Abstract
Background
The purpose of this study was to describe hospital and geographic variation in 30-day risk of surgical complications and death among colorectal cancer (CRC) patients and the extent to which patient-, hospital-, and census-tract-level characteristics increased risk of these outcomes.
Methods
We included patients at least 66 years old with first primary stage I–III CRC from the 2000–2005 National Cancer Institute’s Surveillance, Epidemiology, and End Results data linked with 1999–2005 Medicare claims. A multilevel, cross-classified logistic model was used to account for nesting of patients within hospitals and within residential census tracts. Outcomes were risk of complications and death after a complication within 30 days of surgery.
Results
Data were analyzed for 35,946 patients undergoing surgery at 1,222 hospitals and residing in 12,187 census tracts; 27.2 % of patients developed complications, and of these 13.4 % died. Risk-adjusted variability in complications across hospitals and census tracts was similar. Variability in mortality was larger than variability in complications, across hospitals and across census tracts. Specific characteristics increased risk of complications (e.g., census-tract-poverty rate, emergency surgery, and being African-American). No hospital characteristics increased complication risk. Specific characteristics increased risk of death (e.g. census-tract-poverty rate, being diagnosed with colon (versus rectal) cancer, and emergency surgery), while hospitals with at least 500 beds showed reduced death risk.
Conclusions
Large, unexplained variations exist in mortality after surgical complications in CRC across hospitals and geographic areas. The potential exists for quality improvement efforts targeted at the hospital and/or census-tract levels to prevent complications and augment hospitals’ ability to reduce mortality risk.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 Elderly CRC patients are more likely to die than younger patients, especially during the 30-day perioperative period.2–6 Wide variations in mortality rates across both hospitals and geographic areas suggest that safety and quality of cancer surgery could be improved, but quality improvement efforts are currently hampered by limited understanding of the reasons for such variability.7 To reduce 30-day mortality rates, many organizations, including the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, have focused quality improvement efforts to reduce complications. However, hospitals with high complication rates do not necessarily have high mortality rates.8 Alternately, hospitals with high complication rates but low mortality rates may be implementing successful interventions while hospitals with high mortality rates may not be as proficient at recognizing and managing serious complications once they occur.9
Little is known about factors contributing to increased risk of death after surgical complications.10 Identification of factors underlying observed variations may assist with identifying high-risk groups and opportunities to improve surgical outcomes, thus reducing postoperative mortality. Improving outcomes and reducing mortality are key objectives in CRC surgical management, particularly among elderly patients, those with comorbidities, or others at high risk. Little research has comprehensively evaluated hospital, patient, tumor, and neighborhood characteristics associated with risk of death after complications.10–12 Accordingly, in this study we asked two questions. First, we sought to describe variation in risk of CRC surgical complications and also death after complications across hospitals and geographic areas. Secondly, we wished to examine the extent to which patient sociodemographic, tumor, treatment, hospital, and neighborhood characteristics were associated with increased likelihood of surgical complications and also death after complications.
METHODS
Data Sources
We used 2000–2005 National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) data linked with 1999–2005 Medicare claims. Of SEER cancer patients aged 65 years or older, 94 % are linked with Medicare data.13 These data provide a rich source of information on Medicare patients included in the SEER population-based cancer registries.13 This study included data from 12 SEER registries, representing about 14 % of the US population. The study was reviewed and determined to be exempt from Institutional Review Board (IRB) oversight.
Study Population
We selected patients 66 years of age or older with a first primary invasive stage I–III CRC who had surgery between 2000 and 2005 with both Medicare Parts A and B. We included patients at least 66 years of age to allow for 1 year of complete claims data prior to diagnosis to determine comorbidity. Definitive CRC surgery (e.g. colectomy, proctectomy) was measured by searching inpatient, outpatient, and carrier claims using Healthcare Common Procedure Coding System (HCPCS) and/or International Classification of Diseases (version 9) codes.14 The date of the most extensive surgery was defined as the surgery date. We excluded patients with only autopsy or death certificate records of their cancer diagnosis and health maintenance organization members because claims are not available for the latter groups.
Outcomes
Our retrospective cohort study used two outcomes: (1) incidence of complications (e.g. postoperative pneumonia, surgical-site infection, deep vein thrombosis) within 30 days following CRC surgery identified from Medicare data using a previously developed algorithm and (2) all-cause mortality within 30 days following surgery among patients with one or more complications.15 The complications algorithm was developed by clinical experts and consists of ICD-9 diagnosis and procedure codes representing CRC resection complications and their treatment, including reoperations. Deaths occurring within 30 days of surgery, prior to and after discharge, were included and identified using Medicare data because SEER only includes month and year of death.9, 10, 12, 16
Covariates
We included covariates based on their association with incidence of complications, 30-day mortality, and a quality of care conceptual model.17–19 Patient sociodemographic and area characteristics included sex, race, comorbidity, dual eligibility for Medicare and Medicaid, age, census-tract poverty rate, and diagnosis year. To measure comorbidity (classified as none, one, or two or more), we searched for multiple chronic conditions occurring 1–12 months prior to diagnosis using the Charlson comorbidity index, Klabunde adaptation.20 Dual eligibility was defined as Medicaid eligibility for at least 1 month during the year before diagnosis as proxy for low income. Percentage of the population living in poverty in the census-tract of the patient’s residence at diagnosis was obtained from the 2000 Census. Hospital characteristics where surgery occurred included number of hospital beds and teaching hospital or not, obtained from the CMS Health care Cost Report and Provider of Service files. Tumor characteristics included American Joint Commission on Cancer (AJCC) stage, grade, location, and histology. Tumor location was classified as proximal colon (cecum, ascending), transverse colon (hepatic flexure, transverse colon, splenic flexure), distal colon (descending and sigmoid colon), or rectosigmoid junction or rectum. Treatment characteristics within 30 days included emergency surgery and type of surgery.14, 21, 22
Statistical Analysis
A Bayesian multilevel, cross-classified logistic model was used to account for nesting of CRC patients within hospitals and within patient residential census tract to obtain geographic variability.23 Cross-classified models allow patients to be nested within multiple separate, and unrelated, grouping “levels.” This structure allows patients from the same tracts to be treated at different hospitals and the same hospitals to treat patients from different tracts. Adjusted odds ratios and 95 % confidence intervals were calculated based on all variables entered into multivariable models. Model fit was based on Deviance Information Criterion (DIC), with lower values indicating better fit. The cross-classified Bayesian analysis was performed using WinBUGS (version 1.4.3). After 5000 burn-in iterations, 5000 additional iterations were kept for parameter estimates.
We calculated median odds ratio (MOR) to facilitate interpretation of the variance across tracts and hospitals on a scale directly comparable to odds ratios associated with other variables in the model.24 The MOR is based on the random effects variance component (V) from the regression model: MOR = exp(0.95 ). It can be interpreted as the median value of the ratio of predicted odds of the outcome for two patients randomly selected from different tracts (or hospitals) with equivalent covariates. If the MOR equals 1, it indicates no variation in outcome across tracts or hospitals. We obtained standard errors of census-tract-level and hospital-level variances to compute 95 % credible interval for MORs using Markov Chain Monte Carlo methods.
We calculated census-tract-level predicted values for both outcomes to describe geographic variability for tracts with at least six patients. Similarly, we calculated hospital-level predicted values for both outcomes to describe variability by randomly selecting ten hospitals each from hospitals groups by patient volume: 20–49 or 50 or more. Hospital-level and census-tract level predicted values were computed from multivariable models by averaging patient-level predicted probabilities for all patients residing in a census tract/treated at a hospital.
We calculated population attributable risk of death for all complications to characterize the clinical impact of each complication on risk of death. Population attributable risk is based on incidence of a complication and associated risk of death.25 Attributable risk estimates were calculated using win PEPI.26
RESULTS
Data included 35,946 patients undergoing CRC partial, subtotal, or total colectomy at 1,222 hospitals and residing in 12,187 census tracts. Most were white, not dual enrolled in Medicaid, younger than 85, and lived in census tracts with less than 10 % poverty rate (Table 1). Overall, 4.8 % of patients died. Of all patients, 10,001 (27.2 %) developed at least one complication; 65.8 % of these only during surgery hospitalization, 18.2 % only after discharge, and 16.0 % both during and after hospitalization. Of those with complications, 13.4 % (95 % CI 12.7–14.1) died. Of those who died after a complication within 30 days of surgery, 73.0 % died during CRC surgery hospitalization.
TABLE 1.
Characteristics of the study population of colorectal cancer patients, adjusted risk of development of post-operative complications and death among with at least one complication
| Complication (n = 35,946) |
30-Day postoperative mortality (n = 10,001) |
|||||||
|---|---|---|---|---|---|---|---|---|
| % | aOR | 95 % | CI | % | aOR | 95 % | CI | |
| Sociodemographic characteristics | ||||||||
| Poverty rate (%) | ||||||||
| <10 | 58.5 | 1.00 | 56.0 | 1.00 | ||||
| 10–19 | 25.4 | 1.06 | 1.00 | 1.14 | 26.1 | 1.21 | 1.03 | 1.42 |
| 20+ | 15.6 | 1.06 | 0.98 | 1.15 | 17.4 | 1.25 | 1.02 | 1.53 |
| Sex | ||||||||
| Male | 42.7 | 1.15 | 1.09 | 1.21 | 43.8 | 1.42 | 1.24 | 1.63 |
| Female | 57.4 | 1.00 | 56.2 | 1.00 | ||||
| Race | ||||||||
| White | 86.3 | 1.00 | 85.7 | 1.00 | ||||
| African-American | 7.1 | 1.09 | 0.98 | 1.21 | 8.5 | 0.87 | 0.67 | 1.14 |
| Other | 6.6 | 0.70 | 0.62 | 0.78 | 5.8 | 0.85 | 0.62 | 1.16 |
| Comorbidity | ||||||||
| 0 | 43.0 | 1.00 | 31.7 | 1.00 | ||||
| 1 | 29.3 | 1.39 | 1.31 | 1.48 | 29.0 | 1.09 | 0.93 | 1.29 |
| 2+ | 27.7 | 2.31 | 2.17 | 2.45 | 39.3 | 0.97 | 0.83 | 1.14 |
| Medicaid | ||||||||
| Yes | 15.9 | 1.38 | 1.29 | 1.49 | 20.8 | 0.95 | 0.80 | 1.13 |
| No | 86.1 | 1.00 | 79.2 | 1.00 | ||||
| Age | ||||||||
| 66–74 | 35.7 | 1.00 | 27.7 | 1.00 | ||||
| 75–84 | 45.8 | 1.41 | 1.33 | 1.49 | 46.4 | 1.57 | 1.30 | 1.89 |
| 85+ | 18.6 | 2.15 | 2.01 | 2.31 | 25.9 | 3.04 | 2.50 | 3.71 |
| Year | ||||||||
| 2000 | 16.3 | 1.00 | 15.4 | 1.00 | ||||
| 2001 | 16.8 | 1.06 | 0.96 | 1.16 | 16.4 | 0.97 | 0.77 | 1.22 |
| 2002 | 16.8 | 1.06 | 0.96 | 1.16 | 16.3 | 0.85 | 0.67 | 1.08 |
| 2003 | 17.5 | 1.13 | 1.04 | 1.24 | 17.7 | 0.85 | 0.68 | 1.06 |
| 2004 | 16.8 | 1.19 | 1.09 | 1.30 | 17.8 | 0.83 | 0.67 | 1.05 |
| 2005 | 15.8 | 1.13 | 1.03 | 1.23 | 16.4 | 0.75 | 0.60 | 0.95 |
| Hospital and surgeon characteristics | ||||||||
| Beds | ||||||||
| 1–199 | 25.2 | 1.00 | 25.0 | 1.00 | ||||
| 200–349 | 28.6 | 1.00 | 0.91 | 1.09 | 28.5 | 0.89 | 0.72 | 1.08 |
| 350–499 | 23.8 | 1.03 | 0.93 | 1.14 | 23.6 | 0.90 | 0.71 | 1.13 |
| 500+ | 22.4 | 1.06 | 0.94 | 1.19 | 22.9 | 0.80 | 0.61 | 1.05 |
| Teaching | ||||||||
| Yes | 49.7 | 1.00 | 49.8 | 1.00 | ||||
| No | 35.4 | 1.00 | 0.92 | 1.09 | 35.5 | 1.16 | 0.96 | 1.40 |
| Unknown | 14.9 | 0.89 | 0.89 | 1.09 | 14.7 | 1.24 | 0.98 | 1.57 |
| Surgeon case load | ||||||||
| <21 | 22.9 | 1.14 | 1.05 | 1.23 | 24.9 | 1.21 | 0.98 | 1.50 |
| 21–38 1 | 22.7 | 1.08 | 0.99 | 1.17 | 23.3 | 1.06 | 0.86 | 1.31 |
| 39–69 | 22.1 | 0.97 | 0.90 | 1.05 | 20.8 | 1.10 | 0.88 | 1.37 |
| 70+ | 23.0 | 1.00 | 21.2 | 1.00 | ||||
| Unknown | 9.5 | 9.8 | ||||||
| Tumor characteristics | ||||||||
| AJCC stage | ||||||||
| I | 26.4 | 1.00 | 21.8 | 1.00 | ||||
| II | 39.9 | 1.28 | 1.19 | 1.36 | 43.5 | 1.70 | 1.33 | 1.86 |
| III | 33.7 | 1.20 | 1.12 | 1.29 | 34.8 | 1.52 | 1.24 | 1.86 |
| Grade | ||||||||
| Well differentiated | 8.8 | 1.00 | 8.3 | 1.00 | ||||
| Moderately | 68.1 | 1.00 | 0.91 | 1.09 | 67.5 | 0.97 | 0.74 | 1.27 |
| Poorly | 18.7 | 1.05 | 0.94 | 1.16 | 19.7 | 1.23 | 0.91 | 1.65 |
| Undifferentiated | 1.0 | 1.33 | 1.03 | 1.72 | 1.2 | 1.42 | 0.77 | 2.60 |
| Unknown | 3.5 | 1.03 | 0.87 | 1.21 | 3.3 | 0.93 | 0.59 | 1.47 |
| Location | ||||||||
| Proximal colon | 40.7 | 0.79 | 0.72 | 0.87 | 38.6 | 1.43 | 1.09 | 1.87 |
| Transverse colon | 15.4 | 0.96 | 0.87 | 1.06 | 16.8 | 1.47 | 1.11 | 1.93 |
| Distal colon | 25.5 | 1.02 | 0.94 | 1.11 | 27.0 | 1.59 | 1.25 | 2.03 |
| Rectal | 18.4 | 1.00 | 17.6 | 1.00 | ||||
| Histology | ||||||||
| Mucinous adenocarcinoma | 86.6 | 1.00 | 86.0 | 1.00 | ||||
| Other adenocarcinoma | 12.9 | 1.02 | 0.94 | 1.10 | 13.4 | 1.03 | 0.85 | 1.24 |
| Non-adeno/unknown | 0.5 | 1.34 | 0.97 | 1.87 | 0.7 | 1.07 | 0.52 | 2.22 |
| Treatment characteristics | ||||||||
| Emergency surgery | ||||||||
| Yes | 22.2 | 2.16 | 2.04 | 2.29 | 33.9 | 1.69 | 1.48 | 1.94 |
| No | 77.8 | 1.00 | 66.2 | 1.00 | ||||
| Type of surgery | ||||||||
| Partial colectomy | 43.2 | 1.00 | 42.8 | 1.00 | ||||
| Subtotal colectomy/hemicolectomy | 55.7 | 1.03 | 0.97 | 1.11 | 52.5 | 0.89 | 0.76 | 1.05 |
| Total (procto) colectomy | 4.1 | 1.31 | 1.16 | 1.50 | 4.7 | 1.10 | 0.78 | 1.56 |
aOR adjusted odds ratio, 95 % CI 95 % confidence interval
Risk Factors for Complications and Death After Complications
Characteristics independently associated with increased risk of complications (Table 1) included being male, having comorbid conditions, receiving Medicaid, age 75 or older, more recent CRC diagnosis, lower surgeon case load, stage II or III diagnosis, undifferentiated tumors, emergency surgery, and total proctocolectomy. Patients of other races than white or African-American were less likely to develop complications. No hospital characteristics were significantly associated with risk of complications.
Characteristics of CRC patients with one or more complications at increased risk of dying within 30 days of surgery included living in census tracts with > 10 % poverty rate, being male, older, stage II or III diagnosis, having undifferentiated tumors, colon (vs. rectal) cancer diagnosis, and having emergency surgery (Table 1). Patients diagnosed in 2005 and those with surgery at hospitals with at least 500 beds had a reduced risk of death after complications. Tumor location was not associated with complication risk but did increase the risk of death after a complication.
Characteristics increasing risk of complications but not death after complications included having comorbid conditions and being dual enrolled in Medicaid.
Census-Tract and Hospital Variability
Statistically significant geographic (census-tract) variability in risk of complications (0.09) was present across census tracts, but risk-adjusted geographic variability was six times larger (0.56) for death after complications (Table 2). The MOR for census-tract variability in complications was 1.34, indicating that the odds of complications were greater than 1.34 for 50 % (median) of 2 randomly selected tracts. Hospital variability in complication risk (0.08) was small and the same as census-tract variability. Hospital variability in death after complications (0.16) was two times larger than hospital variability in incidence of complications (0.08). The MOR of 1.45 (95 % CI 1.33–1.58) indicates that the odds of death after complications was greater than 1.45 for 50 % of 2 randomly chosen hospitals. However, geographic variability in death after complications (0.56) was much larger than hospital variability in death after complications (0.16). Geographic and hospital variability remained in multivariable models, suggesting that none of the covariates (including hospital characteristics) accounted for census-tract or hospital variability in either outcome.
TABLE 2.
Adjusted census-tract variability and hospital variability in postoperative complications and 30-day postoperative mortality among colorectal cancer patients
| Complication |
30-day postoperative mortality |
|||||
|---|---|---|---|---|---|---|
| Parameter | 95 % | CI | Parameter | 95 % | CI | |
| Census-tract variability | ||||||
| Variance | 0.09 | 0.06 | 0.12 | 0.56 | 0.28 | 0.83 |
| MOR | 1.34 | 1.28 | 1.40 | 2.03 | 1.67 | 2.39 |
| Hospital variability | ||||||
| Variance | 0.08 | 0.06 | 0.10 | 0.16 | 0.09 | 0.23 |
| MOR | 1.31 | 1.27 | 1.35 | 1.45 | 1.33 | 1.58 |
MOR median odds ratio, 95 % CI 95 %confidence interval
Among the 41 tracts with at least six patients, the observed percentage of patients dying after complications ranged from 0.0 to 50.0 % (mean 12.7 %; median 14.3 %). For 17 of 41 tracts, the observed percentage was higher than predicted, indicating that patients living in these tracts did worse than expected controlling for covariates. For 24 of 41 tracts, the observed percentage was lower than predicted, indicating that patients in these tracts did better than expected.
Among the 30 hospitals with at least 20 patients, the observed percentage of patients who died after complications ranged from 3.2 to 30.0 % (mean 13.2 %; median 13.0 %). For 15 of 30 hospitals, the observed percentage was higher than predicted, indicating that patients treated at these hospitals did worse than expected controlling for covariates. For 15 of 30 hospitals, the observed percentage was lower than predicted, indicating that patients treated at these hospitals did better than expected.
Types of Complications and Death After Complications
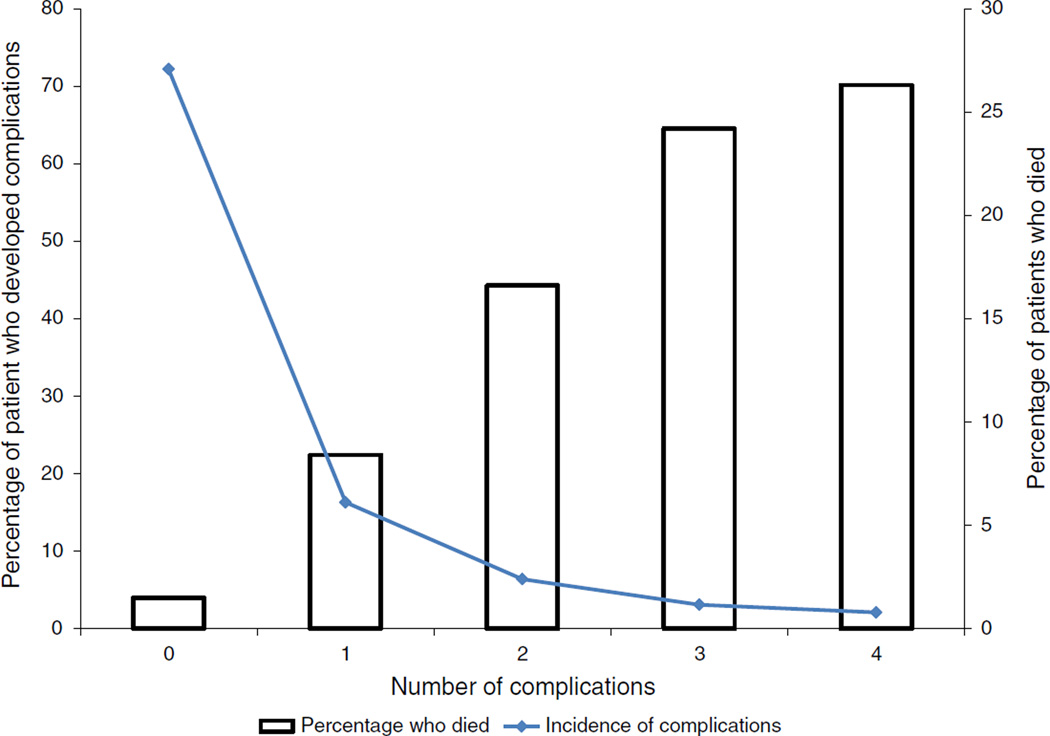
The most common complications were pulmonary (12.0 %, pneumonia and pulmonary failure combined), infections other than surgical-site and pulmonary infections (11.6 %), and surgical-site infections (7.6 %) (Table 3). At least 20 % of patients died after developing pulmonary, metabolic, or cardiac complications. Of the two types of pulmonary complications, 29.7 % died following postoperative pulmonary failure and 16.8 % died after postoperative pneumonia. The attributable risk of death was highest for pulmonary complications (47.9 %), suggesting that preventing pulmonary complications might in turn decrease mortality. Other complications with high attributable risks were postoperative pulmonary failure (38.9 %) and infections other than pulmonary infections and surgical-site complications (25.6 %). Risk of death increased dramatically with increasing number of complications (Fig. 1).
TABLE 3.
Types of complication associated with 30-day postoperative mortality in colorectal cancer patients, SEER-Medicare data, 2000–2005
| Type of complication |
No. CRC patients |
No. deaths |
30-day mortality, % (95 % CI) |
ARp, % (95 % CI) |
|---|---|---|---|---|
| Postoperative pulmonary failurea | 2,515 | 748 | 29.7 (28.0–31.5) | 38.9 (36.6–41.1) |
| Infection other than surgical-site and pulmonary infectionsa | 4,159 | 593 | 14.3 (13.2–15.3) | 25.6 (23.2–28.0) |
| Postoperative pneumoniaa | 2,548 | 429 | 16.8 (15.4–18.3) | 19.0 (17.0–21.1) |
| Metabolic, physiologica | 1,366 | 328 | 24.0 (21.8–26.3) | 15.7 (14.1–17.5) |
| Cardiac (MI or cardiogenic shock)a | 816 | 194 | 23.8 (20.9–26.7) | 9.1 (7.8–10.5) |
| Surgical-site infectiona,b | 2,722 | 262 | 9.6 (8.5–10.7) | 8.2 (6.5–10.0) |
| Deep vein thrombosis, pulmonary embolisma | 1,071 | 115 | 10.7 (8.9–12.6) | 3.8 (2.7–5.0) |
| Hemorrhage (postoperative hemorrhage, splenectomy)a | 186 | 26 | 14.0 (9.0–19.0) | 1.0 (0.5–1.6) |
| Neurologica | 62 | 33 | 53.2 (40.8–65.7) | 1.7 (1.3–2.2) |
| Hospital-acquired injurya | 71 | 14 | 19.7 (10.5–29.0) | 0.6 (0.3–1.1) |
Patients may have more than 1 complication
95 % CI 95 %confidence interval, ARp population attributable risk
p<0.01 for comparison of 30-day mortality between patients with and those without specific complications
Surgical-site infections include inflammation, fistula, drainage of postoperative infection, operative debridement of wound complications, reopening surgical site, reclosure of dehiscence/hernia, fecal diversion, and bowel resection or repair
FIG. 1.
Unadjusted risk of death after complications following colorectal cancer surgery, by number of complications
DISCUSSION
This population-based study describes risk of complications, risk of death after complications, and associated geographic and hospital variability following CRC surgery while integrating sociodemographic, clinical, tumor, treatment, hospital, and geographic characteristics. Our study is the first to demonstrate that variability of patient’s residential location plays a much larger role in risk of death after CRC surgery complications than hospital variability. Thus, regardless of where they were treated and the types of surgical treatment received, the experience of the surgeon, and clinical, tumor, and patient characteristics, CRC patients were at greater risk of death within 30 days following surgery if they lived in areas with worse economic conditions. Thus, in order to reduce risk of death after complications, it becomes important to take into account the residential location of patients upon discharge, particularly in census tracts where patients experienced higher than expected mortality. Future studies should identify reasons for this large geographic variation beyond factors included in this study. Interventions should focus on reducing geographic variability since characteristics of areas where CRC patients live are out of the control of hospitals and may help explain apparent differences in outcomes following complications.
Notably, patient-level factors associated with death after complications were different from factors associated with risk of complications. Death after complications was primarily associated with adverse tumor characteristics (advanced stage, poor or undifferentiated grade, and colon tumors), age 75 or older, and male gender.5 Patients with complications treated in hospitals with at least 500 beds were less likely to die within 30 days after complications, consistent with other studies.27 African-Americans and dual-enrolled Medicaid patients were at increased risk of developing complications, but not in risk of death after complications. Racial disparities were also absent in other studies examining short-term mortality.27
Hospital variation in death after complications was much larger than hospital variation in risk of complications, consistent with other studies.11 Hospital variability in death was not explained by our measured covariates, suggesting that quality of care may play a role. Quality of care may be improved by implementing multidisciplinary patient management (geriatricians or hospitalists in addition to surgeons), interventions shown to be effective in other types of surgery; reducing patient-to-nursing ratio; enhancing recovery after surgery; and implementing electronic medical records (EMR) models.28–34 Recently, “enhanced recovery after surgery” or “fast-tracking” initiatives (e.g. perioperative and postoperative interventions, improving patient education, preoperative nutrition, and pain management, so patients can be mobilized and resume normal diets earlier) designed to attenuate surgery stress and enable rapid recovery, reduced incidence of complications but not mortality.32, 33 With increasing EMR use, validated models could identify high-risk patients for evidence-based interventions such as multidisciplinary management.34 However, this may not be feasible in smaller low-resource hospitals.
As previously reported, adverse pulmonary events were the most common complication.35 Respiratory complications (including pulmonary failure and infections) were associated with higher 30-day mortality risk. Hospitals with higher rates of death after complications might focus on aggressive management of these high-risk complications with fluid management and early mobilization interventions.36, 37 Greater surveillance among patients at increased risk of death following complications and rapid and appropriate intervention might increase the likelihood of survival.38 Alternatively, hospitals could focus on preventing pulmonary and other high-mortality complications, such as interventions to reduce ventilator-assisted pneumonia.39
Study strengths include integrating multiple patient, tumor, geographic, hospital, and treatment factors into a single model estimating both hospital and geographic variability. However, our sample included only fee-for-service Medicare-insured patients, limiting generalizability. Also, claims data likely underreport complications; thus true incidence and attributable risk may be higher.40 In some instances it may be impossible to tell if a surgical-site infection is reported because the patient is in the hospital, or if the patient is in the hospital because of the surgical-site infection. Clinical and laboratory variables were not available. Serum albumin, creatinine, and inflammatory markers might explain observed variations, if they varied across hospitals or tracts.30, 41, 42 We also could not measure preoperative physical status, which is associated with 30-day mortality.30, 41–44
In conclusion, we identified large, unexplained variation in 30-day mortality after CRC surgery, across hospitals and especially geographic areas. These results suggest significant potential for hospital- and/or geographically targeted quality improvement efforts to prevent complications and augment hospitals’ ability to rescue patients after complications.
ACKNOWLEDGMENT
This work was supported by grants from the National Cancer Institute at the National Institutes of Health (Grant No. CA112159); and the Health Behavior, Communication and Outreach Core; the Core is supported in part by the National Cancer Institute Cancer Center Support Grant (Grant No. P30 CA91842) to the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri. Dr. Davidson was supported in part through grants HL-38180, DK-56260, and Digestive Disease Research Core Center DK-52574. We gratefully acknowledge James Struthers for his data management and programming services. We thank the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Health Behavior, Communication, and Outreach Core. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
REFERENCES
- 1.Cancer facts and figures. Atlanta: American Cancer Society; 2012. American Cancer Society. 2012. [Google Scholar]
- 2.Dekker J, van den Broek C, Bastiaannet E, van de Geest L, Tollenaar R, Liefers G. Importance of the first postoperative year in the prognosis of elderly colorectal cancer patients. Ann Surg Oncol. 2011;18:1533–1539. doi: 10.1245/s10434-011-1671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panis Y, Maggiori L, Caranhac G, Bretagnol F, Vicaut E. Mortality after colorectal cancer surgery: a French survey of more than 84,000 patients. Ann Surg. 2011;254:738–743. doi: 10.1097/SLA.0b013e31823604ac. (discussion 43-4) [DOI] [PubMed] [Google Scholar]
- 4.Fazio VW, Tekkis PP, Remzi F, Lavery IC. Assessment of operative risk in colorectal cancer surgery: the Cleveland Clinic Foundation colorectal cancer model. Dis Colon Rectum. 2004;47:2015–2024. doi: 10.1007/s10350-004-0704-y. [DOI] [PubMed] [Google Scholar]
- 5.Morris EJ, Taylor EF, Thomas JD, Quirke P, Finan PJ, Coleman MP, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut. 2011;60:806–813. doi: 10.1136/gut.2010.232181. [DOI] [PubMed] [Google Scholar]
- 6.Davila J, Rabeneck L, Berger D, El-Serag H. Postoperative 30-day mortality following surgical resection for colorectal cancer in veterans: changes in the right direction. Dig Dis Sci. 2005;50:1722–1728. doi: 10.1007/s10620-005-2925-x. [DOI] [PubMed] [Google Scholar]
- 7.Schootman M, Lian M, Pruitt SL, et al. Variability in 30-day mortality following colorectal cancer surgery. Health Serv Res. doi: 10.1111/1475-6773.12171a. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almoudaris AM, Burns EM, Mamidanna R, Bottle A, Aylin P, Vincent C, et al. Value of failure to rescue as a marker of the standard of care following reoperation for complications after colorectal resection. Br J Surg. 2011;98:1775–1783. doi: 10.1002/bjs.7648. [DOI] [PubMed] [Google Scholar]
- 9.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery: a study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–629. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Ghaferi AA, Dimick JB. Variation in mortality after high-risk cancer surgery: failure to rescue. Surg Oncol Clin N. Am. 2012;21:389–395. doi: 10.1016/j.soc.2012.03.006. vii. [DOI] [PubMed] [Google Scholar]
- 11.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 12.Friese CR, Earle CC, Silber JH, Aiken LH. Hospital characteristics, clinical severity, and outcomes for surgical oncology patients. Surgery. 2010;147:602–609. doi: 10.1016/j.surg.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendren S, Birkmeyer JD, Yin H, Banerjee M, Sonnenday C, Morris AM. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53:1587–1593. doi: 10.1007/DCR.0b013e3181f2f202. [DOI] [PubMed] [Google Scholar]
- 16.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. New Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93:501–515. doi: 10.1093/jnci/93.7.501. [DOI] [PubMed] [Google Scholar]
- 18.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24:2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 19.Donabedian A. The quality of care: How can it be assessed? JAMA. 1988;260:1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. [Accessed 18 July 2011];SEER-Medicare: Calculation of comorbidity weights. Available from URL: http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 21.Diggs JC, Xu F, Diaz M, Cooper GS, Koroukian SM. Failure to screen: predictors and burden of emergency colorectal cancer resection. Am J Manag Care. 2007;13:157–164. [PubMed] [Google Scholar]
- 22.Rabeneck L, Paszat LF, Li C. Risk factors for obstruction, perforation, or emergency admission at presentation in patients with colorectal cancer: a population-based study. Am J Gastroenterol. 2006;101:1098–1103. doi: 10.1111/j.1572-0241.2006.00488.x. [DOI] [PubMed] [Google Scholar]
- 23.Snijders TAB, Bosker RJ. Multilevel analysis An introduction to basic and advanced multilevel modeling. London: Sage; 1999. [Google Scholar]
- 24.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman RL, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluer, Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 26.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers SO, Jr, Wolf RE, Zaslavsky AM, Wright WE, Ayanian JZ. Relation of surgeon and hospital volume to processes and outcomes of colorectal cancer surgery. Ann Surg. 2006;244:1003–1011. doi: 10.1097/01.sla.0000231759.10432.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307:2185–2194. doi: 10.1001/jama.2012.4842. [DOI] [PubMed] [Google Scholar]
- 29.Hinami K, Feinglass J, Ferranti DE, Williams MV. Potential role of comanagement in “rescue” of surgical patients. Am J Manag Care. 2011;17:e333–e339. [PubMed] [Google Scholar]
- 30.Story DA. Postoperative mortality and complications. Best Pract Res Clin Anaesthesiol. 2011;25:319–327. doi: 10.1016/j.bpa.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288:1987–1993. doi: 10.1001/jama.288.16.1987. [DOI] [PubMed] [Google Scholar]
- 32.Spanjersberg W, Reurings J, Keus F, van Laarhoven Cornelis JHM. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database of Systematic Reviews [serial online] 2011;(2) doi: 10.1002/14651858.CD007635.pub2. Available from URL: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007635.pub2/abstract. [DOI] [PubMed] [Google Scholar]
- 33.Varadhan KK, Neal KR, Dejong CHC, Fearon KCH, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Richman JS, Hosokawa PW, Min S-J, Tomeh MG, Neumayer L, Campbell DA., Jr Toward prospective identification of high-risk surgical patients. Am Surg. 2012;78:755–760. [PubMed] [Google Scholar]
- 35.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53:424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 36.Havey R, Herriman E, O’Brien D. Guarding the gut: early mobility after abdominal surgery. Crit Care Nurs Q. 2013;36:63–72. doi: 10.1097/CNQ.0b013e3182753237. [DOI] [PubMed] [Google Scholar]
- 37.Holte K, Foss NB, Andersen J, Valentiner L, Lund C, Bie P, et al. Liberal or restrictive fluid administration in fast-track colonic surgery: a randomized, double-blind study. Br J Anaesth. 2007;99:500–508. doi: 10.1093/bja/aem211. [DOI] [PubMed] [Google Scholar]
- 38.Story DA. Postoperative mortality and complications. Best Pract Res Clin Anaesthesiol. 2011;25:319–327. doi: 10.1016/j.bpa.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 39.To KB, Napolitano LM. Common complications in the critically ill patient. Surg Clin North Am. 2012;92:1519–1557. doi: 10.1016/j.suc.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Romano PS, Schembri ME, Rainwater JA. Can administrative data be used to ascertain clinically significant postoperative complications? Am J Med Qual. 2002;17:145–154. doi: 10.1177/106286060201700404. [DOI] [PubMed] [Google Scholar]
- 41.Longo W, Virgo K, Johnson F, Oprian CA, Vernava AM, Wade TP, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. doi: 10.1007/BF02237249. [DOI] [PubMed] [Google Scholar]
- 42.Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg. 2009;208:1009–1016. doi: 10.1016/j.jamcollsurg.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Al-Refaie WB, Parsons HM, Habermann EB, Kwaan M, Spencer MP, Henderson WG, et al. Operative outcomes beyond 30-day mortality: colorectal cancer surgery in oldest old. Ann Surg. 2011;253:947–952. doi: 10.1097/SLA.0b013e318216f56e. [DOI] [PubMed] [Google Scholar]