in the ovarian tumors from the obese versus non-obese mice. Lipocalin, particularly lipocalin 2, has been previously found to be upregulated in number of different cancers, including OCs [29,30]. The primary function of lipocalin is the transport of small ligands such as steroids, bilins, retinoids and lipids. In addition to its role in lipid transport, lipocalin has also been implicated in the inflammatory response. Another gene significantly upregulated was ectonucleoside triphosphate diphosphohydrolase, which is involved in the extracellular hydrolysis of ATP to generate adenosine, which signals through G-protein coupled receptors and regulates metabolic pathways and inflammation. Chronic inflammation is well known to play a role in obesity-driven cancers which could also explain the increased expression of both lipocalin and ectonucleoside triphosphate diphosphohydrolase in the ovarian tumors of obese KpB mice.
Fatty acid amide hydrolase (FAAH) is a serine hydrolase that metabolizes N-acylethanolamines (i.e. N-arachidonoylethanolamine, N-oleoylethanolamine and N-palmitoylethanolamine), also known as endocannabinoids, to fatty acids plus ethanolamine. The endocannabinoid system is thought to be important in the regulation of cancer cell apoptosis, proliferation, migration, adhesion and invasion. Increased expression of the cannabinoid receptors (CB1R and CB2R) and FAAH has been documented in prostate and breast cancer and has been associated with worse outcomes [31]. FAAH inhibitors are under development for the treatment of pain and inflammation [31], but may also be useful in cancer. Our data suggests that FAAH inhibitors might be a potential targeted agent for obesity-driven cancers.
Other unique, metabolically relevant genes that were associated with obesity and OC development in the KpB mouse model included fatty acid 2-hydroxylase, glycerol-3-phosphate acyltransferase, protein phosphatase, protein kinase C and AMP deaminase. Fatty acid 2-hydroxylase (FA2H) catalyzes the synthesis of 2-hydroxysphingolipids, a subset of sphingolipids that contain 2-hydroxy fatty acids. FA2H is thought to be involved in the cell differentiation of Schwann cells, keratinocytes and adipocytes. Glycerol-3-phosphate acyltransferase is an enzyme that participates in glycerolipid metabolism and glycerophospholipid metabolism. Protein phosphatases are essential to protein phosphorylation, an important form of reversible protein posttranslational modification involved in cell signaling cascades. The protein kinase C (PKC) family represents a number of protein kinase enzymes that are involved in regulating the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins. The PKC family of enzymes has been implicated in the regulation of signal transduction, cell proliferation, metabolism and differentiation through its effects on regulation of the cell cycle. PKC inhibitors are already being evaluated in clinical trials for a variety of different cancers, including OC [32]. AMP deaminase 3 is a highly regulated enzyme that catalyzes the hydrolytic deamination of adenosine monophosphate to inosine monophosphate, a branch point in the adenylate catabolic pathway. AMP deaminase 3 is thought to be a potent regulator of energy metabolism in cells. Increased expression of AMP deaminases has been documented in hepatocellular carcinomas [33] but has not been explored in OC.
Although many metabolically relevant genes were found to be associated with obesity-driven cancers in the KpB mouse model, other up-regulated genes and pathways were identified. This included genes related to cell adhesion, including neurotrimin and desmoglein 1-alpha. Expression of neurotrimin and desmoglein 1-alpha has not been previously documented in OCs. Increased expression of histone 1 in the ovarian tumors was also associated with obesity in the KpB mice. Histones are the chief protein component of chromatin and are critical for gene regulation. Endothelin-1 (ET-1) is a highly potent vaso-constrictive peptide and was found to be upregulated 5.8 fold in the ovarian tumors from obese mice. Overexpression of ET-1 has been implicated in the epithelial–mesenchymal transition, a mechanism by which transformed epithelial cells acquire the ability to proliferate, invade, resist apoptosis and metastasize [34]. In chemoresistant ovarian cancer cells, ET-1 has been found to be upregulated, leading to enhanced signaling through the MAPK and mTOR/Akt pathway, increased cell proliferation and reduced sensitivity to cisplatin and paclitaxel [35]. Endothelin receptor antagonists are being developed as potential chemotherapeutic agents for cancer [34]. In the ovarian tumors from the obese versus non-obese mice, DAVID functional annotation analysis revealed significant enrichment in “phospholipid binding”, “regulation of apoptosis”, “lipid binding”, “endopeptidase activity” and “cell–cell signaling”. Thus, the increase in aggressiveness, as manifested by a tripling of tumor size, in the obese KpB mice was accompanied by upregulation of genes involved in metabolic, apoptotic and cell signaling pathways.
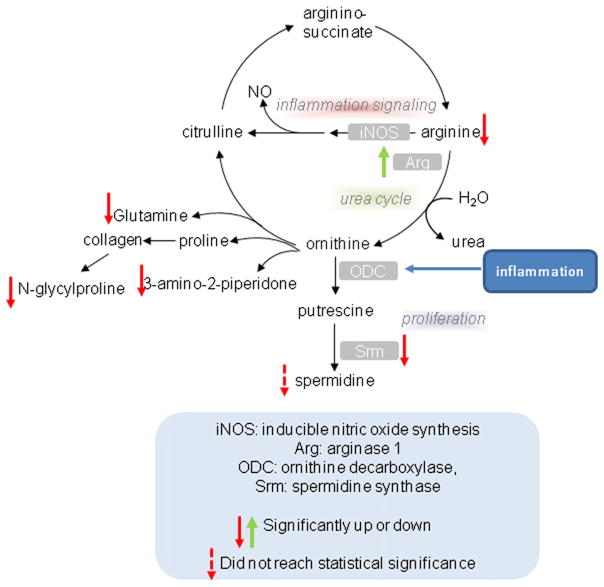
Metabolic analysis revealed that 20 metabolites were identified as significantly regulated. In general, metabolomic analysis revealed that multiple metabolites contributed to separation of non-obese and obese mice with each metabolite being down-regulated in tumors derived from obese mice. Arginase 1 was the most up-regulated gene in obese tumors, which explains the lower detection of arginine concentrations. Catabolic disease states such as sepsis, injury and cancer cause an increase in arginine utilization, which can exceed normal body production, leading to arginine depletion. Arginase 1 converts L-arginine into L-ornithine and urea. Nitric oxide (NO) synthase and arginase compete for the same substrate (L-arginine); hence high arginase activity will blunt NO production, limiting potential pro-inflammatory responses necessary in tumoricidal immune responses. Indeed, arginase 1 is a marker of the M2, alternatively activated, macrophage that is often associated with more aggressive tumors [36]. Arginase also drives polyamine (such as spermidine) synthesis necessary for proliferation. Spermidine synthase in spermidine synthesis was a down-regulated gene in tumors from obese animals, perhaps in a negative feedback mechanism due to elevated delivery of ornithine generated by arginase 1 (30% lower levels of spermidine were detected in ovarian tumors of obese mice but this did not reach statistical significance). Ornithine can also be converted to the delta-lactam 3-amino-2-piperidine, and this was significantly blunted in tumors from obese mice. Finally, arginase generates ornithine which is used to generate proline (necessary for collagen synthesis) and glutamate/glutamine. Glutamate was found at lower levels suggesting that arginase was directing ornithine production to modulate collagen synthesis in tumors derived from obese mice. AMP and arginine both activate AMP kinase (AMPK) which stimulates substrate metabolism, while arginine can also activate mTOR [37,38]. Decreased concentrations of both AMP and arginine in the ovarian tumors from obese versus non-obese mice may be a reflection of increased turnover of these metabolites in the rapidly growing tumors in the obese mice, and potential regulation of substrate metabolism.
N-glycylproline, which had the highest VIP contributing to separation between non-obese and obese tumors, was significantly lower in obese tumors relative to non-obese tumors in KpB mice (Table 2, p = 0.0043). N-glycylproline is an end product of collagen metabolism, but may be recycled into collagen synthesis, and this suggests a potential difference in tissue remodeling between non-obese and obese mice. Overall, Fig. 4 depicts metabolites and genes related to arginine/polyamine/collagen/glutamine metabolism that were decreased in the ovarian tumors from obese mice, suggesting that diet-induced alterations in the stromal components and extracellular matrix are associated with greater growth of the ovarian tumors in obese animals.
Fig. 4.
Obesity-induced alterations in arginine/polyamine/collagen/glutamine metabolism. Metabolomic profiling of ovarian tumors from obese and non-obese KpB mice revealed significant decreases in a number of metabolites related to arginine/polyamine/collagen/glutamine metabolism, suggesting that diet-induced alterations in the stromal components and extracellular matrix are associated with greater growth of the ovarian tumors in obese animals.
Although glutathione disulfide (GSSG) and glutathione (GSH) were significantly regulated by diet, the ratio of the two (as an indicator of oxidative stress) was not significantly different between lean (0.5−/+0.048) and obese (0.45−/+0.284) tumors, suggesting that there was no active oxidative stress. However, a more stable marker of oxidative stress-induced DNA modification, 8-hydroxy-deoxyguanosine, was detected at significantly lower concentrations in obese versus non-obese tumors. Lower concentrations of gluconolactone, an oxidized derivative of glucose, were also found in tumors from obese animals relative to lean, providing further evidence of changes in reduction–oxidation status between the ovarian tumors in the non-obese versus obese group. In sum, in ovarian tumors in obese KpB mice, there appears to be less DNA modification and markers of oxidized metabolites due to oxidative stress, suggesting that oxidative stress is not a major driver of obesity-driven tumorigenesis in the KpB mice or that compensatory mechanisms exist. Alternatively, it could be that the greater growth of ovarian tumors in the obese animals was driven by inflammatory cytokines produced in adipose tissue and distributed to the tumor through the circulation.
Lower concentrations of nucleotides (i.e. cytidine, cytosine, guanosine diphosphate (GDP), adenosine monophosphate (AMP)) may be reflective of increased cell turnover and alterations in utilization and production of these building blocks in the ovarian tumors from obese versus non-obese mice. We postulate that the observed heightened proliferation in the ovarian tumors from the obese versus non-obese mice, as evidenced by a tripling of tumor size, may result in the increased consumption of nucleotides. In the genomic analysis, we also found a 3-fold increase in ectonucleoside triphosphate diphosphohydrolase. This enzyme catalyzes the breakdown of multi-phosphated nucleotides (i.e. ATP, ADP, etc.) and removes free nucleotides and upstream compounds like AMP and GDP, all of which were significantly decreased in the ovarian tumors from the obese mice. In addition, low AMP detected in the ovarian tumors from obese mice suggests possible elevations in anabolic, ATP-burning processes such as lipid synthesis as well as protein, RNA and DNA synthesis.
5-Hydroxyindoleacetic acid (5HIAA) was significantly lower in obese versus non-obese tumors. 5HIAA is a breakdown product of serotonin. Interestingly, the serotonin transporter solute carrier family 6 member 4 (Slc6a4) was upregulated 5.4 fold by obesity. In addition to its function as a neurotransmitter in the central nervous system, increasing evidence suggests that peripheral serotonin may have pro-proliferative and anti-apoptotic effects and act as a mitogen in cancer cells [39,40]; hence, the obesity-mediated regulation of serotonin is of interest.
The catecholamine metabolites, vanillactic acid and phenylethanolamine, were lower in ovarian tumors derived from obese animals. Catecholamines, including epinephrine and norephinephrine, are known to regulate lipolysis [41]. Several studies report that catecholamine responses are blunted in obese versus non-obese individuals at rest and in response to physical activity, suggestive of decreased sympathetic nervous system activity [41]. A decrease in the catecholamine response in the obese mice could lead to reductions in lipolysis and an increase in fat stores that could be advantageous for cancer cell growth.
Succinic acid and glutamate were also significantly decreased by obesity in tumors (Table 2, p = 0.0465 and p = 0.0318). Succinate is a metabolite of the tricarboxylic acid cycle (TCA) cycle and an electron donor to complex II (Succinate-Q oxidoreductase) in oxidative metabolism. Glutamate is also the metabolic intermediate of glutaminolysis, which would feed into the TCA cycle upstream of succinic acid at alpha-ketoglutarate. Interestingly, fructose-6-phosphate did not reach statistical significance (non-obese vs. obese ratio 1.62, p = 0.0684) but contributed to principle component analysis variance (VIP was 1.67). Fructose 6-phosphate is an important intermediate in glycolysis. Taken together, low AMP, succinate, glutamate, and fructose 6-phosphate suggest that KpB tumors in obese mice have a substantially altered metabolic phenotype compared to tumors that have arisen in non-obese controls. We are currently investigating the role of glycolysis and oxidative metabolism, along with AMPK and mTOR signaling, in ovarian cancers from obese and non-obese patients.
Finally, cytidine is a precursor of cytodinetriphosphate, which is needed to create phosphatidylcholine (PC) and phosphatidylethanolamine. Interestingly, lysoPC(16:1(9Z)) was also downregulated in the ovarian tumors from obese versus non-obese mice. Lysophospholipids (LPLs) can play a role in signaling through G-protein coupled receptors, and are a readily accessible fat source for cancer cells [42]. LPLs are generated via inflammatory-responsive phospholipase A (PLA) activity, suggesting that there may be altered inflammatory signaling between non-obese and obese tumors, which is currently being explored. In our genomic analysis, significant enrichment was found in “phospholipid binding” in the ovarian tumors from the obese mice, potentially corresponding to increased utilization of lysophospholipids in the setting of obesity and depletion of cytidine and lysoPC.
In conclusion, we demonstrate that the obese state can promote tumor progression in the KpB mouse model of serous OC, resulting in genomic and metabolic differences between tumors arising in the obese versus non-obese state. Our work suggests that the metabolic consequences of obesity may be crucial in the pathogenesis of OC, resulting in biologically distinct cancers than those that arise in normal weight women. This may have important implications for the treatment of this disease, such that obesity status may be a critical factor in the individualization of management strategies. Further work will be focused on the investigation of the identified obesity-dependent metabolic bio-markers as well as potential novel targets of treatment that may be specific to obesity-driven OCs.
Supplementary Material
HIGHLIGHTS.
Obesity promotes tumor progression in the KpB mouse model of serous ovarian cancer.
Gene expression and metabolomic profiling indicated significant differences between ovarian tumors from obese versus non-obese mice, including metabolically relevant pathways.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2013.12.026.
Footnotes
Presented as an oral presentation at the 2013 Annual Meeting of the Society of Gynecologic Oncology in Los Angeles, CA.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–95. doi: 10.1038/nrc3174. Epub 2011/11/25. [DOI] [PubMed] [Google Scholar]
- 4.Delort L, Kwiatkowski F, Chalabi N, Satih S, Bignon YJ, Bernard-Gallon DJ. Central adiposity as a major risk factor of ovarian cancer. Anticancer Res. 2009;29(12):5229–34. [PubMed] [Google Scholar]
- 5.Pavelka JC, Brown RS, Karlan BY, Cass I, Leuchter RS, Lagasse LD, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006;107(7):1520–4. doi: 10.1002/cncr.22194. [DOI] [PubMed] [Google Scholar]
- 6.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43(4):690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck AR, et al. Body mass index and risk of ovarian cancer. Cancer. 2009;115(4):812–22. doi: 10.1002/cncr.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahmann PH, Cust AE, Friedenreich CM, Schulz M, Lukanova A, Kaaks R, et al. Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126(10):2404–15. doi: 10.1002/ijc.24952. [DOI] [PubMed] [Google Scholar]
- 10.Schouten LJ, Goldbohm RA, van den Brandt PA. Height, weight, weight change, and ovarian cancer risk in the Netherlands cohort study on diet and cancer. Am J Epidemiol. 2003;157(5):424–33. doi: 10.1093/aje/kwf224. Epub 2003/03/05. [DOI] [PubMed] [Google Scholar]
- 11.Fairfield KM, Willett WC, Rosner BA, Manson JE, Speizer FE, Hankinson SE. Obesity, weight gain, and ovarian cancer. Obstet Gynecol. 2002;100(2):288–96. doi: 10.1016/s0029-7844(02)02053-7. Epub 2002/08/02. [DOI] [PubMed] [Google Scholar]
- 12.Chionh F, Baglietto L, Krishnan K, English DR, MacInnis RJ, Gertig DM, et al. Physical activity, body size and composition, and risk of ovarian cancer. Cancer Causes Control. 2010;21(12):2183–94. doi: 10.1007/s10552-010-9638-y. Epub 2010/09/10. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez C, Calle EE, Fakhrabadi-Shokoohi D, Jacobs EJ, Thun MJ. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11(9):822–8. Epub 2002/09/12. [PubMed] [Google Scholar]
- 14.Lubin F, Chetrit A, Freedman LS, Alfandary E, Fishler Y, Nitzan H, et al. Body mass index at age 18 years and during adult life and ovarian cancer risk. Am J Epidemiol. 2003;157(2):113–20. doi: 10.1093/aje/kwf184. Epub 2003/01/11. [DOI] [PubMed] [Google Scholar]
- 15.Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer: a follow-up of 1. 1 million Norwegian women. J Natl Cancer Inst. 2003;95(16):1244–8. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- 16.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. Epub 2007/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang HS, Yoon C, Myung SK, Park SM. Effect of obesity on survival of women with epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2011;21(9):1525–32. doi: 10.1097/IGC.0b013e31822eb5f8. Epub 2011/11/15. [DOI] [PubMed] [Google Scholar]
- 18.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2012;5(7):901–10. doi: 10.1158/1940-6207.CAPR-12-0048. Epub 2012/05/23. [DOI] [PubMed] [Google Scholar]
- 19.Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9(4):e1001200. doi: 10.1371/journal.pmed.1001200. Epub 2012/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engeland A, Bjorge T, Selmer RM, Tverdal A. Height and body mass index in relation to total mortality. Epidemiology. 2003;14(3):293–9. Epub 2003/07/16. [PubMed] [Google Scholar]
- 21.Szabova L, Yin C, Bupp S, Guerin TM, Schlomer JJ, Householder DB, et al. Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 2012;72(16):4141–53. doi: 10.1158/0008-5472.CAN-11-3834. Epub 2012/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30(4):e15. doi: 10.1093/nar/30.4.e15. Epub 2002/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4(10):R70. doi: 10.1186/gb-2003-4-10-r70. Epub 2003/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan L, Qiu Y, Chen T, Lin J, Chi Y, Su M, et al. An optimized procedure for metabonomic analysis of rat liver tissue using gas chromatography/time-of-flight mass spectrometry. J Pharm Biomed Anal. 2010;52(4):589–96. doi: 10.1016/j.jpba.2010.01.046. Epub 2010/02/27. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y, Cai G, Su M, Chen T, Zheng X, Xu Y, et al. Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS. J Proteome Res. 2009;8(10):4844–50. doi: 10.1021/pr9004162. Epub 2009/08/15. [DOI] [PubMed] [Google Scholar]
- 26.Fordahl S, Cooney P, Qiu Y, Xie G, Jia W, Erikson KM. Waterborne manganese exposure alters plasma, brain, and liver metabolites accompanied by changes in stereotypic behaviors. Neurotoxicol Teratol. 2012;34(1):27–36. doi: 10.1016/j.ntt.2011.10.003. Epub 2011/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakar S, Nunez NP, Pennisi P, Brodt P, Sun H, Fallavollita L, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147(12):5826–34. doi: 10.1210/en.2006-0311. Epub 2006/09/09. [DOI] [PubMed] [Google Scholar]
- 28.Ford NA, Dunlap SM, Wheatley KE, Hursting SD. Obesity, independent of p53 gene dosage, promotes mammary tumor progression and upregulates the p53 regulator microRNA-504. PLoS One. 2013;8(6):e68089. doi: 10.1371/journal.pone.0068089. Epub 2013/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho H, Kim JH. Lipocalin2 expressions correlate significantly with tumor differentiation in epithelial ovarian cancer. J Histochem Cytochem. 2009;57(5):513–21. doi: 10.1369/jhc.2009.953257. Epub 2009/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santin AD, Zhan F, Bellone S, Palmieri M, Cane S, Bignotti E, et al. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer. 2004;112(1):14–25. doi: 10.1002/ijc.20408. Epub 2004/08/12. [DOI] [PubMed] [Google Scholar]
- 31.Van Dross R, Soliman E, Jha S, Johnson T, Mukhopadhyay S. Receptor-dependent and receptor-independent endocannabinoid signaling: a therapeutic target for regulation of cancer growth. Life Sci. 2013;92(8–9):463–6. doi: 10.1016/j.lfs.2012.09.025. Epub 2012/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobhia ME, Grewal BK, Ml SP, Patel J, Kaur A, Haokip T, et al. Protein kinase C inhibitors: a patent review (2008–2009) Expert Opin Ther Pat. 2013;23(10):1297–315. doi: 10.1517/13543776.2013.805205. Epub 2013/06/26. [DOI] [PubMed] [Google Scholar]
- 33.Szydlowska M, Roszkowska A. Expression patterns of AMP-deaminase isozymes in human hepatocellular carcinoma (HCC) Mol Cell Biochem. 2008;318(1–2):1–5. doi: 10.1007/s11010-008-9773-x. Epub 2008/05/22. [DOI] [PubMed] [Google Scholar]
- 34.Bagnato A. The endothelin axis as therapeutic target in human malignancies: present and future. Curr Pharm Des. 2012;18(19):2720–33. doi: 10.2174/138161212800626157. Epub 2012/03/07. [DOI] [PubMed] [Google Scholar]
- 35.Bagnato A, Rosano L. Understanding and overcoming chemoresistance in ovarian cancer: emerging role of the endothelin axis. Curr Oncol Rep. 2012;19(1):36–8. doi: 10.3747/co.19.895. Epub 2012/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218–38. doi: 10.1111/j.1600-065X.2012.01151.x. Epub 2012/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong X, Tan B, Yin Y, Gao H, Li X, Jaeger LA, et al. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem. 2012;23(9):1178–83. doi: 10.1016/j.jnutbio.2011.06.012. Epub 2011/12/06. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Song G, Wu G, Gao H, Johnson GA, Bazer FW. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod. 2013;88(5):113. doi: 10.1095/biolreprod.112.105080. Epub 2013/03/15. [DOI] [PubMed] [Google Scholar]
- 39.Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008;31(3):187–99. doi: 10.1111/j.1365-2885.2008.00944.x. Epub 2008/05/13. [DOI] [PubMed] [Google Scholar]
- 40.Liang C, Chen W, Zhi X, Ma T, Xia X, Liu H, et al. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer. 2013;12:14. doi: 10.1186/1476-4598-12-14. Epub 2013/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zouhal H, Lemoine-Morel S, Mathieu ME, Casazza GA, Jabbour G. Catecholamines and obesity: effects of exercise and training. Sports Med. 2013;43(7):591–600. doi: 10.1007/s40279-013-0039-8. Epub 2013/04/25. [DOI] [PubMed] [Google Scholar]
- 42.Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110(22):8882–7. doi: 10.1073/pnas.1307237110. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.