Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease that affects 15 to 30% of children and ~5% of adults in industrialized countries1. Although the pathogenesis of AD is not fully understood, the disease is mediated by an abnormal immunoglobulin E (IgE) immune response in the setting of skin barrier dysfunction2. Mast cells (MCs) contribute to IgE-mediated allergic disorders including AD3. Upon activation, MCs release their membrane-bound cytosolic granules leading to the release of multiple molecules that are important in the pathogenesis of AD and host defense4. More than 90% of AD patients are colonized with Staphylococcus aureus in the lesional skin whereas most healthy individuals do not harbor the pathogen5. Several Staphylococcal exotoxins (SEs) can act as superantigens and/or antigens in models of AD6. However, the role of these SEs in disease pathogenesis remains unclear. Here, we report that culture supernatants of S. aureus contain potent MC degranulation activity. Biochemical analysis identified δ-toxin as the MC degranulation-inducing factor produced by S. aureus. MC degranulation induced by δ-toxin depended on phosphoinositide 3-kinase (PI3K) and calcium (Ca2+) influx, but unlike that mediated by IgE crosslinking, it did not require the spleen tyrosine kinase (Syk). In addition, IgE enhanced δ-toxin-induced MC degranulation in the absence of antigen. Furthermore, S. aureus isolates recovered from AD patients produced high levels of δ-toxin. Importantly, skin colonization with S. aureus, but not a mutant deficient in δ-toxin, promoted IgE and IL-4 production, as well as inflammatory skin disease. Furthermore, enhancement of IgE production and dermatitis by δ-toxin was abrogated in KitW-sh/W-sh MC-deficient mice and restored by MC reconstitution. These studies identify δ-toxin as a potent inducer of MC degranulation and suggest a mechanistic link between S. aureus colonization and allergic skin disease.
Because MCs may play a critical role in the pathogenesis of AD3, we asked first whether S. aureus can release factors that induce MC degranulation. We found that the culture supernatant of S. aureus induced rapid and robust MC degranulation in a dose-dependent manner (Fig.1a, Supplementary Fig.1a,b). Analysis of a panel of Staphylococcus isolates revealed that the culture supernatant of several S. aureus strains as well as of that from S. epidermidis and S. saprophyticus, but not of several Staphylococcus species elicited MC degranulation (Supplementary Fig. 1c). TLR2 stimulation via lipopeptides has been shown by some studies, but not others, to induce MC degranulation7,8. However, neither the culture supernatant of S. aureus deficient in lipoproteins (Δlgt), which lacks TLR2-stimulating activity9, nor that from bacteria deficient in α-, β-, and γ-hemolysins (Δαβγ) were impaired in MC degranulation activity (Supplementary Fig. 1c and 3c). The MC degranulation activity was enriched in the culture supernatant of S. aureus and was sensitive to heat, phenol/chloroform extraction and protease K treatment (Supplementary Fig. 2a). Furthermore, the MC degranulation-inducing factor bound to both diethylaminoethyl and carboxymethyl cellulose matrices and was present in the void fraction on gel filtration at neutral pH (Supplementary Fig. 2b). Based on these observations, we developed a multiple step strategy for biochemical purification of the MC degranulation-inducing factor (Supplementary Fig. 2c). Liquid chromatography-mass spectrometry analysis revealed that δ-toxin (also called δ-hemolysin, PSMγ), a 2.9 kDa peptide secreted by S. aureus that belongs to the peptide toxin family of phenol-soluble modulins (PSMs), was the most abundant and significant protein identified in the purified sample (Supplementary Fig.2c). Mutant analyses in two S. aureus strains revealed that MC degranulation induced by S. aureus culture supernatant required expression of δ-toxin whereas deficiency of related PSMα or PSMβ peptides had minimal or no effect on MC degranulation (Fig. 1b and Supplementary Fig. 3a). Importantly, complementation of the Δhld mutant strain with δ-toxin producing plasmid, but not control plasmid, restored the ability of the culture supernatant to induce MC degranulation (Fig. 1b). Stimulation of MCs with 30 µg/ml of synthetic δ-toxin peptide, a concentration of δ-toxin normally found in S. aureus culture supernatants (Supplementary Fig. 3b), also induced rapid release of histamine (Fig. 1c). Furthermore, transmission electron microscopy revealed classical features of MC degranulation without loss of plasma membrane integrity upon δ-toxin stimulation (Fig. 1d). These results indicate that δ-toxin is the MC degranulation-inducing factor released by S. aureus.
Figure 1. δ-toxin from S. aureus induces MC degranulation in vitro and in vivo.
a, β-Hexosaminidase activity released to the extracellular media of BMCMCs stimulated with medium alone (Control) or indicated stimuli including different concentrations of culture supernatant of S. aureus 8325-4 (S.a sup). b, β-Hexosaminidase activity in supernatants of MC/9 cells stimulated with 10% of culture supernatant from LAC S. aureus wild-type (LAC wt) or isogenic mutants deficient in PSMα peptides (LAC Δpsmα), PSMβ peptides (LAC Δpsmβ), δ-toxin (LAC Δhld), LAC wild-type expressing vector alone (LAC pTXΔ16), LAC deficient in δ-toxin expressing vector alone (LACΔhld pTXΔ16) and strain complemented with δ-toxin plasmid (LACΔhld pTXΔhld). Control represents 10% TSB medium. c, Histamine concentrations in culture supernatant of fetal skin-derived MCs (FSMCs) after stimulation with indicated stimuli including synthetic δ-toxin at 30 µg ml−1 for 15 min. Data represent means ± s.d. of triplicate cultures. Results are representative of at least 3 independent experiments (a-c). P value refers to comparisons between experimental and control groups (a-c). d, Electromicroscopic images of FSMCs stimulated with synthetic δ-toxin (30 µg ml−1) for 15 min. Images of unstimulated (Cont) and ionomycin-treated FSMCs are also shown. Representative of at least 20 images. e, δ-toxin expression in Staphylococcus culture supernatants (0.5 µl per well). Loading of lanes with synthetic δ-toxin (10 ng, 100 ng) is shown as reference. Representative of three experiments. f, C57BL6 (WT) and MC-deficient (Kit w-sh/w-sh) mice were injected intradermally into the left and right ears with δ-toxin (100 µg) or PBS, respectively. One representative mouse for each group is shown. Representative of 8 mice per group. g, Quantification of Evans blue extracted from skin tissue of WT, Kit w-sh/w-sh, Kit w-sh/w-sh reconstituted with BMCMCs is shown. Dots represent individual ear samples from 2 independent experiments. NS; no significant; *P < 0.05; **P < 0.01 ; ***P < 0.001, 2-tailed t test
PSMs, especially PSMα2 and PSMα3 induce cell death and IL-8 release in human neutrophils10,11. In accord with these results10, PSMα2 and PSMα3 induced robust loss of cell viability in MCs (Supplementary Fig. 4a). Non-toxic concentrations of PSMαs did not possess any MC-degranulation activity (Supplementary Fig. 4b). In contrast, stimulation with a concentration of δ-toxin that induces robust MC degranulation did not induce detectable cell death in MCs (Supplementary Fig. 4a,c). Furthermore, formylation of the N-terminus of the δ-toxin peptide was not required for MC degranulation activity, whereas it was essential for the ability of δ-toxin to induce the release of IL-8 from human neutrophils (Supplementary Fig. 4c,d). Consistent with previous results, stimulation of human neutrophils with formylated PSMα2, PSMα3 or δ-toxin induced robust IL-8 release (Supplementary Fig. 4d). Moreover, stimulation of primary mouse macrophages and keratinocytes with PSMα2, but not δ-toxin, triggered robust cell death (Supplementary Fig. 5). Thus, the MC degranulation activity induced by δ-toxin is not associated with cell death and is different from other activities triggered by PSMα2 and PSMα3. Immunoblotting confirmed that the presence of δ-toxin in S. aureus supernatants correlated with MC degranulation activity (Fig. 1e). Notably, the supernatant from S. epidermidis, a bacterium that is present in normal skin, possessed weak MC degranulation which correlated with smaller amounts of δ-toxin when compared to that from S. aureus strains (Fig. 1e and Supplementary Fig. 6). Furthermore, deficiency of δ-toxin had a larger effect on MC degranulation in S. aureus than in S. epidermidis (Supplementary Fig. 6). To assess whether δ-toxin induces MC degranulation in vivo, we injected synthetic δ-toxin into the skin of mouse ears and monitored MC degranulation by the vascular leakage of Evan's blue dye into the extravascular space using the passive cutaneous anaphylaxis (PCA) assay. Intradermal administration of δ-toxin induced Evan’s blue dye leaking at the site of injection in wild-type mice, but not in MC-deficient KitW-sh/W-sh mice (Fig. 1f,g). Importantly, reconstitution of the skin of KitW-sh/W-sh mice with bone marrow-derived cultured MCs (BMCMCs) restored leaking of the dye upon administration of δ-toxin (Fig. 1g). Moreover, the culture supernatant from the δ-toxin positive LAC strain induced Evan’s blue dye leaking whereas that from δ-toxin negative LAC Δhld and SA113 strains did not (Supplementary Fig. 7). These results indicate that δ-toxin induces MC degranulation in vitro and in vivo.
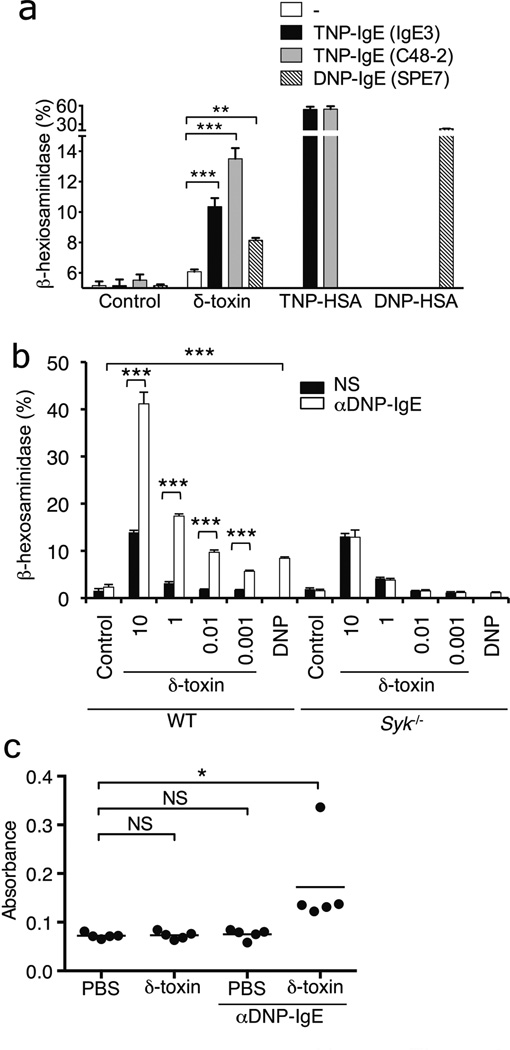
δ-toxin triggers Ca2+ influx through FPR2 in human neutrophils11. Because Ca2+ influx is an essential step in MC degranulation, we analyzed whether δ-toxin induces Ca2+ influx in MCs. Stimulation of MCs with ionomycin or DNP plus anti-DNP IgE induced rapid Ca2+ influx (Fig. 2a). Likewise, δ-toxin triggered Ca2+ influx and this was abrogated by treatment with the Ca2+ chelator ethylene glycol tetraacetic acid (EGTA) (Fig. 2a). EGTA also blocked MC degranulation induced by ionomycin, DNP plus anti-DNP IgE or δ-toxin (Fig. 2b). Similarly, MC degranulation induced by DNP plus anti-DNP IgE or δ-toxin was inhibited by the PI3 kinase inhibitor, LY294002 (Fig. 2c). However, unlike antigen plus IgE, MC degranulation induced by δ-toxin did not require Syk (Fig. 2d). Fpr1, Fpr2 and related family members were expressed in mouse MCs although their expression was higher in neutrophils (Supplementary Fig. 8). WRW4, a peptide antagonist of formyl peptide receptor 2 (FPR2), blocks human and mouse neutrophil activation induced by PSMs including δ-toxin11. Notably, WRW4 inhibited mouse MC degranulation induced by δ-toxin both in vitro and in vivo (Supplementary Fig. 9a,b). Cyclosporin H, an antagonist of human FPR1, also partially inhibited mouse MC degranulation induced by δ-toxin (Supplementary Fig. 9c). However, human FPR2 ligands, MMK1 and Lipoxin A4, did not induce mouse MC degranulation (Supplementary Fig. 10a). Furthermore, treatment with pertussis toxin (PTX), an inhibitor of G-protein coupled receptors, reduced partially MC degranulation induced by δ-toxin (Supplementary Fig. 10b). However, MCs from wild-type and Fpr2−/− mice exhibited comparable MC degranulation induced by δ-toxin (Supplementary Fig. 10c). Collectively, these results suggest that δ-toxin induces MC degranulation via a signaling pathway that is different from that induced through antigen and IgE.
Figure 2. δ-toxin-induced MC degranulation depends on Ca2+ influx/PI3K pathway, but is independent of Syk.
a, FSMCs loaded with the fluorescent Ca2+ indicator Fluo-4AM with or without EGTA were stimulated for 50 sec. Baseline fluorescence (red) was measured, and then the MCs were stimulated with indicated stimuli and fluorescence shift (green) was measured. RFU, relative fluorescence units. b, c, β-Hexosaminidase activity in culture supernatants of FSMCs pretreated with EGTA (b) or LY294002 (c) stimulated with medium alone (Crtl), ionomycin, DNP-HSA (DNP) plus anti DNP-IgE or δ-toxin (10 µg ml−1). d, β-Hexosaminidase activity in culture supernatants of FSMCs derived from Syk−/− and wild-type (WT) mice stimulated with indicated concentration of δ-toxin (µg ml−1). Data represent means ± s.d. of triplicates cultures and representative of at least 3 independent experiments (b-d). NS; no significant; *P < 0.05; **P < 0.01 ; ***P < 0.001, 2-tailed t test
Stimulation with IgE and antigen, but not monomeric IgE, induces robust MC degranulation4. Notably, pre-incubation of MCs with anti-DNP or anti-TNP IgE alone increased markedly the degranulation activity of δ-toxin (Fig. 3a). The synergistic effect of monomeric IgE and δ-toxin was abrogated in MCs deficient in Syk (Fig. 3b). To test whether the synergism between monomeric IgE and δ-toxin could be observed in vivo, we injected monomeric IgE and δ-toxin (at concentrations that do not induce MC degranulation) into the skin of mice and monitored MC degranulation in vivo with the PCA assay. At these inactive concentrations, δ-toxin induced Evans blue dye leaking at the site of injection in mice pretreated with anti-DNP (Fig. 3c). These results indicate that IgE increases the MC degranulation activity of δ-toxin in the absence of antigen.
Figure 3. Antigen-independent IgE signaling enhances δ-toxin-induced MC activation.
a, β-Hexosaminidase activity in culture supernatants of FSMCs stimulated with or without anti DNP-IgE or TNP-IgE and then re-stimulated with δ-toxin (0.01 µg ml−1), DNP-HSA (DNP) or TNP-HSA (TNP). b, β-Hexosaminidase activity in culture supernatants of FSMCs derived from Syk−/− and wild-type mice (WT) pretreated with or without anti DNP-IgE, and then stimulated with indicated concentration of δ-toxin (µg ml−1). Representative of at least 3 independent experiments. **P < 0.01; ***P < 0.001, 2-tailed t test (a,b). c, Quantification of Evans blue extracted from skin tissue of C57BL6 mice injected intradermally into the left and right ears with δ-toxin (5 µg) or PBS, respectively. Data represent means ± s.d. of triplicate cultures and representative of at least 3 independent experiments (a,b). Dots represent individual ear samples. Representative of 2 independent experiments (c). NS; no significant; *P < 0.05, one-way ANOVA with Tukey post-hoc test for multiple comparisons
δ-toxin is encoded by RNAIII, a regulatory RNA that is induced via the agr quorum-sensing system of S. aureus 12. Notably, supernatants from 26 S. aureus strains isolated from the lesional skin of AD patients produced δ-toxin (Supplementary Fig. 11a). Moreover, RNAIII expression was detected in lesional skin colonized with S. aureus, but not normal skin, of AD patients (Supplementary Fig. 11b-c). To test whether δ-toxin plays a role in allergic skin disease, we used a modified epicutaneous disease model in which the skin of BALB/c mice was colonized with wild-type or δ-toxin-deficient S. aureus and then challenged once with ovalbumin (OVA) to assess antigen-specific IgE production (Fig. 4a). One week after colonization with wild-type S. aureus, the mice developed severely inflamed reddened skin at the site of application (Fig. 4b,c). Expression of hld was detected in the skin on day 4 after bacterial colonization using a bioluminescent reporter S. aureus strain (Supplementary Fig. 12). Histological analysis revealed spongiosis and parakeratosis and marked neutrophil-rich inflammatory infiltrates in the skin of mice colonized with wild-type S. aureus (Fig. 4c,d). In contrast, mice colonized with S. aureus lacking δ-toxin exhibited a significantly reduced skin inflammatory cell infiltrate and disease score (Fig. 4c, 4b,d). Complementation of the Δhld mutant with a plasmid producing δ-toxin restored the disease score to levels comparable to those observed with the wild-type bacterium (Supplementary Fig. 13). The differential ability of wild-type and mutant S. aureus to promote inflammatory disease was not explained by differences in skin colonization (Supplementary Fig. 14a, b). Furthermore, mice colonized with wild-type S. aureus developed greater amounts of total serum IgE and IgG1, but not IgG2a, as well as IL-4 in the skin than mice inoculated with the δ-toxin mutant bacterium (Fig. 4e and Supplementary Fig. 14c and Supplementary Fig. 15). At three weeks, there was a slight increase in IgG1 production in mice colonized with the δ-toxin mutant bacterium compared to PBS control (Supplementary Fig.15c), suggesting the existence of a minor S. aureus-dependent, but δ toxin-independent pathway for IgG1 production. In addition, pre-colonization with wild-type, but not the δ-toxin-deficient S. aureus, enhanced the production of OVA-specific IgE (Fig. 4f). Colonization with S. aureus without disrupting the skin barrier by stripping also induced inflammatory disease and enhanced IgE responses (Supplementary Fig. 16). Pre-colonization with δ-toxin producing S. aureus was important to elicit antigen-specific IgE because administration of OVA prior to or concurrent with S. aureus colonization did not enhance OVA-specific IgE production (Supplementary Fig. 17). To test whether δ-toxin is sufficient to trigger allergic skin disease, we epicutaneously sensitized the skin of mice with OVA in the presence and absence of δ-toxin and challenged the mice with OVA alone or OVA plus δ-toxin 3 weeks later. We found that δ-toxin triggered inflammatory skin disease including OVA-specific IgE and IgG1 production whereas challenge with OVA alone did not (Supplementary Fig. 18) C57BL/6 mice colonized with wild-type S. aureus also developed higher serum IgE levels and more severe inflammatory skin disease than mice inoculated with the bacterium deficient in δ-toxin (Fig. 4g,h). Importantly, MC-deficient KitW-sh/W-sh mice inoculated with wild-type S. aureus showed reduced IgE serum levels and skin inflammation when compared to wild-type mice (Fig. 4g,h). Adoptive transfer of MCs into the skin of KitW-sh/W-sh mice restored skin disease and increase IgE production in mice colonized with wild-type, but not S. aureus lacking δ-toxin (Fig. 4g,h and Supplementary Fig. 19). There were increased numbers of S. aureus and total bacteria in the skin of KitW-sh/W-sh mice (Supplementary Fig. 19), suggesting that mast cells can regulate bacterial colonization under our experimental conditions. Microscopic analysis showed that the dermal MC densities in the skin of KitW-sh/W-sh recipient mice were ~50% of those found in age-matched C57BL/6 mice (Supplementary Fig. 19>). Furthermore, toluidine-positive granules associated with MC degranulation were present in the skin of mice colonized with wild-type, but not δ-toxin-deficient, S. aureus (Supplementary Fig. 19). Taken together, these results indicate that δ-toxin from S. aureus promotes allergic skin disease via activation of MCs.
Figure 4. Staphyloccocus δ-toxin promotes IgE production and inflammatory skin disease via mast cells.
a, S. aureus colonization and OVA sensitization protocol. Mice were colonized epicutaneously with 108 CFU S. aureus using a gauze patch for 1 week. For OVA sensitization, a patch containing OVA or PBS was applied to the same skin site 2 weeks after S. aureus inoculation. b, Skin disease score 1 week post colonization with wild-type and δ-toxin mutant (Δhld) S. aureus or treated with PBS. **P < 0.01; ***P < 0.001, Kruskal-Wallis test with post-hoc Dunn’s test for multiple comparisons. c, Skin phenotype and histopathology of BALB/c mice colonized with S. aureus or treated with PBS. Skin sections were stained with H&E. Bar = 100 µm. Inset shows high power image with neutrophil-rich inflammation. Representative of 14 mice per group. d, Number of inflammatory cells in skin of BALB/c mice colonized with S. aureus or treated with PBS. Results depicted as number of inflammatory cells per high power field (hpf). Error bars represent means ± s. e. m. e, Serum levels of IgE in BALB/c mice colonized with S. aureus or treated with PBS at 1 and 3 weeks post colonization with S. aureus. f, Serum levels of OVA-specific IgE after OVA sensitization in BALB/c mice colonized with S. aureus or treated with PBS. g, Skin disease score in C57BL/6 (B6), MC-deficient (KitW-sh/W-sh) and MC-deficient (KitW-sh/W-sh) mice reconstituted with MCs at 1 week after the inoculation with S. aureus. h, Serum levels of total IgE 1 week after colonization of B6, KitW-sh/W-sh and KitW-sh/W-sh mice reconstituted with MCs with wild-type and δ-toxin mutant (Δhld) S. aureus or treated with PBS. Dots represent individual mice pooled from two independent experiments. *P < 0.05; **P < 0.01 ; ***P < 0.001, one-way ANOVA with Tukey post-hoc test for multiple comparisons (e-h)
The δ-toxin transcript is contained within RNAIII, a regulatory RNA that governs S. aureus virulence genes13,14. The role of δ-toxin in the growth of S. aureus is not understood. Because δ-toxin can form pores on the surface of certain bacteria15, one possibility is that it promotes pathogen colonization by killing competing bacteria. Our results suggest that the host senses S. aureus through the detection of δ-toxin to promote innate and adaptive Th2 immune responses via MC degranulation. Although clinical studies are needed to determine the role of δ-toxin in AD, our results in mouse models suggest that in the setting of genetic defects associated with the disease2, δ-toxin may promote allergic immune responses and that strategies to inhibit δ-toxin might be beneficial for the treatment of AD.
Method Summary
Culture of mast cells and degranulation
Preparations of BMCMCs and fetal skin-derived mast cells (FSMCs) were previously described16. The purity of MCs was > 95 % as assessed by surface expression of FcεRI and CD117 (eBioscience). Degranulation of MCs was assessed by β-hexosaminidase assay as described16.
Passive cutaneous anaphylaxis (PCA) assay
PCA assay was performed as described with minor modifications17.
Epicutaneous sensitization with S. aureus
The dorsal skin of 6–8 week old female mice was shaven and stripped using a transparent bio-occlusive dressing (Tegaderm®; 3M). 108 CFU of S. aureus strains were placed on a patch of sterile gauze and attached to the shaved skin with another transparent bio-occlusive dressing (Tegaderm®; 3M). Each mouse was exposed to S. aureus for 1 week through the patch. After a 2-week interval, each mouse was challenged once with 100 µg ovalbumin epicutaneously for 1 week and the animals were sacrificed for analyses.
Animal study
All animal studies were performed according to approved protocols by the University of Michigan Committee on the Use and Care of Animals.
Statistical analysis
All analyses were performed using GraphPad Prism. Differences were considered significant when p values were less than 0.05.
Methods
Bacterial strains
S. aureus strain 8325-4 and its isogenic toxin mutant (Δαβγ) have been previously described18. S. aureus strains SA113 and Newman, and isogenic mutants deficient in lipoprotein diacylglyceryl transferase (Δlgt) have also been previously described19. S. aureus strains LAC and MW2, their isogenic δ-toxin mutants (Δhld), the psm gene deleted mutants (Δpsmα, Δpsmβ), and LAC agr mutant (Δagr) have been previously described10. The isogenic Δhld mutant of S. epidermidis 1457, a clinical isolate20 was produced by an allelic replacement procedure21. This was done in a way analogous to the S. aureus Δhld mutants used herein, abolishing translation by exchanging the third base in the hld start codon from ATG to ATA (to avoid interfering with the function of RNAIII). LAC P3-lux was constructed by integration of the S. aureus LAC agr P3 promoter fused to the luxABCDE operon of Photorhabdus luminescens with codon usage optimized for staphylococci22 into the Φ11 attB site of the S. aureus genome, using a procedure described by Luong and Lee23. Plasmid pTXΔhld was constructed by cloning the hld coding sequence containing the ribosomal binding site region in the BamH1/Mlu1 sites of plasmid pTXΔ10. The hld gene was amplified from the genomic DNA of the respective strain, because the δ-toxin sequence differs in one amino acid in position 10 (serine or glycine) in these two strains. The δ-toxin is constitutively expressed in these plasmids. See Supplementary Table 1 for all oligonucleotides used in generation of the strains. Clinical isolates of S. aureus from children diagnosed with AD were obtained originally from the Department of Laboratory Medicine and Pathobiology at the University of Toronto24. S. epidermidis (NI335), S. cohnii (NI446), S. saprophyticus (NI488), S. xylosus (NI987), S. sciuri (NI981), S. succinus (NI534), S. lentus (NI487) and S. fleuretti (NI533) were isolated by plating on BHI after culturing at 37°C for two days under aerobic conditions. Identification of bacterial species was verified by 16S rRNA gene sequencing as described25. Bacterial supernatants were produced by overnight culture with shaking in tryptic soy broth (TSB) followed by filtration through a 0.2 µm filter.
Mice
C57BL/6, C57BL/6-KitW-sh/KitW-sh (B6.CG-KitW-sh/HNihrJaeBsmJ), and BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). Syk+/− mouse breeders were a gift of Dr. Steven Teitelbaum (Washington University School of Medicine, St. Louis, MO) and Syk−/− embryos were generated by intercrossing. We used 4–12 week old age-matched female mice for in vivo experiments. Mice were allocated randomly into experimental groups. All mouse strains were housed under pathogen-free conditions. The animal studies were conducted under approved protocols by the University of Michigan Committee on Use and Care of Animals.
Materials
The synthetic peptides fPSMα2 (fMGIIAGIIKVIKSLIEQFTGK), fPSMα3 (fMGIIAGIIKFIKGLIEKFTGK), fδ-toxin (fMAQDIISTIGDLVKWIIDTVNKFTKK), (WRWWWW-CONH2) and MMK-1 (LESIFRSLLFRVM) were purchased from American Peptide. Unformylated δ-toxin (MAQDIISTIGDLVKWIIDTVNKFTKK) was synthesized at The University of Michigan Protein Structure Facility. Polyclonal anti-δ-toxin antibody was produced in rabbits by immunization with a synthetic multiple antigenic peptide displaying an 18 amino acid peptide (IGDLVKWIIDTVNKFTKK) (Sigma-Genosys) from the full length δ-toxin sequence. Rabbit IgG was purified from rabbit serum on Protein A (Pierce) according to the manufacturer’s protocol.
Protein purification from S. aureus culture supernatant
S. aureus was cultured in 700 ml chemical defined medium supplemented with 2% yeast extract26. Filtrated cultured supernatant was incubated with carboxymethyl cellulose equilibrated with 10 mM sodium citrate (pH 5.5), and eluted with a linear gradient of 0–1 M NaCl. Fractions containing β-hexosaminidase activity were collected and adjusted at pH 7.4, 100 mM HEPES. The sample was concentrated using Amicon Ultra-15, 5 kDa filter (Millipore). Concentrated sample was further fractionated with a Superdex 200 10/300 GL column (GE). Final positive fractions were pooled and concentrated using an Amicon Ultra-15 filter (Supplementary Fig. 2b).
Protein identification by LC-tandem MS
Purified sample was denatured in 8 M urea, reduced by incubation with 10 mM DTT at 37 °C for 30 min and alkylated using 50 mM iodoacetamide at room temperature for 30 min. The protein sample was digested with sequencing grade trypsin (Promega) overnight at 37 °C. The reaction was terminated by acidification with trifluoroacetic acid (0.1% v/v) and peptides were purified using a SepPak C18 cartridge following the manufacturer’s protocol (Waters Corporation). Eluted peptides were directly introduced into an ion-trap mass spectrometer (LTQ-XL, Thermo Fisher) equipped with a nano-spray source. The mass spectrometer was operated in data-dependent MS/MS mode to acquire a full MS scan (400–2000 m/z) followed by MS/MS on the top 6 ions from the full MS scan. Dynamic exclusion was set to collect 2 MS/MS spectra on each ion and exclude it for a further 2 min. Raw files were converted to mzXML format and searched against S. aureus NCTC 8325 database appended with decoy (reverse) database using X! Tandem with k-score plug-in, an open-source search engine developed by the Global Proteome Machine (www.thegpm.org). Search parameters included a precursor peptide mass tolerance window of 1 Da and fragment mass tolerance of 0.5 Da. Oxidation of methionine (+16 Da), and carbamidomethylation of cysteines (+57 Da) were considered as variable modifications. Search was restricted to tryptic peptides with one missed cleavage. Results of the X! Tandem search were then subjected to Trans-Proteomic Pipeline (TPP) analysis, a suite of software including PeptideProphet and ProteinProphet. All proteins with a ProteinProphet probability of >0.9 were considered positive and verified manually.
Culture of mast cells and degranulation
Preparations of BMCMCs and fetal skin-derived mast cells (FSMCs) were previously described16. Bone marrow cells from Fpr2−/− mice were generously provided by Dr. Ji Ming Wang (Center for Cancer Research, National Cancer Institute, US). The purity of MCs was >95% as determined by surface expression of FcεRI and CD117 (eBioscience). Degranulation of MCs was assessed by β-hexosaminidase assay as previously described16. Briefly, MCs (2 × 106 ml−1) were preloaded with or without IgEs (anti-DNP IgE{clone; SPE7}; 0.3 µg ml−1, anti-TNP IgE{clone; IgE3 and C48-2}; 0.5 µg ml−1) in RPMI with IL-3 for 15 h. The cells were resuspended in Tyrode’s buffer (Sigma) at 2 × 104 cells per 100 µl for FSMCs or 1 × 105 cells per 100 µl for BMCMCs and MC/9 cells, aliquoted in triplicate into a 96 well U-bottom plate and incubated with EGTA (1 mM, Sigma), LY294002 (100 µM, Sigma), WRW4 (10 µM) and Cyclosporine H (10 µM, Alexis Biochemicals) for 30 min, and then stimulated DNP-HSA (30 ng ml−1) TNP-HSA (30 nM) for 30 min, Ionomycin (1 µM, Sigma), δ-toxin (indicated concentrations), PSMαs (indicated concentrations) or FPR2 ligands for 15 min. Results of various stimuli are given as a relative percentage, where freeze and thaw of total cell culture represents 100%.
MC-reconstitution in KitW-sh/W-sh mice
For BMCMC reconstitution experiments, 106 BMCMCs (cell purity was > 95 %) were injected into the ear skin. 4 × 106 BMCMCs in 50 µl × 8 injections were injected into the shaved back skin of non-randomized KitW-sh/W-sh mice as described27. 4–6 weeks later, the mice were subjected to experimental passive cutaneous anaphylaxis assay or epicutaneous S. aureus sensitization. The number of animals per group (n= 5–8) was chosen as the minimum likely required for conclusions of biological significance, established from prior experience. The reconstitution rate of cutaneous MCs was quantified blindly by an independent observer and scored as number of MCs per low power field in toluidine blue stained tissue slides by microscope. The average rate of reconstituted MCs was ~40% in the ear pina and ~50% in the back skin (Supplementary Fig. 19 and 20).
Passive cutaneous anaphylaxis assay
PCA assay was performed as previously described with minor modifications17. Ears of non-randomized mice were injected intradermally with or without αDNP-IgE in 40 µl saline and 15 h later, mice were challenged with 20 µl saline with or without synthetic δ-toxin (100 µg or 5 µg) or TSB bacteria supernatants. The number of animals per group (n= 5–8) was chosen based on previous experience as the minimum likely required for conclusions of biological significance. After inoculation, 0.1 ml of 5 mg ml−1 Evans blue dye was injected intravenously. Extravasation of Evans blue dye was monitored for 30 min, and 4 mm of punched-out biopsies were incubated at 63°C overnight in 200 µl formamide. Quantitative analysis of extracts was determined by measuring the absorbance at 600 nm.
Ca2+ influx assay
FSMCs (2 × 106 ml−1) were preloaded with or without anti-DNP-IgE (0.3 µg ml−1) in RPMI with IL-3 for 15 h. Cells were washed and then loaded with Fluo-4AM (5 µM, Life Technologies) for 30 min. Cells were washed again and further incubated in Tyrode’s buffer with or without EGTA (1 mM) for 30 min. DNP-HSA (30 ng ml−1), Ionomycin (1 µM) or δ-toxin (30 µg ml−1) were used to induce calcium flux in these cells. Ca2+ flux was measured using a flow cytometer (FACSCalibur, BD Biosciences) to monitor RFU (relative fluorescence units) as described28.
Epicutaneous sensitization with S. aureus or OVA
We performed epicutaneous colonization with S. aureus by shaving the dorsal skin of non-randomized 6–8 week old female mice and 3-time stripping using a transparent bio-occlusive dressing (Tegaderm®; 3M). Sample size (n = 5–8 per group) was based on prior experience as the size necessary for conclusions of biological significance and adequate statistical analysis. After overnight culture at 37°C with shaking, S. aureus were cultured in fresh TSB medium for 4 hrs at 37°C with shaking, washed and resuspended in PBS at 108 CFU of S. aureus LAC or LAC (Δhld) strains. 100 µl of the S. aureus suspension was placed on a patch of sterile gauze (1 × 1 cm) and attached to the shaved skin with transparent bio-occlusive dressing. Each mouse was exposed to S. aureus for 1 week through the patch. After a 2-week interval, each mouse was challenged once with 100 µg OVA (Grade V, Sigma) epicutaneously for 1 week and the animals were sacrificed for analyses. For OVA sensitization model, BALB/c mice were sensitized epicutaneously with OVA (100 µg) with or without synthetic δ-toxin (100 µg) for 1 week. After 2 week interval, mice were challenged with OVA (100 µg) with or without synthetic δ-toxin (100 µg) at the same skin site.
Skin disease score
The severity of skin lesions was scored according to defined macroscopic diagnostic criteria in a blind fashion29. In brief, the total clinical score of skin lesions was designated as the sum of individual scores, graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) for thickness, erythema, edema, erosion, and scaling.
Histology
Skin tissue was formalin fixed, paraffin embedded and sectioned for H&E and Toluidine blue staining.
Cytokine and immunoglobulin levels
Chemokines and cytokines were measured with enzyme-linked immunoabsorbent assay (ELISA) kits (R&D Systems). For tissue cytokines, skin tissue (5 × 10 mm area) was removed and homogenized. The skin homogenates were centrifuged and supernatants were collected for cytokine measurements by ELISA. Serum IgG1 and IgG2a were measured with ELISA kit (Cayman chemical). Serum IgE was measured with ELISA kit (Bethyl Laboratories). ELISA for OVA-IgE was described previously30.
RNA isolation from human skin samples
Wash fluid derived from lesional and normal skin of AD patients was collected using a 2.5-cm-diameter polypropylene chamber as reported31. 100 µl of the samples were mixed with an equal volume of RNAprotect Bacteria Reagent (QIAGEN) and RNA extracted with Bacterial RNA Kit (OMEGA). The human studies were approved by the Indiana University Institutional Review Committee31. Informed consent was obtained from all subjects.
Quantitative real time RT-PCR
cDNA was synthesized using High Capacity RNA-to-cDNA Kit (Applied Biosystems), according to the manufacturer’s instructions. Quantitative real time RT-PCR (qPCR) was performed using a SYBR green PCR master mix (Applied Biosystems) and StepOne Real-time PCR system (Applied Biosystems). Primers to amplify mouse Fpr genes32 and bacterial genes (RNAIII, gyrB, 16S rRNA) have been described33,34. Expression of mouse Fpr genes was normalized to that of Gapdh (F; 5-CCTCGTCCCGTAGACAAAATG-3, R; 5-TCTCCACTTTGCCACCTGCAA-3) and expression were analyzed by the 2−ΔΔCt method. Expression of RNAIII expression in human skin samples was normalized to that of S. aureus gyrB and that of gyrB to universal bacterial 16S rRNA and relative expression calculated by the 2−ΔCt method. RNAIII and gyrB expression in some human skin samples were below the detection limit and arbitrarily given a value of zero for statistical analysis. LAC wt and LAC Δagr cultured for 24 hrs were used as reference controls.
Measurement of P3-lux expression
For determination of the levels of P3-lux expression in culture, 105 ml−1 LAC P3-lux strain was suspended in TSB and luminescence emitted from P3-lux-expressing bacteria was measured using a LMax luminometer (Molecular Devices). For in vivo bioluminescence imaging (BLI), mice were sacrificed, the skin dressing removed and immediately placed into the light-tight chamber of the CCD camera system (IVIS200, Xenogen). Luminescence emitted from lux-expressing bacteria in the tissue was quantified using the software program living image (Xenogen).
Supplementary Material
Acknowledgments
We thank S. Koonse for animal husbandry, J. Whitfield for ELISA assays, S. Meshinchi for electron microscopy analysis, V. Basrur for mass spectrometry analysis, K. Kidwell for advise with statistical analysis, M.K. Oyoshi and R.S. Geha for experimental advise, Vee Y. Tan for help with constructing the LAC P3-lux strain, and A. Burberry for critical review of the manuscript. Y. Nakamura was supported by fellowships from Chiba University Global COE Program, the Cell Science Research Foundation and Kanae Foundation for the Promotion of Medical Science. J. Oscherwitz and K. Cease were supported by Department of Veterans Affairs Merit Award I01BX000429. A. E. Villaruz, G. Y. C. Cheung and M. Otto were supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), NIH. This work supported by NIH grants R01AR059688 to G. N. and R01HL062996 to J. B. T. and funds to the Michigan Comprehensive Cancer Center Immunology Monitoring Core from the University of Michigan's Cancer Center Support Grant.
Footnotes
Author Contributions
Y.N., N.I., and G.N. designed the research. Y.N. conducted the experiments and analyzed data with the help of R.M.-P., S. M. C., and M.H. J.O., K.B.C., J. B. T., and M.J.M. generated and provided critical reagents or material. A.E.V, G.Y.C., and M.O. engineered bacterial strains. Y.N and G.N. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Williams H, Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol. 2006;118:209–213. doi: 10.1016/j.jaci.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 2.Elias PM, Steinhoff M. "Outside-to-inside" (and now back to "outside") pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:298–310. doi: 10.1007/s12016-011-8252-4. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;351:1715–1721. doi: 10.1016/S0140-6736(97)12082-7. [DOI] [PubMed] [Google Scholar]
- 6.Leung DY, Walsh P, Giorno R, Norris DA. A potential role for superantigens in the pathogenesis of psoriasis. J Invest Dermatol. 1993;100:225–228. doi: 10.1111/1523-1747.ep12468941. [DOI] [PubMed] [Google Scholar]
- 7.Supajatura V, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selander C, Engblom C, Nilsson G, Scheynius A, Andersson CL. TLR2/MyD88-dependent and -independent activation of mast cell IgE responses by the skin commensal yeast Malassezia sympodialis. J Immunol. 2009;182:4208–4216. doi: 10.4049/jimmunol.0800885. [DOI] [PubMed] [Google Scholar]
- 9.Schmaler M, et al. Lipoproteins in Staphylococcus aureus mediate inflammation by TLR2 and iron-dependent growth in vivo. J Immunol. 2009;182:7110–7118. doi: 10.4049/jimmunol.0804292. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 11.Kretschmer D, et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 13.Morfeldt E, Tegmark K, Arvidson S. Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol Microbiol. 1996;21:1227–1237. doi: 10.1046/j.1365-2958.1996.751447.x. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama Y, et al. Changes in the agr locus affect enteritis caused by methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2009;47:1528–1535. doi: 10.1128/JCM.01497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cogen AL, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada N, Matsushima H, Tagaya Y, Shimada S, Katz SI. Generation of a large number of connective tissue type mast cells by culture of murine fetal skin cells. J Invest Dermatol. 2003;121:1425–1432. doi: 10.1046/j.1523-1747.2003.12613.x. [DOI] [PubMed] [Google Scholar]
- 17.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- 18.Nilsson IM, Hartford O, Foster T, Tarkowski A. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect Immun. 1999;67:1045–1049. doi: 10.1128/iai.67.3.1045-1049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoll H, Dengjel J, Nerz C, Gotz F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun. 2005;73:2411–2423. doi: 10.1128/IAI.73.4.2411-2423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack D, et al. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis KP, et al. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68:3594–3600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Luong TT, Lee CY. Improved single-copy integration vectors for Staphylococcus aureus. J Microbiol Methods. 2007;70:186–190. doi: 10.1016/j.mimet.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung M, et al. Identification of major clonal complexes and toxin producing strains among Staphylococcus aureus associated with atopic dermatitis. Microbes Infect. 2011;13:189–197. doi: 10.1016/j.micinf.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa M, et al. Differential release and distribution of Nod1 and Nod2 immunostimulatory molecules among bacterial species and environments. J Biol Chem. 2006;281:29054–29063. doi: 10.1074/jbc.M602638200. [DOI] [PubMed] [Google Scholar]
- 26.Miller RD, Fung DY. Amino acid requirements for the production of enterotoxin B by Staphylococcus aureus S-6 in a chemically defined medium. Appl Microbiol. 1973;25:800–806. doi: 10.1128/am.25.5.800-806.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimbaldeston MA, et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vig M, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung DY, et al. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol. 1990;85:927–933. doi: 10.1016/0091-6749(90)90079-j. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima S, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol. 2012;129:1048–1055. doi: 10.1016/j.jaci.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travers JB, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy Clin Immunol. 2010;125:146–152. e141–e142. doi: 10.1016/j.jaci.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 33.Seidl K, et al. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:5631–5639. doi: 10.1128/AAC.05251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barman M, et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.